Abstract
Background: One of the main characteristics that differentiate Asian and European cultures is self-construal, the former has a higher tendency to have interdependent, and the latter tends to have an independent self. Recent structural brain imaging studies have shown that the Prefrontal Cortex (PFC) is thinner in Asian than European individuals, attributed to a need for reduction of reward relevant to self to maximize the reward relevant to the group. There is more to find out about differential cortical thickness and behavioral correlates across these cultural groups
Aim: This study was performed to investigate the associations between cultural group membership, age, cortical thickness, and two sets of reward-related behaviors (e.g., reward responsiveness and prosocial behaviors) in a national sample of 9/10-year-old children in the U.S.
Materials and Methods: For this cross-sectional study, we used demographic, socioeconomic, structural, and behavioral data from the Adolescent Brain Cognitive Development (ABCD) study. Our analytical sample included 5942 American children between the ages of 9 and 10 who were either European (n=5741) or Asian (n=201). The cortical thickness for various Regions Of Interest (ROIs) was measured using Structural Magnetic Resonance Imaging (sMRI). Two aspects of the behavioral profile, reward sensitivity, and prosocial behaviors, were also measured using self-report data. As a proxy of self-construal, culture was the independent variable or the moderator variable, depending on the model. Mixed-effects regression models were used for data analysis to adjust for nested data across families and study sites.
Results: In the overall sample, asian children had a smaller thickness of cortical regions across all ROIs than European children. Age did not interact with culture on cortical thickness, suggesting a similar rate of pruning across cultures. Culture showed statistically significant interactions with cortical thickness across ROIs within and beyond PFC on children’s reward responsiveness and prosocial behaviors, indicating stronger associations for Asian than European children.
Conclusion: Compared to European children, Asian children show lower cortical thickness across ROIs, a phenomenon that is not limited to PFC and is not due to a differential rate of age-related pruning. There are stronger associations between the thickness of several cortical areas with prosocial behaviors and reward responsiveness in Asian children relative to European children, extending the existing literature on culture, cerebral cortex, and reward salience. Our findings support the hypothesis that Asian children’s cortical volume changes are a cultural adaptation to maximize conformity and harmony with the group in Asian culture by reducing the relevance of reward salience of self and maximizing it for the community. More research is needed on cultural differences in behavioral correlates of structural and functional measures of cortical regions among European and Asian children.
Keywords: Population groups; Prefrontal cortex; Cerebral cortex; Morphometry; cortical thickness; Culture; Ethnic groups; Asian; European
Background
Cultural psychologists such as Markus and Kitayama [1] have introduced self-construals of interdependence versus independence as a primary distinction of Asian from European culture [2]. Also described as one of the main East-West differences [3], a considerable body of research has shown that Asian individuals differ in their definition and view of self, relative to others [4]. One of the most robust findings on the contrasts between these two cultural patterns are seen in the higher social orientation of Asian than European culture, a finding repeatedly documented by multiple surveys comparing Asian and European individuals inside and outside the United States [5]. Asians place higher relative importance on others relative to self, compared to Europeans [1]. Although some questions have been raised regarding the validity and reliability of these findings and constructs [3,5,6], the consensus is that this cultural difference between Asians and Europeans is both valid and significant [7].
The systematically higher significance of social orientation in Asian culture than European culture has implications for behavioral profiles of Asian versus European cultures. Some of the behavioral implications of social orientation might be differences in down-regulation of urges and emotion regulation to maximize conformity, social harmony, and pursuing rewards for the group rather than self [8]. However, we are not aware of any studies that have compared Asian and European cultures for the associations between brain structure and reward-related behaviors.
To prioritize relationship with others (one's social orientation toward a community) above one's self-interest [1,9,10], Asians may have a higher need for suppressing their emotions and motivations, which may be in contrast to the benefit of their group [10]. Asian’s interdependence culture, viewing the self as connected to others, emphasizing harmonious relations with others, and favoring the community's good all may require minimizing the rewards that are merely relevant to self [10]. The reward system should be consistently down-regulated, and emotion regulation should be empowered in Asians [10]. In contrast to Asian culture, European’s independent culture emphasizes uniqueness, views self as separate from others, and favors pursuing own reward independent of group [1]. As such, Europeans require far less down-regulation and regulation of emotions [11,12]. These differences may have implications for reward-related behaviors between and within Asian and European cultures [1].
Some recent research has shown that Prefrontal Cortex (PFC) differences may exist between Asian and European individuals, supporting the above hypothesis of Asian culture adaptation to these cultural differences [11,12]. In one study, Kitayama et al. studied 135 Japanese young adults and collected data on structural magnetic resonance imaging and self-construal [11]. They measured (a) independent and interdependent self-construal, (b) the degree to which individuals form vivid images of external objects (object imagery), and (c) the PFC volume, particularly for the Orbitofrontal Cortex (OFC) [11]. The authors investigated OFC because of its role in value-based decision-making. The authors hypothesized that OFC thickness, which has a role in personal goals and desires, would be inversely linked to interdependent self-construal scores. The highest level of interdependent self-construal was associated with lower OFC volume and high object imagery in that study. The authors argued that their findings are consistent with previous evidence that interdependence, as realized via obligation and duty, requires reduced self-interest and maximizes cognitive attunement to environmental context [11]. In another study [12], Kitayama et al. analyzed data of 132 young adults (both European Americans and Asian-born East Asians) for self-construal, structural MRI, and genetic data [12]. Authors found that gray matter volume of the medial prefrontal cortex and the orbitofrontal cortex were smaller among Asian than European individuals. Moreover, the difference in gray matter volume was significantly more pronounced among carriers of the 7/2-R allele of the dopamine (DRD4) gene than among non-carriers. This pattern was robust in an alternative measure assessing cortical thickness. The authors also found that among Asian carriers, the number of years spent in the U.S. was predictive of increased gray matter volume in the OFC cortex. Both these studies have provided evidence consistent with a view that culture shapes the cortical thickness [11,12].
For at least five reasons, there is a need for additional studies in this field. First, most of this literature is on adults, and less is known about the relevance of brain structure for culture in children. Second, given the replication crisis in psychological studies, there is a need to replicate a study that has recently emerged. Third, most of these studies have a small sample size, and there is a need for investigations that have larger statistical power. Fourth, past research is mainly on PFC. However, it is likely that this is a general pattern that holds for PFC and other cortical regions. As such, there is a need to explore these cultural variations in cortical thickness across Regions of Interest (ROIs). Finally, these studies have not tested differential correlations between brain structures and reward-related behaviors such as reward responsiveness and prosocial behaviors between Asian and European children.
Aims
In a national sample of 9/10-year-old American children (general population), the current study was performed with three aims in mind. First, to compare Asian and European children for cortical thickness across Regions of Interest (ROIs), particularly PFC thickness. Second, to investigate cultural differences in the associations between cortical thickness with age. Third, to investigate cultural differences in the associations between cortical thickness with reward responsiveness and prosocial behaviors. Cortical thickness across Regions of Interest (ROIs), including but not limited to PFC was expected to be smaller in Asian than European culture (Hypothesis 1). Smaller cortical thickness and stronger associations between cortical thickness and reward responsiveness and prosocial behaviors in Asian than European children are based on the existing hypothesis on differences between Asians and Europeans in group orientation, sympathy, conformity, and reward dependence, as described by Kitayama and others [1]. We also expect cortical thickness to show a stronger inverse association with rage for Asian than European children, suggesting faster pruning in Asian than European children, which would contribute to the thinner cortex in Asians than Europeans (Hypothesis 2). We also expect cortical thickness to show stronger associations with reward responsiveness and prosocial behaviors for Asian than European children (Hypothesis 2).
Materials and Methods
Design and Setting
With a cross-sectional design, this study applied a secondary analysis of data from the Adolescent Brain Cognitive Development (ABCD) study [13-17]. The ABCD is a national brain development study of American children [13,18].
Sample and Sampling
The ABCD participants were sampled from 21 sites in multiple cities across different states in the United States. The ABCD sample is mainly enrolled through the U.S. school system. The ABCD sampling strategy applied a careful design of pre-adolescents sampling across various sites [13,14,16,18-33]. To ensure that the ABCD sample is representative, the ABCD has used a weight (propensity score). Using weights (propensity scores), the final ABCD results are generalizable to the U.S., and the weighted participants are a close approximation of national sociodemographic, sex, culture, race, and ethnicity. A full description of the ABCD sample and sampling is published here [34].
Analytical Sample
This study included 5938 9/10-year-old children who had data on our study variables, including negative urgency. Children from European or Asian cultures were included. Participants from other cultural and ethnic groups such as Black, Native American, Hispanic/Latino, or other/mixed were excluded. No additional eligibility criteria were considered.
Measures and Measurements
Cerebral cortex thickness. For various Regions of Interest (ROIs), the cerebral cortex thickness was measured using harmonized sMRI across 21 study sites. Harmonization and standardization of ABCD imaging modalities are well described here [35]. The ABCD centers conducted high-resolution T1-weighted structural MRI scans (1-mm isotropic voxels) with one of the following scanners: Philips Healthcare (Andover, Massachusetts), GE Healthcare (Waukesha, Wisconsin), or Siemens Healthcare (Erlangen, Germany) [14]. All the structural MRI data were processed using FreeSurfer version 5.3.0 [36,37], in line with the standard processing pipelines [14]. The process included the removal of nonbrain tissue, the segmentation of gray and European matter [38] and the parcellation of the cerebral cortex [39]. Every scan session underwent a radiological review. Anextended quality control protocol was implemented, which included a visual inspection of T1 images and Free Surfer outputs for an acceptable quality [40]. MRI images that did not pass the quality control were excluded. The cortical parcellation in this study was based on the Desikan-Killiany atlas of ROIs [40]. Although the primary ROIs in this study were PFC and OFC, we reported values for the volumetric data provided by the ABCD data for all ROIs in cerebral cortex. One of the confounders was intra-cranial volume to adjust for the differences between skull and whole-brain size across cultures.
Culture: Culture, identified by parents, was a categorical variable with the following levels: Asian and European (reference group). This variable was the independent variable for aim 1 and the effect modifier for the other aims.
Parental educational attainment: Parental educational attainment was a five-level categorical variable. Responses included 1= less than high school diploma; 2 = high school diploma or GED; 3 = some college; 4 = college degree; and 5 = some graduate education.
Parental marital status: The household's marital status was a dichotomous variable: married = 1 and non-married = 0.
Family income: Family income was a three-level categorical variable. The item used to measure parental educational attainment was: "What is your total combined parental educational attainment for the past 12 months? This should include income (before taxes and deductions) from all sources, wages, rent from properties, social security, disability and veteran's benefits, unemployment benefits, workman". Levels were 1= less than $50,000; 2 = $50,000 to $99,000; 3 = $100,000 or more.
Age: Age was measured in months and was a continuous measure.
Sex: Sex, 1 = males and 0 = females, was a dichotomous variable.
Data Analysis
We used the Data Analysis and Exploration Portal (DEAP) for data analysis. Developed as a part of the National Data Archive (NDA), National Institutes of Health (NIH), DEAP is a data analysis platform that uses R software to perform analysis of the ABCD data. As ABCD participants are nested within families, who are themselves sampled across 21 sites, DEAP uses mixed (random) effect models for analysis of data. Such an approach adjusts for the ABCD data's nested nature. Standard Errors (SEs) are estimated for various levels of analysis (individual, family, site). To describe our sample, we reported mean Standard Deviation (SD) for continuous variables, frequencies, and percentages for categorical variables in the pooled sample and by culture. We used Chi-square or independent sample t-test for bivariate analysis. Mixed-effects multivariable models were performed. In these models, cortical thickness for ROIs was the outcome, culture was the moderator, and sex, age, household income, parental education, and family structure were covariates. All these models controlled for study site and family ID as well. For models in the pooled sample, we also controlled for whole-brain size (intracranial volume). First, we ran models with culture as the main independent variable and ROI cortical thickness as the outcomes. Then we ran models within each culture (culture as strata), with cortical thickness across ROIs as the predictor and reward responsiveness or prosocial behavior as the outcomes. We also ran models with and without interaction for regions that showed culture-specific correlations with reward responsiveness or prosocial behavior. Regression coefficient (b), and p-values were reported for each model parameter. Appendix 1 shows the formula used in the DEAP system. Appendix 2 is a fit of our models. Appendix 3 reports the results of our regression to test the effect of culture on ROIs without whole-brain volume as a covariate.
Ethical Aspect
For this study, we used a fully de-identified data set and therefore the study was exempted from a full review Institutional Review Board (IRB). However, the main study protocol, the ABCD, was approved by the IRB at the University of California, San Diego (UCSD), and several other institutions. Participants signed consent or assent depending on their age [18].
Results
Descriptive Data
Table 1 depicts the summary statistics of the pooled sample and by culture. The current analysis was performed on 5942, 9/10-year-old children, from which 5741 were European and 201 were Asian.
Level
All
White
Asian
p
N
5942
5741
201
Mean (SD)
Mean (SD)
Mean (SD)
Age (Month)
119.12 (7.51)
119.11 (7.49)
119.47 (7.87)
0.008
Intracranial volume
1543187.28 (143168.77)
1545817.78 (142809.28)
1468054.21 (132960.39)
<0.001
n(%)
n(%)
n(%)
Sex
F
2811 (47.3)
2710 (47.2)
101 (50.2)
0.556
M
3131 (52.7)
3031 (52.8)
100 (49.8)
Parental Education
<HSDiploma
27 (0.5)
26 (0.5)
1 (0.5)
<0.001
HS Diploma/GED
174 (2.9)
172 (3.0)
2 (1.0)
Some College
1085 (18.3)
1071 (18.7)
14 (7.0)
Bachelor
1870 (31.5)
1814 (31.6)
56 (27.9)
Post Graduate Degree
2786 (46.9)
2658 (46.3)
128 (63.7)
Married Family
No
1019 (17.1)
994 (17.3)
25 (12.4)
<0.001
Yes
4923 (82.9)
4747 (82.7)
176 (87.6)
Household income
<50K
757 (12.7)
731 (12.7)
26 (12.9)
<0.001
>=50K&<100K
1797 (30.2)
1748 (30.4)
49 (24.4)
>=100K
3388 (57.0)
3262 (56.8)
126 (62.7)
Table 1: Descriptive data overall and by culture.
Table 2 shows values of cortical thickness across ROIs of right and left hemispheres for European and Asian participants. As this table shows, the thickness of all the ROIs was larger for European than Asian children. This pattern was universal and did not have any exceptions.
Right
Left
All
White
Asian
p
All
White
Asian
p
N
5942
5741
201
5942
5741
201
Bankssts
2.91 (0.18)
2.91 (0.18)
2.86 (0.19)
<0.001
2.82 (0.17)
2.82 (0.17)
2.77 (0.18)
< 0.001
Caudal anterior cingulate
2.74 (0.21)
2.74 (0.20)
2.75 (0.22)
0.006
2.88 (0.24)
2.88 (0.24)
2.85 (0.23)
0.056
Caudal middle frontal
2.85 (0.16)
2.85 (0.16)
2.79 (0.17)
<0.001
2.88 (0.16)
2.88 (0.16)
2.84 (0.16)
< 0.001
Cuneus
2.11 (0.16)
2.11 (0.16)
2.02 (0.15)
<0.001
2.08 (0.15)
2.09 (0.15)
2.00 (0.13)
< 0.001
Entorhinal
3.58 (0.37)
3.58 (0.37)
3.57 (0.37)
<0.001
3.45 (0.32)
3.46 (0.32)
3.39 (0.31)
0.002
Fusiform
2.98 (0.13)
2.98 (0.13)
2.92 (0.15)
<0.001
2.97 (0.13)
2.98 (0.13)
2.93 (0.13)
< 0.001
Inferior parietal
2.82 (0.15)
2.82 (0.15)
2.73 (0.18)
<0.001
2.79 (0.16)
2.79 (0.15)
2.73 (0.17)
< 0.001
Inferior temporal
3.12 (0.16)
3.12 (0.16)
3.00 (0.18)
<0.001
3.09 (0.16)
3.10 (0.16)
2.99 (0.18)
< 0.001
Isthmus cingulate
2.66 (0.18)
2.66 (0.18)
2.61 (0.19)
<0.001
2.71 (0.18)
2.71 (0.18)
2.66 (0.20)
< 0.001
Lateral occipital
2.42 (0.16)
2.43 (0.15)
2.29 (0.17)
<0.001
2.36 (0.15)
2.37 (0.15)
2.24 (0.17)
< 0.001
Lateral orbitofrontal
2.98 (0.16)
2.98 (0.16)
2.94 (0.16)
<0.001
3.00 (0.16)
3.01 (0.16)
2.92 (0.15)
< 0.001
Lingual
2.25 (0.13)
2.26 (0.13)
2.20 (0.11)
<0.001
2.22 (0.13)
2.22 (0.13)
2.16 (0.12)
< 0.001
Medial orbitofrontal
2.75 (0.18)
2.75 (0.18)
2.77 (0.18)
<0.001
2.73 (0.17)
2.73 (0.17)
2.74 (0.18)
0.19
Middle temporal
3.23 (0.19)
3.23 (0.18)
3.10 (0.21)
<0.001
3.20 (0.19)
3.21 (0.19)
3.10 (0.23)
< 0.001
Para hippocampal
2.98 (0.24)
2.99 (0.24)
2.84 (0.25)
<0.001
3.03 (0.27)
3.04 (0.27)
2.90 (0.28)
< 0.001
Para central
2.76 (0.14)
2.77 (0.14)
2.73 (0.16)
<0.001
2.77 (0.16)
2.77 (0.16)
2.70 (0.16)
< 0.001
Pars opercularis
2.90 (0.15)
2.91 (0.15)
2.86 (0.15)
<0.001
2.92 (0.14)
2.92 (0.14)
2.85 (0.16)
< 0.001
Pars orbitalis
3.11 (0.21)
3.11 (0.21)
3.06 (0.20)
<0.001
3.13 (0.21)
3.13 (0.21)
3.07 (0.23)
< 0.001
Pars triangularis
2.81 (0.17)
2.81 (0.17)
2.76 (0.18)
<0.001
2.83 (0.16)
2.83 (0.16)
2.79 (0.17)
< 0.001
Pericalcarine
1.77 (0.15)
1.78 (0.15)
1.69 (0.14)
<0.001
1.78 (0.15)
1.79 (0.15)
1.68 (0.13)
< 0.001
Postcentral
2.32 (0.16)
2.32 (0.16)
2.25 (0.18)
<0.001
2.35 (0.16)
2.36 (0.16)
2.27 (0.16)
< 0.001
Posterior cingulate
2.73 (0.14)
2.73 (0.14)
2.73 (0.14)
<0.001
2.78 (0.15)
2.78 (0.15)
2.76 (0.16)
0.066
Precentral
2.77 (0.15)
2.77 (0.15)
2.69 (0.18)
<0.001
2.80 (0.15)
2.81 (0.15)
2.73 (0.18)
< 0.001
Precuneus
2.73 (0.13)
2.73 (0.13)
2.68 (0.13)
<0.001
2.73 (0.13)
2.73 (0.13)
2.65 (0.13)
< 0.001
Rostral anterior cingulate
3.06 (0.22)
3.06 (0.22)
3.05 (0.21)
<0.001
3.17 (0.21)
3.17 (0.21)
3.08 (0.22)
< 0.001
Rostral middle frontal
2.70 (0.16)
2.70 (0.16)
2.65 (0.16)
<0.001
2.74 (0.16)
2.74 (0.16)
2.69 (0.16)
< 0.001
Superior frontal
3.10 (0.15)
3.11 (0.15)
3.07 (0.16)
<0.001
3.14 (0.16)
3.15 (0.16)
3.10 (0.16)
< 0.001
Superior parietal
2.51 (0.14)
2.51 (0.14)
2.43 (0.15)
<0.001
2.51 (0.14)
2.51 (0.14)
2.43 (0.14)
< 0.001
Superior temporal
3.14 (0.16)
3.15 (0.16)
3.06 (0.17)
<0.001
3.12 (0.18)
3.12 (0.17)
3.02 (0.20)
< 0.001
Supramarginal
2.87 (0.18)
2.88 (0.18)
2.75 (0.22)
<0.001
2.88 (0.18)
2.88 (0.18)
2.79 (0.21)
< 0.001
Frontal pole
3.18 (0.31)
3.18 (0.31)
3.14 (0.30)
<0.001
3.22 (0.32)
3.23 (0.32)
3.19 (0.31)
0.105
Temporal pole
3.95 (0.32)
3.95 (0.31)
3.84 (0.31)
<0.001
3.82 (0.30)
3.82 (0.30)
3.75 (0.27)
0.001
Transverse temporal
2.80 (0.20)
2.80 (0.20)
2.72 (0.19)
<0.001
2.77 (0.21)
2.78 (0.20)
2.71 (0.22)
< 0.001
Insula
3.31 (0.16)
3.31 (0.16)
3.24 (0.17)
<0.001
3.31 (0.14)
3.32 (0.14)
3.28 (0.13)
< 0.001
Table 2: Descriptive data overall and by culture.
Culture and Cortical Thickness (ROI analysis)
Table 3 summarizes our mixed-effects regression model that adjusted for the nested nature of the data and tested the effect of culture on cortical thickness across various ROIs. These models were performed in the overall (pooled) sample that included European and Asian children. As shown, for most ROIs, Asian culture was associated with a negative and significant beta coefficient indicating lower cortical thickness in Asian than European children, while covariates were adjusted.
ROI name
Negative log10 P-value (lh)
Beta Weights (lh)
Negative log10 P-value (rh)
Beta Weights (rh)
Bankssts
3.2301
-0.0424
2.3142
-0.0366
Caudal anterior cingulate
0.5672
-0.0186
0.0053
-0.0002
Caudal middle frontal
1.4709
-0.0234
3.4714
-0.0413
Cuneus
11.4608
-0.0737
9.4859
-0.0707
Entorhinal
2.6938
-0.0711
0.0248
-0.0018
Fusiform
4.9847
-0.0400
5.9545
-0.0459
Inferior parietal
4.8452
-0.0478
10.7796
-0.0722
Inferior temporal
15.7576
-0.0949
18.0939
-0.0996
Isthmus cingulate
4.6350
-0.0559
4.1065
-0.052
Lateral occipital
20.2574
-0.0974
20.8165
-0.1005
Lateral orbitofrontal
10.3002
-0.0736
3.8933
-0.0435
Lingual
8.0137
-0.0535
5.9903
-0.0463
Medial orbitofrontal
0.0641
0.0021
0.0720
0.0024
Middle temporal
9.8454
-0.0875
15.6412
-0.1069
Para hippocampal
14.5201
-0.1547
20.5992
-0.161
Para central
5.5265
-0.0521
2.0778
-0.0273
Pars opercularis
5.1400
-0.0454
2.3286
-0.0304
Pars orbitalis
4.4253
-0.0615
3.5169
-0.0532
Pars triangularis
2.4077
-0.0323
2.3735
-0.0346
Pericalcarine
16.5966
-0.0882
10.944
-0.0715
Postcentral
8.1079
-0.0627
4.3371
-0.0453
Posterior cingulate
0.2867
-0.0071
0.3056
-0.0067
Precentral
6.4715
-0.0539
6.6704
-0.0536
Precuneus
11.6518
-0.0654
7.1194
-0.0497
Rostral anterior cingulate
7.1207
-0.0812
1.3442
-0.0307
Rostral middle frontal
2.7681
-0.0346
2.472
-0.0333
Superior frontal
2.6024
-0.0345
2.0743
-0.0289
Superior parietal
7.9063
-0.0571
7.6605
-0.0553
Superior temporal
9.7974
-0.0781
8.5657
-0.0683
Supramarginal
6.5838
-0.0642
13.0203
-0.0949
Frontal pole
0.6475
-0.0278
1.1316
-0.0394
Temporal pole
3.1758
-0.0727
5.0889
-0.1012
Transverse temporal
4.9308
-0.0641
8.4705
-0.0864
Insula
3.087
-0.0341
5.8246
-0.0546
Table 3: Association between Asian ethnicity and cortical thickness (ROIs) (Intracranial volume controlled).
Cortical Thickness and Reward Responsiveness (ROI analysis)
Table 4 summarize regression coefficients in our two mixed-method regression models performed in Asian and European children, respectively. These models showed stronger associations between cortical thickness in Asian than European children.
Asian
European
Left
Right
Left
Right
ROI name
Negative log10 P-value (lh)
Beta Weights (lh)
Negative log10 P-value (rh)
Beta Weights (rh)
Negative log10 P-value (lh)
Beta Weights (lh)
Negative log10 P-value (rh)
Beta Weights (rh)
Bankssts
0.0488
-0.1505
0.2672
-0.592
0.3576
-0.1706
0.2556
0.1242
Caudal anterior cingulate
0.2469
-0.4712
0.0067
0.017
1.2769
-0.3168
0.5106
-0.1895
Caudal middle frontal
1.35
2.351
0.1331
0.3676
1.0391
-0.4098
0.9976
-0.3834
Cuneus
0.2877
0.9513
0.6825
1.6181
0.5705
-0.2859
0.1229
-0.0764
Entorhinal
0.394
-0.5236
0.0256
-0.0369
1.0455
-0.2038
0.133
-0.0352
Fusiform
0.0332
0.1421
0.2036
0.6482
0.2432
-0.172
0.014
-0.0114
Inferior parietal
1.4706
2.3187
0.6056
1.2256
1.2734
-0.4692
0.9546
-0.3962
Inferior temporal
1.1176
1.845
0.4307
0.9417
5.3473
-1.0797
1.964
-0.6159
Isthmus cingulate
0.1822
-0.4466
0.5693
-1.0833
0.0177
-0.0105
0.3984
-0.1781
Lateral occipital
1.6664
2.706
1.8074
2.756
0.8555
-0.3797
0.3681
-0.1972
Lateral orbitofrontal
1.04
-2.1045
0.5153
-1.2186
2.9373
-0.7955
1.8366
-0.5906
Lingual
0.1495
-0.5825
0.3435
-1.3126
0.6638
0.3637
0.0583
0.0457
Medial orbitofrontal
1.211
-2.0091
1.8852
-2.6035
1.6586
-0.519
2.1929
-0.5929
Middle temporal
1.3867
1.681
0.0721
0.1767
0.6521
-0.2378
0.1708
-0.0865
Para hippocampal
0.3708
-0.5277
0.0332
-0.0705
1.3502
-0.2835
0.1114
-0.0468
Para central
0.7876
1.6202
1.231
2.1821
0.7908
-0.3369
0.3636
-0.2083
Pars opercularis
0.0227
-0.0753
0.2633
-0.7378
0.4801
-0.2578
1.3592
-0.5089
Pars orbitalis
0.0956
0.2071
0.8185
1.3516
0.6634
-0.2285
1.1415
-0.3363
Pars triangularis
0.4741
1.0977
0.3813
-0.8758
0.7588
-0.3332
0.2386
0.1253
Pericalcarine
0.0985
0.3789
0.6588
1.6947
1.2184
0.4916
0.0628
-0.0442
Postcentral
1.8134
2.727
1.0149
1.712
0.0307
0.0208
0.3109
0.1646
Posterior cingulate
0.0377
0.1287
0.2131
0.6737
0.3466
0.1889
0.0259
0.0205
Precentral
1.1317
1.9381
1.192
1.9773
1.4757
-0.5277
0.4312
-0.2312
Precuneus
0.8339
2.1814
0.0711
0.2867
0.4386
-0.2644
0.8558
-0.4343
Rostral anterior cingulate
0.0376
-0.0911
0.1793
-0.3931
2.1397
-0.4918
0.5049
-0.1783
Rostral middle frontal
0.3093
0.8735
0.1162
0.3523
0.845
-0.3548
0.8909
-0.3646
Superior frontal
0.4213
1.0424
0.8051
1.6835
0.5508
-0.2553
1.2846
-0.4844
Superior parietal
1.1816
2.3982
1.447
2.6602
0.4179
-0.2321
0.3161
-0.1897
Superior temporal
0.195
0.444
0.2379
-0.6233
0.3899
-0.18
0.0142
-0.0094
supramarginal
1.48
1.8586
0.4092
0.7591
0.6205
-0.2498
0.0987
0.053
Frontal pole
0.5738
-0.7088
1.3534
-1.318
1.0468
-0.2011
1.4421
-0.2597
Temporal pole
0.9926
-1.1301
0.3872
-0.4889
0.158
-0.0508
1.4097
-0.2518
Transverse temporal
0.0059
0.0142
1.0795
1.6818
0.2781
0.119
0.1189
-0.0565
insula
0.1497
0.567
0.1218
0.3863
1.4893
-0.5679
0.9273
-0.3792
Table 4: Association between reward responsiveness and cortical thickness (ROIs) in Asian and White children.
Cortical Thickness and Prosocial Behaviors (ROI analysis)
Table 5 summarizes regression coefficients in our two mixed-effects regression models that adjusted for the nested nature of the data. These models were performed in Asian and European children. These models showed larger associations between cortical thicknesses in Asian than European children. Compared to European children, Asian children showed a stronger negative association between cortical thickness at the regions right superior frontal (Model 5-a) and right medial orbitofrontal (Model 5-g) with prosocial behaviors. Compared to European children, Asian children showed a stronger positive association between cortical thickness at the regions left inferior temporal (Model 5-b) and left lateral occipital (Model 5-c), right superior frontal (Model 5-e), left middle temporal (Model 5-f), right lateral occipital (Model 5-h), right paracentral (Model 5-i), and left precentral (Model 5-k) with reward responsiveness (Table 7).
Asian
White
Left
Right
Left
Right
ROI name
Negative log10 P-value
Beta Weight
Negative log10 P-value
Beta Weight
Negative log10 P-value
Beta Weight
Negative log10 P-value
Beta Weight
Bankssts
0.1331
0.0376
0.2868
-0.0666
0.4633
-0.0205
0.0403
-0.0023
Caudal anterior cingulate
0.6301
0.1014
1.7191
0.2129
0.3752
0.0127
0.8204
-0.0259
Caudal middle frontal
0.4149
-0.1042
1.8948
-0.2859
1.3999
-0.049
0.5079
-0.0233
Cuneus
1.4939
0.3267
0.4755
0.128
0.0296
0.0021
0.0096
0.0006
Entorhinal
0.2711
-0.0402
0.9538
0.0866
0.0577
-0.0018
0.56
0.0111
Fusiform
0.9625
-0.264
0.404
-0.1235
0.0192
-0.0016
0.7396
0.0372
Inferior parietal
0.0625
-0.02
0.2154
-0.0597
0.2821
0.0152
0.0189
0.0013
Inferior temporal
0.03
0.0095
0.2758
0.0709
0.0624
-0.0039
0.3876
0.0193
Isthmus cingulate
1.3355
-0.2127
2.0848
-0.2803
0.7622
-0.0277
1.9662
-0.0518
Lateral occipital
0.4104
0.1035
0.1846
0.0543
0.2417
-0.014
0.0521
-0.0034
Lateral orbitofrontal
1.3724
-0.2677
0.19
-0.0588
1.587
-0.053
1.1463
-0.0424
Lingual
0.3148
-0.1139
0.0007
-0.0004
0.6873
0.0361
0.1934
0.013
Medial orbitofrontal
0.6858
-0.1378
0.7281
-0.144
0.8729
-0.0334
0.5614
-0.0232
Middle temporal
1.261
0.1644
0.0458
-0.0122
0.3871
-0.016
0.1373
-0.007
Para hippocampal
0.6872
0.0889
0.9985
0.1313
1.1833
-0.0251
0.98
-0.0256
Para central
0.2526
-0.0709
0.6138
-0.1436
0.3903
-0.0194
1.1259
-0.046
Pars opercularis
0.0995
-0.0315
2.1317
-0.3476
0.3357
-0.0192
1.454
-0.0515
Pars orbitalis
1.67
-0.199
0.0646
0.0178
0.3399
-0.0134
0.8224
-0.0261
Pars triangularis
0.2085
0.0597
1.165
-0.2125
0.3324
-0.0174
0.1973
-0.0104
Pericalcarine
0.014
0.006
0.8581
0.2141
0.0812
0.0054
0.3936
-0.0209
Postcentral
1.0279
-0.1977
0.6554
-0.1296
0.5257
-0.0246
0.5475
-0.025
Posterior cingulate
0.2159
-0.0663
0.1589
-0.0567
0.449
-0.0224
2.0249
-0.0706
Precentral
0.0224
-0.0069
0.0817
-0.0241
0.4898
-0.0239
0.0925
-0.0061
Precuneus
0.3464
-0.1207
0.2982
-0.1089
0.32
0.0201
0.0106
0.0009
Rostral anterior cingulate
0.059
-0.0152
0.0394
0.0106
0.0325
0.0016
0.7407
-0.0231
Rostral middle frontal
1.491
-0.2726
1.3301
-0.2542
0.9214
-0.0369
0.6286
-0.0278
Superior frontal
1.0819
-0.2132
1.7026
-0.2985
0.8798
-0.0348
1.5042
-0.0525
Superior parietal
0.4942
-0.1365
0.34
-0.1019
0.124
-0.0082
0.3276
-0.0189
Superior temporal
0.2063
0.0499
0.037
0.0124
1.4281
-0.0445
1.0875
-0.0397
supramarginal
0.1917
-0.0439
0.6964
-0.1197
0.2047
-0.0102
0.1126
-0.0059
Frontal pole
0.2904
-0.0442
0.6115
-0.0779
0.2349
-0.0064
0.3177
0.0085
Temporal pole
0.2425
-0.0401
0.031
0.0056
0.2391
0.0069
0.3573
0.009
Transverse temporal
0.7616
-0.1199
0.1134
-0.0301
0.8301
-0.0265
0.3107
-0.0126
Insula
0.962
-0.2535
0.6734
-0.162
0.1542
0.0101
0.9834
0.0386
Table 5: Association between prosocial behaviors and cortical thickness (ROIs) in Asian and White children.
ROI name
Negative log10 P-value (lh)
Beta Weights (lh)
Negative log10 P-value (rh)
Beta Weights (rh)
Negative log10 P-value (lh)
Beta Weights (lh)
Negative log10 P-value (rh)
Beta Weights (rh)
Bankssts
0.2788
0.001
0.1
0.0005
5.2373
-0.0014
3.2385
-0.0011
Caudal anterior cingulate
0.3732
0.0017
0.1594
-0.0008
2.9414
-0.0014
5.3541
-0.0017
Caudal middle frontal
0.1021
0.0004
0.1594
0.0006
0.8853
0.0004
0.8911
0.0004
Cuneus
0.0119
0
0.4356
-0.0012
17.839
-0.0023
18.3632
-0.0025
Entorhinal
0.021
-0.0002
0.2732
-0.0021
0.3425
0.0004
1.4353
0.0014
Fusiform
0.7895
0.0015
1.0482
0.0022
8.4526
-0.0013
3.6518
-0.0009
Inferior parietal
0.0403
0.0002
0.1666
0.0007
3.7789
-0.0011
1.9072
-0.0007
Inferior temporal
0.2657
0.001
0.9488
0.0026
0.7791
-0.0004
0.5498
-0.0003
Isthmus cingulate
1.5084
-0.0037
0.3482
-0.0013
2.7966
-0.001
10.0745
-0.0021
Lateral occipital
0.6327
0.0017
0.4467
0.0014
3.2398
-0.0009
1.5573
-0.0006
Lateral orbitofrontal
0.0701
0.0003
0.0807
0.0003
6.7518
-0.0014
9.0631
-0.0017
Lingual
0.2916
-0.0007
0.7233
-0.0013
18.3967
-0.0021
16.2414
-0.002
Medial orbitofrontal
0.1219
0.0005
0.1182
0.0005
11.0468
-0.002
10.4659
-0.0021
Middle temporal
0.1017
0.0005
0.6082
0.0022
0.1986
-0.0002
0.3354
-0.0002
Para hippocampal
0.2703
0.0016
0.121
-0.0007
2.1801
-0.0013
0.6597
-0.0005
Para central
0.6155
0.0017
1.0359
0.0025
2.0815
-0.0007
4.1057
-0.001
Pars opercularis
0.2312
0.0008
0.2753
0.0009
1.4295
-0.0005
0.3017
-0.0002
Pars orbitalis
0.3002
0.0014
0.4109
-0.0015
5.0801
-0.0016
3.1397
-0.0012
Pars triangularis
0.4992
0.0015
0.1099
0.0004
5.621
-0.0013
0.7233
-0.0004
Pericalcarine
0.9137
-0.0018
0.7524
-0.0017
2.6285
-0.0008
2.6804
-0.0008
Postcentral
0.1092
-0.0004
0.4426
-0.0015
2.8475
-0.0009
2.1011
-0.0008
Posterior cingulate
0.4265
0.0012
0.2314
0.0007
2.1815
-0.0007
4.8134
-0.001
Precentral
0.4953
0.0016
0.4008
0.0013
2.4878
0.0008
2.1676
0.0007
Precuneus
0.7363
0.0015
0.7498
0.0015
10.0874
-0.0015
13.7462
-0.0018
Rostral anterior cingulate
0.0723
-0.0004
1.3513
-0.0038
4.6528
-0.0016
11.9773
-0.0027
Rostral middle frontal
0.4999
-0.0013
0.1416
-0.0005
1.3063
-0.0006
3.1505
-0.001
Superior frontal
0.1254
0.0005
0.2029
0.0007
0.0427
0
1.0838
-0.0005
Superior parietal
0.5152
0.0013
0.2102
0.0007
4.1397
-0.001
2.7658
-0.0008
Superior temporal
0.5328
0.0019
0.9002
0.0023
0.616
0.0004
0.0369
0.0000
supramarginal
0.3743
0.0016
0.2242
0.001
0.0807
-0.0001
0.0144
0.0000
Frontal pole
0.3365
-0.002
0.5068
-0.0026
2.8277
-0.0018
2.1344
-0.0015
Temporal pole
0.3315
0.0018
1.2292
0.0054
0.0273
0
1.5491
0.0012
Transverse temporal
0.0128
-0.0001
0.3439
-0.0013
4.3702
-0.0015
10.6994
-0.0025
Insula
0.1904
-0.0005
0.0352
0.0001
8.7824
-0.0015
5.9694
-0.0014
Table 6.a: Association between age(month) and cortical thickness (ROIs) in Asian and White children.
Compared to European children, Asian children showed a stronger negative association between cortical thickness at the regions right superior frontal (Figure 5-a) and right medial orbitofrontal (Figure 5-g) with prosocial behaviors. Compared to European children, Asian children showed a stronger positive association between cortical thickness at the regions left inferior temporal (Figure 5-b) and left lateral occipital (Figure 5-c), right superior frontal (Figure 5-e), left middle temporal (Figure 5-f), right lateral occipital (Figure 5-h), right paracentral (Figure 5-i), and left precentral (Figure 5-k) with reward responsiveness.
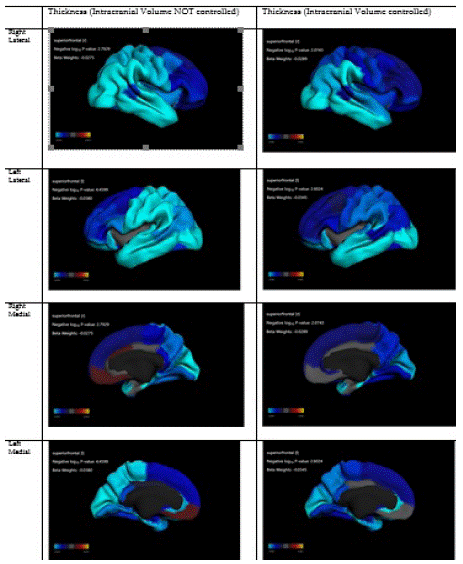
Figure 1: Association between Asian American ethnicity and cortical thickness.
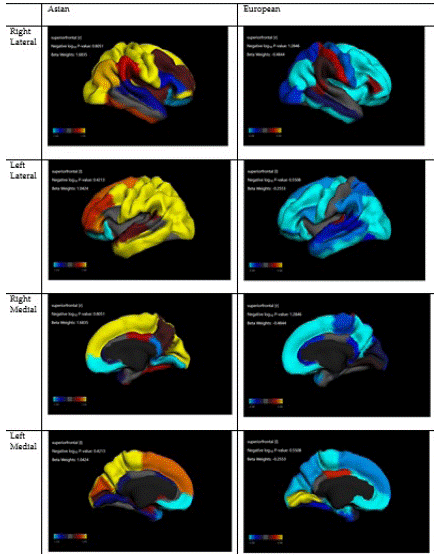
Figure 2: Association between reward responsiveness and cortical thickness in Asians vs Europeans.
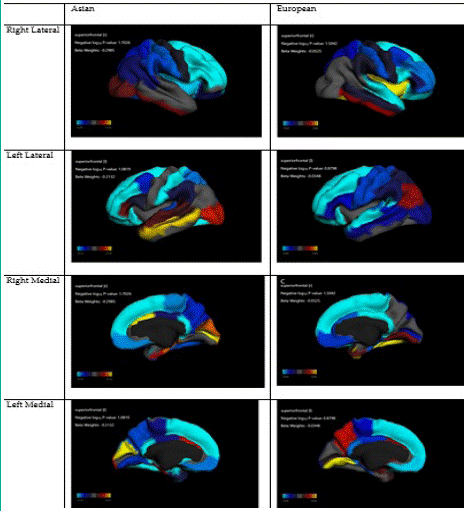
Figure 3: Association between prosocial and cortical thickness in Asian and European children.
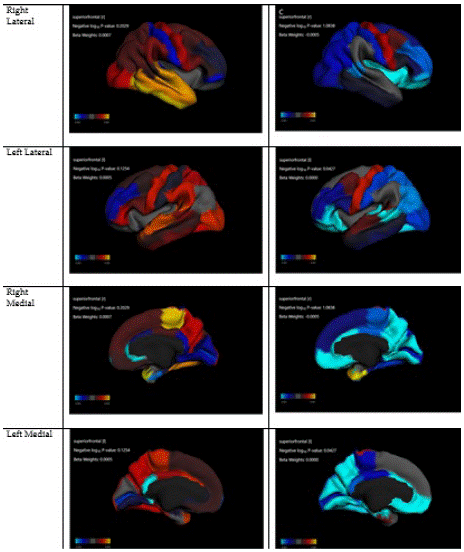
Figure 4: Association between age (month) and cortical thickness in Asians vs Europeans.
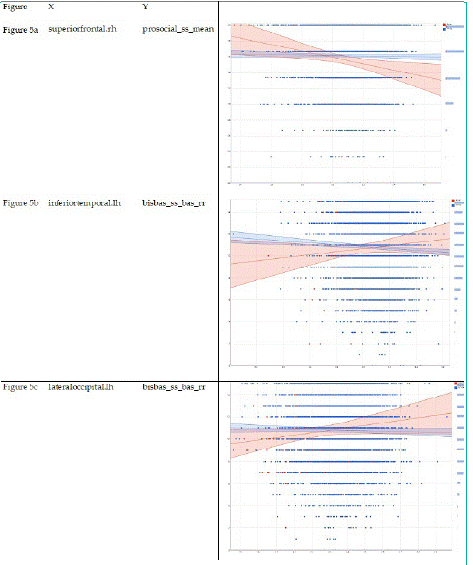
Figure 5: Interactions between culture and cortical thickness on behavioral outcomes (reward responsiveness and prosocial behaviors.
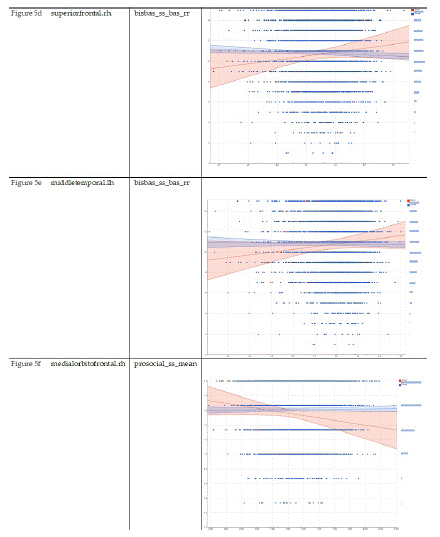
Figure 5.1:
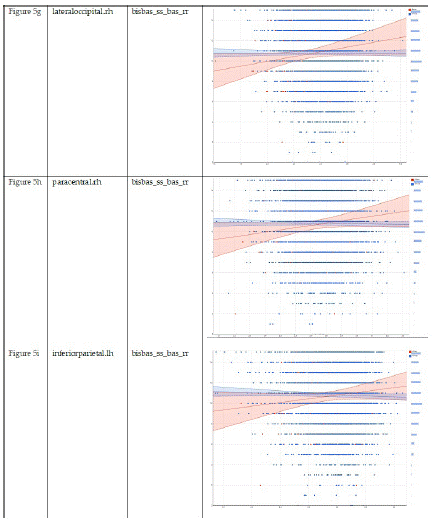
Figure 5.2:
Figure 5.3:
Discussion
Findings regarding our aim 1 showed that Asian culture is associated with a reduced cortical thickness across ROIs, a pattern which was not limited to PFC or OFC. Regarding our aim 2, the association between age and cortical thickness did not significantly differ between Asian and European children. Regarding our aim 3, we found stronger associations between cortical thickness across several ROIs with reward sensitivity and prosocial behaviors for Asian children than European children. Thus, while our hypotheses 1 and 3 were supported, our hypothesis 2 was rejected.
In the overall sample, Asian children had smaller cortical thickness across multiple ROIs than European children (aim 1). This finding is in line and is also an extension of recent research [11,12] on this topic. It is a replication of past work because Kitayama and others have reported thinner cortex in PFC or OFC in Asian compared to European individuals. It is an extension because they believed that this thin cortex in Asian versus European culture is limited to PFC or OFC. We, however, showed that the thin cortex of Asian children relative to European children is not limited to PFC or OFC and is seen across cortical regions.
Our finding is in line with the findings reported by Kitayama and colleagues [12] who analyzed data of self-construal, structural MRI, and genetics among young adults who were either European Americans and Asian-born East Asian [12]. Authors found smaller gray matter volume of the medial prefrontal cortex and the orbitofrontal cortex among Asian than European individuals. The difference in gray matter volume was more pronounced among carriers than non-carriers of the 7/2-R allele of the DRD4 gene. The study also showed that among Asian carriers, the number of years spent in the U.S. was positively correlated with gray matter volume in the OFC cortex [12]. In another study, Kitayama et al. collected structural magnetic resonance imaging, object imagery (the degree to which individuals form vivid images of external objects), and self-construal data of 135 Japanese young adults [11]. The highest level of interdependent self-construal was associated with lower OFC volume and high object imagery in that study. The authors argued that their findings are consistent with previous evidence that interdependence, as realized via obligation and duty, requires reduced self-interest and maximizes cognitive attunement to environmental context [11]. In addition to these studies, ours also proposes that as a cultural adaptation, PFC differences may exist between Asian and European individuals [11,12]. All these studies suggest that culture may correlate with cortical thickness [11,12]. The study by Kitayama et al included a sample size of 132 [12] and 135 [11], while our analysis included 5942 individuals. Another difference is that we used data of children, while the past studies are mainly adults. Finally, while our participants had a homogenous age cohort, which is very important for structural studies of the brain, past studies by Kitayama et al included participants with a wider age range.
Compared to the past work [11,12], we did not limit our analysis to PFC or OFC. Our results also showed that Asian culture is associated with thinner cortex beyond PFC and OFC. Most of past work is only focused on PFC and OFC as Kitayama and others have argued about their role in value-based decision making [11,12]. As such, those investigators have mainly focused on the PFC and OFC which have a role in personal goals and desires that would be inversely linked to the score on interdependent self-construal. Thus, this study extends what was reported by previous studies and shows that this pattern is not limited to PFC or OFC.
Our finding that culture alters the association between cortical thickness and children's reward responsiveness and prosocial behaviors is in line with the cultural moderation hypothesis [41-43]. Associations between Socioeconomic Status (SES), negativity, anger, and other biological markers are shown to differ between European and Asian individuals. For example, there has been Asian European variation in the link between social status and ability to express negative emotions against others [41-43]. These are in line with our observation on stronger inverse associations between cortical thickness and children's reward responsiveness and prosocial behaviors in Asian than European children.
Kitayama, Markus [44], and others [45] have conducted extensive work on cultural aspects of self, behaviors, and brain. Our work introduces culture as a factor that moderates both brain morphometry and their associated factors, a growing field [11,12]. Cortical thickness in the OFC/PFC and beyond may be linked to cultural orientation and self-construals [11,12]. The cerebral cortex, particularly PFC, has a major implication for decision making, emotion regulation, social behaviors, and regulation of impulsive urges [46,47]. The central role of PFC in affect regulation, emotion processing, and reward-seeking are replicated across multiple animal [48] and human [49] studies. The cerebral cortex may also have clinical implications given findings that altered PFC function [50] and structure [51] in mood disorders such as Major Depressive Disorder (MDD). Both structural [52] and functional [49] alterations of PFC as a correlate of disorders that regulate reward salience is well-established in children, youth, adults, and older adults. However, not only clinical disorders [53] but also social context shapes PFC morphometry and function. A primary social determinant of PFC is stress exposure [53], as chronic stress alters PFC function and structure [53]. Future research should investigate the additive and multiplicative effects of clinical diagnoses, stress, context, and culture as determinants and correlates of the cerebral cortex.
More research is needed on cultural correlates of structural and functional measures of PFC among European and Asian children. One hypothesis is that Asian children’s changes in PFC volumetric features and their behavioral correlates are a cultural adaptation to a relative increase in the salience of conformity and harmony with the group. Such a goal would require reduced relevance of salience of reward for self, to maximize the gain of the communities. This may, however, vary across cautious Asian sub-cultures and may reduce as they adopt the US culture through acculturation.
This study is not without methodological limitations. The first limitation is the cross-sectional design. The sample was not random as a result; we cannot generalize the results to all US children. However, we used the ABCD propensity score to maximize the comparability of cultural groups and also the generalizability of results. Our sample size was also imbalanced, with the largest sample in European children, and the smallest in Asian children. Despite the limitations listed above, our study is among the first to explore cultural variation of the link between MDD and cortical morphometry. Strength of this study was using a large national diverse sample of children.
Our result has implications for future research on culture, race/ethnicity, and neuroscience. Researchers have recently shown that cultural adaptation shapes cortical morphometry and function [11,12]. As PFC and cortical thickness have major implications for a wide range of clinical and behavioral manifestations, cultural variation in cortical features may have relevance with clinical and psychological utility. Changes in PFC and other brain regions that reduce the salience of reward may also explain why Asian culture is associated with a lower prevalence of depression and anxiety [54].
Our findings, in line with other work, suggest that researchers on brain morphometry may not reduce culture or culture to a control variable. Culture has direct effects on brain development and human behavior; however, some of their effects are through indirect effects that can be explained by contextual effects of culture. As a result, not only culture is linked to brain morphometry but also alters the correlates of brain morphometric indicators. The results may help us explain how culture alters brain structure and function through socialization in culturally diverse groups of children. Altered changes in PFC thickness in Asian culture may be an adaptation for maximizing emotion regulation, which is needed in Asian culture and can increase the individuals’ chance of conformity with others within-the group. In contrast, European independent culture may afford larger PFC thickness, which is needed for value judgment and personal decisions to maximize reward that is related to self, regardless of the group-level gains.
Additional theoretical and empirical research is needed on the heterogeneity of brain morphometry across cultures. As culture intersects with SES, class, sex, and other features, intersectional research should explore how the morphometric brain features observed here to replicate across intersectional groups. Finally, some of these cultural differences may be due to third factors such as context, life experiences, place, or SES that vary across cultural groups. It is still unknown what clinical implications such cultural variations in brain morphometry have. Research may link the brain's altered features due to culture, resilience, and vulnerability to stress and trauma. While under normal situations, one set of brain morphometry may be an asset, the same feature may become a vulnerability factor, when toxic stress is observed. This becomes more challenging as some cultures are linked to higher stigma, so psychiatric care may be delayed when needed [55]. As such, depression and some other mental health problems tend to remain untreated for a longer period in some cultures such as Asians. Thus, research should investigate societal and clinical consequences of such variations under normal development and when excessive adversity increases a psychiatric disorder's likelihood.
Conclusions
Asian and European cultural groups of children differ in their cortical thickness, which may adapt to their cultural values and needs. Asian culture emphasizes interdependence (salience of group), which reduces the relevance of individual-level rewards, while European culture emphasizes independence (salience of self), which maximizes individual-level reward’s relevance. These variations may have implications for links between the brain and behavior. While our findings replicate some of the previous work in the field, it extends the field by showing that these cortical differences are not limited to the cortical thickness of a specific brain region, as we could see the same pattern for various ROIs, within and beyond PFC and OFC.
Author Statements
Funding
Shervin Assari research is partially supported by the National Institutes of Health (NIH) research excellence award with the grant number 1R16GM145544-01. Assari is also partially supported by funds provided by The Regents of the University of California, Tobacco-Related Diseases Research Program, Grant Number No. T32IR5355.
Acknowledgments
The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/Consortium_Members.pdf. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in this report's analysis or writing. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. As such, the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH Data Archive or the National Institutes of Health. DEAP Data: The data available on DEAP is a copy of the published data of the ABCD study on the NIMH Data Archive (NDA). The data is pre-processed and merged into a single large database that is easiest understood as a spreadsheet. This process is documented on a public repository at github.com/ABCD-STUDY/analysis-nda17. Tat the time of this analysis, DEAP used the Curated Annual Release 2.0 (ABCD Release 2.0 package) under the doi: 10.15154/1503209. The ABCD data repository grows and changes over time. Check the ABCD Release 2.0 Notes for more detail. DEAP is a software provided by the Data Analysis and Informatics Center of ABCD located at the UC San Diego with generous support from the National Institutes of Health and the Centers for Disease Control and Prevention under award number U24DA041123. The DEAP project information and links to its source code are available under the resource identifier RRID: SCR_016158.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Markus HR, Kitayama S. Culture and the self: Implications for cognition, emotion, and motivation. Psychological review. 1991; 98: 224-253.
- Markus HR, Kitayama S. The cultural psychology of personality. Journal of cross-cultural psychology. 1998; 29: 63-87.
- Kitayama S, Karasawa M, Grossmann I, Na J, Varnum ME, et al. East-West Differences in Cognitive Style and Social Orientation: Are They Real?. 2019; 1.
- Markus HR, Kitayama S. The cultural construction of self and emotion: Implications for social behavior. 1994.
- Dong X, Talhelm T, Ren X. Teens in rice county are more interdependent and think more holistically than nearby wheat county. Social Psychological and Personality Science. 2019; 10: 966-976.
- San Martin A, Schug J, Maddux WW. Relational mobility and cultural differences in analytic and holistic thinking. Journal of personality and social psychology. 2019; 116: 495-518.
- Salvador CE, Kraus BT, Ackerman JM, Gelfand MJ, Kitayama S. Interdependent self-construal predicts reduced sensitivity to norms under pathogen threat: An electrocortical investigation. Biol Psychol. 2020; 157: 107970.
- Na J, Grossmann I, Varnum ME, Karasawa M, Cho Y, et al. Culture and personality revisited: Behavioral profiles and within-person stability in interdependent (vs. independent) social orientation and holistic (vs. analytic) cognitive style. Journal of personality. 2020; 88: 908-924.
- Triandis HC. The self and social behavior in differing cultural contexts. Psychological review. 1989; 96: 506-520.
- Varnum ME, Grossmann I, Kitayama S, Nisbett RE. The origin of cultural differences in cognition: The social orientation hypothesis. Current directions in psychological science. 2010; 19: 9-13.
- Kitayama S, Yanagisawa K, Ito A, Ueda R, Uchida Y, et al. Reduced orbitofrontal cortical volume is associated with interdependent self-construal. Proc Natl Acad Sci USA. 2017; 114: 7969-7974.
- Yu Q, Abe N, King A, Yoon C, Liberzon I, et al. Cultural variation in the gray matter volume of the prefrontal cortex is moderated by the dopamine D4 receptor gene (DRD4). Cereb Cortex. 2019; 29: 3922-3931.
- Alcohol Research: Current Reviews Editorial, S. NIH’s Adolescent Brain Cognitive Development (ABCD) Study. Alcohol Res. 2018; 39: 97.
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev Cogn Neurosci. 2018; 32: 43-54.
- Karcher NR, O’Brien KJ, Kandala S, Barch DM. Resting-State Functional Connectivity and Psychotic-like Experiences in Childhood: Results From the Adolescent Brain Cognitive Development Study. Biol Psychiatry. 2019; 86: 7-15.
- Lisdahl KM, Sher KJ, Conway KP, Gonzalez R, Feldstein Ewing SW, et al. Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev Cogn Neurosci. 2018; 32: 80-96.
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, et al. Adolescent neurocognitive development and impacts of substance use: Overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 2018; 32: 67-79.
- Auchte AM, Hernandez Mejia M, Heyser CJ, Shilling PD, Jernigan TL, et al. A description of the ABCD organizational structure and communication framework. Dev Cogn Neurosci. 2018; 32: 8-15.
- Asaad SK, Bjarkam CR. The Aalborg Bolt-Connected Drain (ABCD) study: a prospective comparison of tunnelled and bolt-connected external ventricular drains. Acta Neurochir (Wien). 2019; 161: 33-39.
- ABCD. ABCD Protocl Brocure - Baseline.
- Feldstein Ewing SW, Chang L, Cottler LB, Tapert SF, Dowling GJ, et al. Approaching Retention within the ABCD Study. Dev Cogn Neurosci. 2018; 32: 130-137.
- Werneck AO, Agostinete RR, Cayres SU, Urban JB, Wigna A, et al. Association between Cluster of Lifestyle Behaviors and HOMA-IR among Adolescents: ABCD Growth Study. Medicina (Kaunas). 2018; 54: 96.
- Fine JD, Moreau AL, Karcher NR, Agrawal A, Rogers CE, et al. Association of Prenatal Cannabis Exposure With Psychosis Proneness Among Children in the Adolescent Brain Cognitive Development (ABCD) Study. JAMA Psychiatry. 2019; 76: 762-764.
- Dick AS, Garcia NL, Pruden SM, Thompson WK, Hawes SW, et al. Author Correction: No evidence for a bilingual executive function advantage in the ABCD study. Nat Hum Behav. 2019; 3: 1124.
- Dick AS, Garcia NL, Pruden SM, Thompson WK, Hawes SW, et al. Author Correction: No evidence for a bilingual executive function advantage in the nationally representative ABCD study. Nat Hum Behav. 2019; 3: 999.
- Michelini G, Barch DM, Tian Y, Watson D, Klein DN, et al. Delineating and validating higher-order dimensions of psychopathology in the Adolescent Brain Cognitive Development (ABCD) study. Transl Psychiatry. 2019; 9: 261.
- Gray JC, Schvey NA, Tanofsky-Kraff M. Demographic, psychological, behavioral, and cognitive correlates of BMI in youth: Findings from the Adolescent Brain Cognitive Development (ABCD) study. Psychol Med. 2019.
- Beauchaine TP. Editorial: Family History of Depression and Child Striatal Volumes in the ABCD Study: Promise and Perils of Neuroimaging Research With Large Samples. J Am Acad Child Adolesc Psychiatry. 2020; 59: 1133-1134.
- Buscemi S, Corleo D, Vasto S, Buscemi C, Massenti MF, et al. Factors associated with circulating concentrations of irisin in the general population cohort of the ABCD study. Int J Obes (Lond). 2018; 42: 398-404.
- Exuperio IN, Agostinete RR, Werneck AO, Maillane-Vanegas S, Luiz-de-Marco R, et al. Impact of Artistic Gymnastics on Bone Formation Marker, Density and Geometry in Female Adolescents: ABCD-Growth Study. J Bone Metab. 2019; 26: 75-82.
- Lynch KR, Anokye NK, Vlachopoulos D, Barbieri FA, Turi-Lynch BC, et al. Impact of sports participation on incidence of bone traumatic fractures and health-care costs among adolescents: ABCD - Growth Study. Phys Sportsmed 2019; 48.
- Dick AS, Garcia NL, Pruden SM, Thompson WK, Hawes SW, et al. No evidence for a bilingual executive function advantage in the nationally representative ABCD study. Nat Hum Behav. 2019; 3: 692-701.
- Hoffman EA, Howlett KD, Breslin F, Dowling GJ. Outreach and innovation: Communication strategies for the ABCD Study. Dev Cogn Neurosci. 2018; 32: 138-142.
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, et al. Recruiting the ABCD sample: Design considerations and procedures. Dev Cogn Neurosci. 2018; 32: 16-22.
- Hagler DJ, Hatton S, Cornejo MD, Makowski C, Fair DA, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019; 202: 116091.
- Vargas T, Damme KSF, Mittal VA. Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. Neuroimage. 2020; 220: 117086.
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999; 9: 195-207.
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33; 341-355.
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, et al. Automatically parcellating the human cerebral cortex. Cerebral cortex. 2004; 14: 11-22.
- Hagler DJ, Hatton S, Cornejo MD, Makowski C, Fair DA, et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 2019; 202: 116091.
- Park J, Kitayama S, Markus HR, Coe CL, Miyamoto Y, et al. Social status and anger expression: The cultural moderation hypothesis. Emotion. 2013; 13: 1122-1131.
- Kitayama S, Park J, Boylan JM, Miyamoto Y, Levine CS, et al. Expression of anger and ill health in two cultures: An examination of inflammation and cardiovascular risk. Psychological science. 2015; 26: 211-220.
- Kitayama S, Park J. Emotion and biological health: The socio-cultural moderation. Current Opinion in Psychology. 2017; 17; 99-105.
- Savani K, Markus HR, Naidu N, Kumar S, Berlia N. What counts as a choice? US Americans are more likely than Indians to construe actions as choices. Psychological Science. 2010; 21: 391-398.
- Wexler BE. Brain and culture: Neurobiology, ideology, and social change; Mit Press: 2008.
- Staudinger MR, Erk S, Walter H. Dorsolateral prefrontal cortex modulates striatal reward encoding during reappraisal of reward anticipation. Cerebral cortex. 2011; 21: 2578-2588.
- Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016; 351: aac9698.
- Nashed MG, Seidlitz EP, Frey BN, Singh G. Depressive-like behaviours and decreased dendritic branching in the medial prefrontal cortex of mice with tumors: A novel validated model of cancer-induced depression. Behav Brain Res. 2015; 294: 25-35.
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008; 63: 369-376.
- Masuda K, Nakanishi M, Okamoto K, Kawashimwsrpa C, Oshita H, et al. Different functioning of prefrontal cortex predicts treatment response after a selective serotonin reuptake inhibitor treatment in patients with major depression. J Affect Disord. 2017; 214: 44-52.
- Lu S, Xu R, Cao J, Yin Y, Gao W, et al. The left dorsolateral prefrontal cortex volume is reduced in adults reporting childhood trauma independent of depression diagnosis. J Psychiatr Res. 2019; 112: 12-17.
- Kozel FA, Johnson KA, Nahas Z, Nakonezny PA, Morgan PS, et al. Fractional anisotropy changes after several weeks of daily left high-frequency repetitive transcranial magnetic stimulation of the prefrontal cortex to treat major depression. J ECT. 2011; 27: 5-10.
- Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, et al. Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry. 2015; 77; 285-294,
- Okazaki S. Sources of ethnic differences between Asian American and White American college students on measures of depression and social anxiety. Journal of Abnormal Psychology. 1997; 106: 52-60.
- Lauber C, Rössler W. Stigma towards people with mental illness in developing countries in Asia. International review of psychiatry. 2007; 19: 157-178.
