
Research Article
Austin Aging Res. 2017; 1(1): 1002.
Depletion of Gut Granules Diminishes Lifespan in C. Elegans
Kumar J1,2*, Srivastava P², Awasthi A³, Seervi M², Singh P4, Parween N5, Anwar A5 and Prasad B5
¹The Buck Institute for Aging Research, USA
²DBT-PU-IPLS Programme, Department of Botany/ Biotechnology, Patna University, India
³Department of Biology, School of Engineering, Presidency University, India
4National Institute of Technology, India
5Department of Botany/Biotechnology, Patna University, India
*Corresponding author: Jitendra Kumar, Department of Botany/Biotechnology, Patna University, India
Received: September 19, 2016; Accepted: February 21, 2017; Published: February 22, 2017
Abstract
Gut granules are prominent Lysosome-Related Organelles (LROs) having acidic interior, reported in the intestinal wall of nematode C. elegans. The most important function of these large and abundant organelles is to store the fat as well as zinc but the role of these organelles has not been explored in relation to life span and health span. In order to investigate the role of gut granules with respect to lifespan, we used three different C. elegans mutants i.e. glo-1(zu391), glo-3 (zu446) and pgp-2 (gk112) with lesser number of gut granules compared to wild type. These mutants exhibited decreased lifespan. In addition to lifespan, these mutants also showed decrease in mobility, instantaneous touch response and heat stress. Altogether this study suggests that gut granules in C. elegans modulates life span and health span. Furthermore, lack of gut granules also enhances the Aβ aggregation and paralysis in Alzheimer’s model animals. This study describes importance of gut granules in relation with life span as well as health span.
Keywords: Caenorhabditis elegans; Gut granules; Alzheimer’s Disease (AD); Lifespan, Aging
Introduction
Lysosomes are membrane bound cytoplasmic organelle function as intracellular protein degradator and also act as a major degradative compartment. It contains acid hydrolases which degrade waste materials and cellular debris in eukaryotes [1,2]. Acid hydrolases present within lysosome help to break down all the macromolecules such as proteins, lipids, nucleic acids and oligosaccharides with the help of more than fifty acid-dependent hydrolases (e.g. proteases, lipases and glycosidases) present in its lumen [3]. Lysosomal activity facilitates cellular repair by participating in the events such as autophagy and apoptosis and along with this, it also aids in immune response by assisting endocytosis and phagocytosis. Lysosomal digestive proteins are formed in endoplasmic reticulum and golgi bodies through secretory pathway; and extracellular material is taken in through endocytic pathway.
Genetical, biochemical and structural analysis revealed that a group of cell type-specific compartments with distinct morphology, composition and functions share most of the properties with lysosome and these are known as “Lysosome-Related Organelles (LROs)”. Such organelles include melanosomes, MHC class II compartments, basophil granules, lytic granules, Weibel-Palade bodies and Drosophila pigment granules. Features that LROs share with lysosomes include low intra organellar pH, specific membrane proteins or other components and a common pathway of formation [4]. LROs share compositional and functional similarity with lysosomes but still these are specialized to confer cell type specific functions. LROs often co-exist with conventional lysosomes and the dysfunction of both has been reported to be associated with many genetic disorders in humans like Hermansky-Pudlak Syndrome (HPS) and Chediak-Higashi Syndrome (CHS) [4].
Gut granules are cell type - specific LROs with acidic interior that present in large numbers within the intestinal cells of Caenorhabditis elegans [5]. They contain birefringent and auto-fluorescent material, whose accumulation is coincident with their formation during embryogenesis [6,7]. Although gut granules share some characteristics and proteins with conventional lysosomes, but these two organelles are distinct and coexist within intestinal cells [8-10]. Gut granules can be stained with lysosome-specific fluorescence dyes such as Lyso-Tracker [9]. Gut granules are present in the intestinal cells of C. elegans and function as a major site for fat storage [11]. Pathways for biogenesis of gut granules are reported to be conserved from C. elegans to mammals. However, the role of gut granules in relation to longevity have not been explored [5].
In this proposed study, we examined the role of gut granules on lifespan and health span of animals. We found that gut granules are essential and vital for lifespan and health span in wild type animals. We further found that lack of gut granules enhances Aβ aggregation dependent paralysis phenotype in the worm model of Alzheimer’s Disease (AD). There may be a similar mechanism involved in mice and humans. These findings explore the importance of gut granules in aging and age related neurodegenerative diseases such as Alzheimer’s disease.
Material and Method
Strains and maintenance
C. elegans wild type strain (N2 Bristol) and mutant strains glo- 1(zu391), glo-3 (zu446) and pgp-2 (gk112) were obtained from Caenorhabditis Genetics Center (CGC) which is funded by the NIH National Center for Research Resources (NCRR). Animals were maintained on Nematode Growth Medium (NGM) seeded with E. coli strain OP50 as food source at 20°C.
Lifespan and health-span analysis
Synchronized population of L4 animals were grown on OP50 spotted NGM plates. The animals were transferred to fresh NGM plates at regular interval of every 2 days and number of live animals scored every alternate day until death. Animals that failed to display touch-provoked movement were scored as dead. Animals that died from causes other than aging, such as sticking to the walls of the plate, internal hatching and bursting in the vulval region, were not scored as dead. All the lifespan experiments were performed at 20°C. The results of four independent trials with each test comprising of approximately 100 animals were averaged together to obtain the mean life span. Health-span was assayed by counting the number of body bends per minute and spontaneous touch response on fifth and tenth day of aged adults respectively. For both the assays, three independent trials were carried out with 25-30 animals per trial and results have been generated.
Statistical analysis
Log-rank tests were performed for survival curves by using the Prism 4 software. A paired two-tailed student’s t-test was used for comparing the data sets. The value of p<0.001 was considered as significant.
Results
Lysosome related gut granules regulate lifespan and health-span of C. elegans
Lysosome related gut granules (LROs) are one of the important organelles that function as major site for fat as well as zinc storage in C. elegans [13]. To investigate the effect of gut granules on life span of C. elegans, we performed the life span assay on three gut granule mutants i.e. glo-1(zu391), glo-3 (zu446) and pgp-2 (gk112) on NGM plates spotted with E. coli OP50 at 20°C. These three mutants were well characterized for the loss of gut granules function. All these three mutants are reported to have less number of gut granules compared to wild type. The mean lifespan was expressed as mean±S.D (Standard Deviation). For wild type animals, mean lifespan was 17.18 ± 0.26 days, whereas glo-1(zu391), glo-3 (zu446), pgp-2 (gk112) animals had mean lifespan of 15.2±0.24 days (p<0.0001, log-rank test), 15.28±0.31days (p<0.0001, log-rank test), 15.28±0.31days (p<0.0001, log-rank test) respectively (Figure 1A, Table 1). This result shows that gut granules of C. elegans are vital for lifespan of C. elegans. Life span is closely associated with health span.
Genotype
Mean life span (±SD)
Log Rank test (P value)
Number of nematodes (n)
Wild type (Control)
17.18±0.26
N.A.
342
glo-1(zu391)
15.20±0.24
<0.0001
365
glo-3 (zu446)
15.31±0.31
<0.0001
371
pgp-2 (gk112)
15.28±0.23
<0.0001
339
P value denotes log-rank test results for survival curves. Results of four independent experimental trials with approximately 100 animals per trial were used for the analysis. Log-rank tests were performed for survival curves by using the Prism 4 software. The value of p<0.001 was considered as significant.
Table 1: Results of lifespan assay summarized.
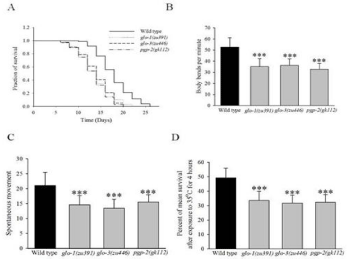
Figure 1: Gut granules mutant animals modulate lifespan and health-span of C. elegans.
(A) Survival curve of wild type and gut granule mutants glo-1(zu391), glo-3(zu446) and pgp-2(gk112) animals. The gut granules mutant animals show a significant
decrease in lifespan. (B) Effect of gut granules mutants on motility of 5 days old adult animal. Body bends per minute of 5 days adult animals were analyzed for
wild type and gut granules mutants glo-1(zu391), glo-3(zu446) and pgp-2(gk112) animals (C) Effect of the spontaneous movement of wild type and gut granules
mutants glo-1(zu391), glo-3(zu446), pgp-2(gk112) animals in response to touch for 10 days old adult animals. Spontaneous movements of 10 days old adult
animals were analyzed for control is significantly more in comparison of gut granules mutant animals. (D) Effect of gut granules mutant animals on thermo tolerance
for 5 days old adult animal. Gut granules mutant animals were exposed to 35°C for 4 hours, animals were allowed to recover at 20°C and were scored the next day
for the number of dead animals. Data is represented as mean ± S.D, (**p<0.001, ***p<0.0001, t-test values).
Further, to investigate the effect of gut granules on health, we quantified some of the health hallmarks of C. elegans such as motility, spontaneous movements and thermo tolerance in animals. We measured motility of 5 days old animals by counting the number of body bends per minute in wild type and gut granules mutant animals. Number of body bends per minute was expressed as mean±S.D. The mean value of body bends per minute observed for control was 52.5±7.2, whereas gut granules mutant animals glo-1(zu391), glo- 3 (zu446) and pgp-2 (gk112) showed values of 35.1±7.1, 36.1±6.0 (p<0.0001, t-test) and 32.8±4.9 (p<0.0001, t-test) respectively (Figure 1B). The motility (body bends per minute) observed in gut granules mutant animals is found to be significantly lower as compared to the wild type animals.
To investigate the spontaneous movement, 10 days adult animals were analyzed for their body movement in response to touch. Results of spontaneous movements assays are also expressed as mean±S.D by averaging the results of three independent trials carried out with 25-30 animals each test. The mean value observed for spontaneous movement in control (wild type) was 21.04±4.4 and gut granules mutant animals glo-1(zu391), glo-3 (zu446) and pgp-2 (gk112) showed respective values of 14.1±3.1, 13.1±2.9 (p<0.0001, t-test), 9.8±3.2 (p<0.0001, t-test) (Figure 1C).
The thermo-tolerance assay was carried out on five days adult wild type as well as on mutant animals grown at 20°C which were subsequently shifted to 35oC for four hours. After 4 hours of treatment at 35oC, animals were shifted back to 20°C for recovery. Percentage of worms survived after temperature stress was scored. Three independent trials with approximately 100 worms per trial were assayed. The survival percentage of animals for wild type was 49.2±6.6, whereas mutant animals glo-1(zu391), glo-3 (zu446) and pgp-2 (gk112) showed 33.5±6.1 (p<0.001, t-test), 31.7±5.4 (p<0.0001, t-test), 32.3±5.3 (p<0.001, t-test) respectively (Figure 1D).
The animals which were mutant for gut granules showed a higher rate of decline in physiological processes such as mobility, touch response and thermal tolerance compared to the wild type. Overall, all these results show that the mean lifespan and health span has been decreased due to lack of gut granules in animals.
Gut granules modulate proteotoxicity mediated paralysis phenotype in Alzheimer’s disease model animals
Numerous lines of evidence have been reported that gut granules are associated with protein homeostasis. There is direct relation of healthy aging and protein homeostasis. Furthermore, to investigate the possible role of gut granules in protein aggregate formation, we used CL4176 animals, a transgenic model of Alzheimer’s Disease (AD). The animals express human Aβ (1-42) peptide in the muscle cells. At 16°C temperature, the animals do not express Aβ, but shifting to 25°C induces Aβ expression and shows paralysis phenotype in the CL4176 [14]. Age synchronized eggs from CL4176 animals were placed on RNAi plate of glo-1 for 36 hours at 16°C, followed by temperature shift to 25°C to induce Aβ expression [15]. The number of paralyzed CL4176 C. elegans were scored 20 hours post 25°C temperature shift. Animals that did not move at all or only moved their head when gently touched with a platinum loop were scored as paralyzed. A significant increase in paralysis phenotype was observed on knock down of gut granules in animals, compared to control animals (p<0.001) (Figure 2). The reproducibility of paralysis at 25°C temperature in individual’s trials was consistent. These results demonstrate that knock down of gut granules enhance Aβ aggregation and thus resulting in lower rate of paralysis phenotype in AD model animal.
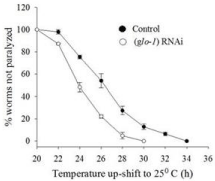
Figure 2: Loss of gut granules effects proteotoxicity mediated paralysis
phenotype in Alzheimer’s disease model animals.
Time course of paralysis in Cl4176 worms on RNAi of gut granules. The
paralysis was scored at 2 hours intervals and continued until all the animals
were paralyzed. Scoring of paralysis was initiated 18-20 hours post incubation
at 25°C. Data are expressed as percentage of non-paralyzed worms from
at least three different independent experiments. The animals having
knockdown of gut granules gene show increase in Aβ induced paralysis.
Discussion
Aging is a universal feature of an organism at variable rate resulting in the diverse lifespan. The identification and analysis of compounds that influence aging and lifespan has become an important aspect of gerontology research. In this study, we demonstrated the impact of gut granules on lifespan as well as health span of C. elegans. In this assessment, we have used three different gut granule mutants of C. elegans glo-1(zu391), glo-3 (zu446) and pgp-2 (gk112). These three mutants have lesser number of gut granules as compared to the wild type. In this report, presence of gut granules of C. elegans shows close relation with lifespan as well as health span in terms of mobility, touch response and thermal tolerance. This finding suggests that the LROs gut granules is directly associated with the lifespan as well as health span.
Furthermore, to validate the effect of declined lifespan of C. elegans on its health span, three different set of experiments such as mobility test (5 days mature animals were used and their body bent per minute were analysed which was lesser in mutant organism as compared to wild type organism), touch response (20 no. of 10 days old animals were used in three independent trials and observed a significant decrease of the touch response in mutant compared to control), thermal tolerance (100 no. of 5 days mature animals were maintained at 20°C and then shifted to 35°C for 4 hrs and subsequently shifted back to 20°C for recovery and showed survival percentage is decreased in mutant species compared to control) were conducted and results have been generated.
Over the last few years, biology of aging and age related disorders gained a great potential to study the animal development and behaviour by using C. elegans as model organism in research. This soil nematode has been widely used as a research model system due to its small size (1.5-mm-long adult), short life span, self-fertilization, simple anatomy, small nervous system, body transparency, constancy of cell number, and ease to cultivate in the laboratory [16]. The study of aging in C. elegans reveals that animal’s life span is influenced by genes, environment and stochastic factors. Aging of C. elegans is characterized by a progressive decline in locomotion, both in speed and coordination [17-19] and decreased defecation and pharyngeal pumping rate [17,20, 21]. Old nematodes, like old humans, show progressive tissue and organ deterioration. Muscles, gonad, intestine and connective tissues degenerate in aged worms [17, 22].
It is a proven fact that several pathways are known to control C. elegans aging such as insulin signaling, germ-line signaling, chemosensory signaling, mitochondrial function, dietary restriction (DR) [12, 23]. Since 30 years, various studies have revealed an association between aging and decreased fatty acid oxidation [24]. Triglycerides and various products of incomplete lipid oxidation accumulate during old age, leading to so-called lipotoxicity and this accumulation is associated with age-related disorders, such as type 2 diabetes, metabolic syndrome and Alzheimer’s disease [14].
Conclusively, we found that the mutant variants of C. elegans exhibited a higher rate of decline in lifespan as well as health span (mobility, touch response and thermal tolerance) as compared to the control. Altogether, this study revealed a very promising result that the lifespan as well as health span have close relation with gut granules of C. elegans.
Acknowledgments
We are thankful to Buck Institute for Research on Aging for lab facilities; DBT-PU-IPLS (BT / PR4577 / INF / 22 / 149 /2012), Department of Biotechnology (DBT), Govt. of India and DST for young scientist award (SB/YS/LS-166/2014) for financial support. The funders had no role in the study designing, data collection and analysis, decision to publish or preparation of the manuscript.
Author Contributions
Conceived and designed the experiments: JK AA. Performed the experiments: JK. Analyzed the data: JK AA MS. Wrote the paper: JK AA PS MS PS NP AA BP. The authors of this paper have no conflict of interest.
References
- Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. Journal of Cell Biology. 1987: 105; 1253-1265.
- Tappel AL. Lysosomal enzymes and other components. Lysosomes in biology and pathology. 1969; 2: 207-244.
- de Duve C. The lysosome concept. In Lysosomes editors. de Reuck AVS, Cameron MP. Churchill. London. 1963; 1-35.
- Dell’ Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. The FASEB Journal. 2000; 14: 1265-1278.
- Hermann GJ, Schroeder LK, Hieb CA, Kershner AM, Rabbitts BM, Fonarev P, et al. Genetic analysis of lysosomal trafficking in Caenorhabditis elegans. Molecular biology of the cell. 2005; 16: 3273-3288.
- Laufer JS, Bazzicalupo P, Wood WB. Segregation of developmen–tal potential in early embryos of Caenorhabditis elegans. Cell. 1980; 19: 569- 577.
- Bossinger O, Schierenberg E. Transfer and tissue-specific accumulation of cytoplasmic components in embryos of Caenorhabditis elegans and Rabditis dolichura: in vivo analysis with a low-cost signal enhancement device. Development. 1992; 114: 317-330.
- Clokey GV, Jacobson LA. The autofluorescent “lipofuscin granules” in the intestinal cells of Caenorhabditis elegans are secondary lysosomes. Mech Ageing Dev. 1986; 35: 79-94.
- Levitte S, Salesky R, King B, Coe Smith S, Depper M, Cole M, Hermann GJ. A Caenorhabditis elegans model of orotic aciduria reveals enlarged lysosome-related organelles in embryos lacking umps-1 function. FEBS J. 2010; 277: 1420-1439.
- Hermann GJ, Scavarda E, Weis AM, Saxton DS, Thomas LL, Salesky R, et al. C. elegans BLOC-1 functions in trafficking to lysosome-related gut granules. PLoS One. 2012; 7: e43043.
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003; 421: 268-272.
- Yuan L. Alzheimer’s Disease, the Nematode Caenorhabditis elegans, and Ginkgo Biloba Leaf Extract. Life Sciences. 2006; 78: 18.
- Kumar J, Barhydt T, Awasthi A, Lithgow GJ, Killilea DW, Kapahi P. Zinc Levels Modulate Lifespan through Multiple Longevity Pathways in Caenorhabditis elegans. Plos One. 2016; 11.
- Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci USA. 1995; 92: 9368-9372.
- Kumar J, Park KC, Awasthi A, Prasad B. Silymarin Extends Lifespan and Reduces Proteotoxicity in C. elegans Alzheimer’s Model. CNS & Neurological Disorders. 2015; 14: 295-302.
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974; 77: 71- 94.
- Collins JJ, Huang C, Hughes S, Kornfeld K. The measurement and analysis of age-related changes in Caenorhabditis elegans. WormBook. 2008; 1-21.
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation. Genetics. 2002; 161:1101-1112.
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2004; 101: 8084-8089.
- Klass MR. Aging in the nematode Caenorhabditis elegans: Major biological and environmental factors influencing life span. Mech Ageing Dev. 1977; 6: 413-429.
- Gerstbrein B, Stamatas G, Kollias N, Driscoll M. In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell. 2005; 4: 127-137.
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002; 419: 808-814.
- Sonani RR, Singh NK, Awasthi A, Prasad B, Kumar J, Madamwar D. Phycoerythrin extends life span and health span of Caenorhabditis elegans. 2014; 36: 9717.
- Singh NJ, Sonani RR, Awasthi A, Prasad B, Patel AR, Kumar J, et al. Phycocyanin moderates aging and proteotoxicity in Caenorhabditis elegans. J Appl Phycol. 2016; 28: 2407-2417.