
Research Article
Ann Agric Crop Sci. 2020; 5(2): 1064.
Towards Development of Mungbean [Vigna radiata (L.) Wilczek] Genotypes with Combined Resistance to Bruchid (Callosobruchus maculatus) and Mungbean Yellow Mosaic Virus (MYMV)
Majhi PK1*, Mogali SC2 and Bhoi TK3
1Department of Genetics and Plant Breeding, University of Agricultural Sciences, India
2AICRP on MULLaRP, University of Agricultural Sciences, Karnataka, India
3Division of Entomology, ICAR-Indian Agricultural Research Institute (IARI), India
*Corresponding author: Prasanta Kumar Majhi, Department of Genetics and Plant Breeding, University of Agricultural Sciences, College of Agriculture, Dharwad-580005, Karnataka, India
Received: Oct 02, 2020; Accepted: Oct 23, 2020; Published: Oct 30, 2020
Abstract
Mungbean is an important pulse crop that is grown throughout Asia. Among the biotic constraints, Bruchids and Mungbean Yellow Mosaic Virus are the most destructive in mungbean. Bruchid causes serious damage during storage whereas MYMV disease is a serious threat under field condition. The yield loss is observed up to 100% in both cases with a severe infestation. The seeds derived from eight F3 families of mungbean were screened against C. maculatus in a CRD design under ideal laboratory conditions during rabi-2017. Breeding lines from the same crosses were laid out in an RBD design and screened against MYMV resistance in F4 generation under field conditions in summer-2018. The cross derivatives of V-02-802xDGGV-7 and V-02-802xDGGV-2 were resistant to C. maculatus with susceptibility index 0.039 and 0.043 respectively. The breeding lines from V-02-802xDGGV-2 were found to be resistant to MYMV, with a percent disease incidence of 7.60. The promising progeny lines selected from the cross V-02-802xDGGV-2 shown resistance to both bruchid and MYMV with yield per plant is 10.0 g (plant code no. 5S-2) and 10.7g (plant code no. 5S-10). These resistant breeding lines were selected separately and it needs special consideration to develop genotypes with combined resistance to C. maculatus and MYMV disease.
Keywords: Mungbean; Bruchid; Mungbean yellow mosaic virus; Combined resistance
Introduction
A variety of pulses are cultivated in India as well as in the world and among these pulse crops, mungbean occupies the third position in terms of acreage, production and productivity after chickpea and pigeon pea. India is the largest consumer and producer of mungbean, which alone accounts for 65% of the world acreage and 54% of global production [1]. Mungbean [Vigna radiata (L.) Wilczek; Family: Fabaceae] is a diploid species (2n=2x=22) and predominantly selfpollinated. This is an excellent source of high-quality protein with easy digestibility, consumed as whole grains, dal and sprouted in a variety of ways (Tiwari, 2016). The nutritional value of mungbean seeds contains approximately protein (25-28%), carbohydrates (62- 65%) and fiber (3.5-4.5%) on a dry weight basis. The concentration of sulfur-containing amino acids like cysteine and methionine are in low quantity but lysine contents are comparatively high, which makes the protein of mungbean is an excellent complement to rice in terms of balanced human nutrition [2]. There are several major constraints in mungbean production like narrow genetic variability, lack of different plant types for different seasons, low harvest index and susceptibility to a number of biotic and abiotic stresses, which limits achieving the goal of higher productivity. Among various biotic stresses; Bruchid (Callosobruchus spp.) and Mungbean Yellow Mosaic Virus (MYMV) disease which has been renowned in India for more than five decades are the most brutal for mungbean production [3].
Bruchid or pulse beetle (Callosobruchus spp. F; Coleoptera: Bruchidae) causes serious damage and yield loss in pulse crops including mungbean in field condition as well as during storage [4]. Generally the infestation rate in field condition is low but during storage, the infestation rate is very rapid [5]. There are four developmental stages observed in bruchid viz., egg, larva, pupa and adult. The adult insects lay the egg on the surface of the seeds and from egg to larva developed. The larval stage is the most destructive stage of bruchid for the pulses. From the pupa, adult insects emerged and again lay eggs on the seed surface causing secondary infestation with the rapid multiplication of the pest population during storage and resulting up to 100% grain loss [6]. There are several species of Bruchid that infect the pulse crops, but in the present investigation, we focused on the species Callosobruchus maculatus. The wild species of mungbean (Vigna radiata var. sublobata) accession TC1966 is completely reported to C. maculatus and C. chinensis [7], but due to the crossability barrier, a satisfactory level of result is not observed. The cultivated varieties of mungbean V02802 and V02709 were reported highly resistant to both species of bruchid [8,9]. Therefore, in the present study, these two cultivated resistant sources were used to improve the elite breeding lines of mungbean. Other than bruchid, Mungbean Yellow Mosaic Virus (MYMV) is one of the most devastating and destructive diseases that severely affect the production and productivity of mungbean throughout Asia, including India [10,11]. The yield loss in pulse due to viral diseases accounts for up to 80 percent, while the yield loss due to MYMV in mungbean alone accounts 80-100 percent [12]. The virus belongs to the family geminiviridae and genus Begomovirus with bipartite genome (having two different components, DNA-A and DNA-B) as single-stranded circular DNA of genome size about 2.7kb [13,14,15,16]. The MYMV disease is mainly transmitted by the polyphagous pest whitefly (Bemisia tabaci; Hemiptera: Aleyrodidae) in a persistent manner, but not much by the sap, seed or soil. The insect vector and virus survive on different alternate and collateral hosts, including the main crop which serves as the primary source of inoculum to spread the disease all-round the year.
There are several approaches followed to manage bruchid (chemical treatment like methyl bromide, Carbon disulfide, botanicals, etc.) [17] and MYMV (vector control by spraying the insecticides and applications of different plant extracts) [15]. But, the application of chemical pesticides is highly toxic, environmentally undesirable, and poses a threat to food safety whereas on the other side botanicals, plant extracts are slow-release in action and cause a problem in the germination of seed and growth of the plant [18]. Thus, managing these two problems up to 100% is quite difficult. The wide host range, genome size variation of the virus, quantitative nature of inheritance of the disease make the task more challenging to develop mungbean varieties resistant to MYMV [15]. The identification of resistance source is the most reliable, reasonable and environmentally friendly method for the management of bruchid and MYMV in mungbean through innate resistance. Even though some genotypes and germplasms have been identified as a resistance source against bruchid and MYMV separately, but lack of durable resistance has been noticed in most of the cases. Hence, it needs continuous screening in all the seasons to identify the resistance/tolerance lines against bruchid and MYMV. Therefore, considering these constraints into account, the present investigation was aimed at serious attention to screen the advanced breeding lines of mungbean against bruchid (C. maculatus) during storage and against the menace of MYMV disease under natural field condition. The yield levels of all the breeding lines were also analyzed to develop superior genotypes with combined resistance/tolerance to bruchid and MYMV disease.
SI. No.
Treatments
Checks
Releasing Centers of the Variety (Check)
Reaction of Checks to MYMV
Reaction of Parents to Bruchid (C. maculatus)
1.
DGGV-2xV-02-709
DGGV-2 (Parent)
University of Agricultural Sciences, Dharwad
Susceptible
Susceptible
2.
DGGV-2xV-02-802
DGGV-7 (Parent)
University of Agricultural Sciences, Dharwad
Susceptible
Susceptible
3.
DGGV-7xV-02-709
V-02-802 (Parent)
Asian Vegetable Research Development Centre, Taiwan
Moderately susceptible
Resistant
4.
DGGV-7xV-02-802
V-02-709 (Parent)
Asian Vegetable Research Development Centre, Taiwan
Moderately susceptible
Resistant
5.
V-02-802xDGGV-2
IPM-409-4
Indian Institute of Pulses Research (IIPR), Kanpur
Moderately resistant
-
6.
V-02-802xDGGV-7
IPM-2-14
Indian Institute of Pulses Research (IIPR), Kanpur
Moderately resistant
-
7.
V-02-709xDGGV-2
LGG-460
Andhra Pradesh Agricultural University (APAU), Lam
Resistant
-
8.
V-02-709xDGGV-7
SML-1815
Punjab Agricultural University (PAU), Ludhiana
Resistant
-
Table 1: Experimental materials and their checks used for screening to the reaction of MYMV and Bruchid(C. maculatus).
Materials and Methods
Experimental materials
The genetic materials for the present investigation included the F3 and F4 families of mungbean breeding lines. There are eight crosses (four direct and four reciprocal) that have been made among four parents viz., DGGV-2, DGGV-7, V-02-802 and V-02-709. The desirable features of experimental materials along with their checks, centers responsible for releasing are mentioned in detail in Table 1. The genotypes DGGV-2 and DGGV-7 are agronomically superior and high yielding but when grow in Northern parts of Karnataka they are susceptible to MYMV under the natural condition and to bruchid during storage. Genotypes V-02-802 and V-02-709 are contributed by AVRDC [19], Taiwan and are resistant to bruchid [Callosobruchus maculatus (F.) and C. chinensis] but moderately susceptible to MYMV. So, an attempt was made in the present study to identify superior breeding lines with combined resistance to bruchid and MYMV resistance from the direct and reciprocal crosses among these four parents. The experiment was conducted at Main Agricultural Research Station (MARS), AICRP on MULLaRP, University of Agricultural Sciences, Dharwad, Karnataka, India which is geographically located 15° 12' N latitude and 75° 07' E longitudes with an altitude of 678 m above mean sea level.
Methodology for Bruchid Screening
The seeds from eight F3 families from the above-mentioned crosses (Table 1) were used for screening against C. maculatus. The experiment was laid in a Complete Randomized Design (CRD) under ideal laboratory conditions [25±50C and 65±5% RH and photoperiod (16L:8D)] in rabi-2017. The procedure for maintenance of stock culture, bioassay test by free choice test and force choice test (Figure 1a) were practiced according to Ponnusamy et al., [20]; and Soumia et al., [21]. The adult C. maculatus species were collected from Pulse storehouse, AICRP on MULLaRP, Dharwad, and reared with very susceptible genotypes of mungbean for mass multiplication. There is an equal number of seeds from each family were taken for free choice tests and further the resistance reaction was screened under the forced-choice test. All the biological parameters like the number of eggs laid on each seed surface, number of adult emergence, weight loss, percent infestation and susceptibility index were taken into consideration from egg-laying to adult emergence up to one complete life cycle. Susceptibility index or growth index which was calculated by using the formula given by Howe (1971), Growth Index=(Log S)/T;
Where, S is the percent adult emergence, T is the mean developmental period (days). The lines were classified into different groups based on their Susceptibility Index (SI) value as resistant (<0.05), moderately resistant (0.051-0.060), moderately susceptible (0.061-0.070), susceptible (0.071-0.080) and highly susceptible (>0.081) [22]. The detailed procedure of screening is mentioned and published by Majhi and Mogali, [23]. Therefore, it is suggested that to follow the previously published research article "Characterization and Selection of Bruchid [Callosobruchus maculatus (F.)] tolerant Greengram [Vigna radiata (L.) Wilczek] Genotypes" by Majhi and Mogali [23]. The statistical analysis for CRD design was accomplished by using WASP software version 1.0. The mean value of each character was compared with the CD values @ p=0.05.

Figure 1: Screening of Bruchid (Callosobruchus maculatus) resistance
genotypes in mungbean: a) Screening through force choice test; b) selected
resistant progeny lines; c) reaction of parental lines to bruchid infestation.

Figure 2: Field screening for MYMV resistance in mungbean: a) infected row
method of sowing; b) green and uniform pods of the resistant plant; c) yellow
and shriveled pods of the highly susceptible plant; d) MYMV symptoms on
leaf samples (where, R-Resistant, MR-Moderately resistant, S-Susceptible,
HS-Highly susceptible); e) MYMV symptoms on pod samples (where,
R-Resistant, MR-Moderately resistant, HS-Highly susceptible).

Figure 3: Promising breeding lines selected for MYMV resistance/tolerance
in mungbean: a) selected resistant breeding lines from the cross V-02-
802XDGGV-2; b) general field view of mungbean; c) selected moderately
susceptible lines from the cross DGGV-7XV-02-802.
Methodology for MYMV disease screening
The same eight F3 families which were screened for bruchid resistance were again grown in the field to give F4 population. These eight F4 families of mungbean were used for screening against MYMV disease resistance under natural field conditions in summer-2018. The experiment was laid out in an RBD design. Each row length was 4m with the row to row spacing 30cm and plant to plant spacing 10cm. Each F4 family was sown along with their resistant and susceptible checks as well as their parents (Figure 2a). The genotypes DGGV-2 and DGGV-7 were used as infector lines after every five test entries to study the level of infestation of MYMV under field condition as suggested by Mogali et al. [24]. The observations were recorded at regular intervals. All the agronomical practices followed to raise a good crop. The spraying of chemical insecticide is prevented to control the whitefly population as we need host plant resistance. All the symptoms were noted down with critical observation from a single plant basis. The Percent Disease Incidence (PDI) was calculated by using the formula given by Basir et al., [25] and the breeding lines were categorized into different reaction groups based on their respective PDI values (Table 2).
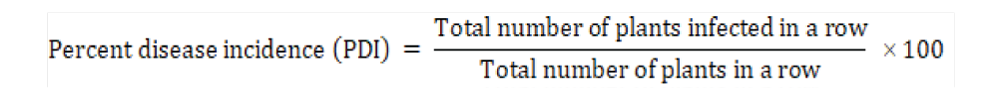
Percent Disease Incidence(PDI)
Results and Discussion
Screening for Bruchid resistance
The Susceptibility Index (SI) or growth index was calculated for the eight test entries and was categorized as resistant, moderately resistant, moderately susceptible, susceptible, and highly susceptible based on the estimation of susceptibility index given by Howe [22]. The highest susceptibility index values were recorded for the highly susceptible ones whereas resistant genotypes have shown the lowest susceptibility index. The parents were evaluated as a check to test their resistance/susceptibility reaction to bruchid infestation. The SI value of two susceptible parents was observed and DGGV-2 was more susceptible than DGGV-7 with SI value 0.073 and 0.076 respectively; in contrast among the resistant parents V-02-802 (SI=0.039) and V-02-709 (SI=0.046) respectively (Figure 1c). The cross derivatives of V-02-802×DGGV-7 and V-02-802×DGGV-2 were resistant with susceptibility index 0.039 and 0.043 respectively (Figure 1b). This result may be due to the physical appearance of the seed (shining appearance, color and size) which prevents egg laying or even though eggs laid on the seed coat, which is prevented from hatching by the biochemical substances present in the seed. Moderately resistant reaction was observed in four sets of cross derivatives to bruchid with susceptibility index value viz., V-02-709×DGGV-2 (SI=0.050), DGGV-2×V-02-709 (SI=0.055), DGGV-2×V-02-802 (SI=0.056) and DGGV-7×V-02-709 (SI=0.058); and two moderately susceptible lines viz., DGGV-7×V-02-802 and V-02-709×DGGV-7 with susceptibility index value 0.061 (Table 3). The weight loss was observed highest for the cross V-02-709×DGGV-7 (44.25%) and lowest for the cross V-02- 802×DGGV-2 (13.49%); among the segregating lines. The percent infestation was highest for the cross DGGV-7×V-02-802 (90) while lowest cross V-02-802×DGGV-7 (27.66), followed by 81.66 percent infestation in the breeding lines of V-02-709×DGGV-7. The highest infestation of these breeding lines was due to the dull color of the seed coat and large seed size which is very suitable for colonization and oviposition of bruchid. More than one adult has emerged from large size seeds. The reports of Somta et al., [9], Duraimurugan et al. [26], Chakraborty [27] and Sekar and Nalini [28] were similar to the present study on oviposition, mean developmental period and weight loss for resistant and susceptible lines. The ovipositional preference of C. maculatus has significantly differed among the treatment genotypes. The number of eggs laid by the female beetle was recorded on 100 seeds in each genotype. The number of eggs on 100 seeds ranged between 8.00 to 181.66 on different genotypes. More numbers of eggs laid on the 100 seeds were observed for the cross derivatives DGGV-7×V-02-802 (135.33), V-02-709×DGGV-7 (133.33) and V-02-802×DGGV-7 (121.66). The other five breeding lines showed less than 1 egg per seed. Two susceptible parents viz., DGGV-2 and DGGV-7 showed 181.66 and 180.33 number of eggs per 100 seeds; while the resistance parents viz., V-02-802 and V-02-709 recorded 10.66 and 8.00 number of eggs respectively.
Disease Scale
Percent infection
Visual symptoms
Category
Reaction group
0
All plants free of virus symptoms
Complete absence of symptoms
Highly resistant
HR
1
1-10 % infection
Small yellowish spots scattered on some leaves
Resistant
R
2
11-20 % infection
Yellowish bright spots common on leaves, easy to observe
Moderately resistant
MR
3
21-30 % infection
Yellowish bright specks on leaves, easy to observe with larger patches of symptoms
Moderately Susceptible
MS
4
30-50 % infection
Bright yellow specks or spots on all leaves, minor stunting of plants and less number of pods
Susceptible
S
5
50% and more infection
Yellowing or chlorosis of all leaves on whole plants, Shortening of internode, severe stunting of plants with no yield or few flowers and deformed pods produced with small, immature and shriveled seeds
Highly Susceptible
HS
Table 2: Disease scoring scale (0-5) for Mungbean Yellow Mosaic Virus (MYMV) based on the Percentage of Disease Incidence (PDI) under field condition [25].
Sl. No.
Treatments
No. of eggs/ 100 seeds
Adult emergence (S) (%)
Mean developmental period (days) (T)
Weight loss (%)
Infestation (%)
Susceptibility Index
(S.I)Categorization
1.
DGGV-2×V-02-802
88.66±2.61d
54.89±2.15cd
30.97±0.12e
18.95±0.86cd
48.00±1.32c
0.056
Moderately Resistant (MR)
2.
DGGV-2×V-02-709
56.00±0.76ef
65.81±3.15b
32.93±0.12d
28.07±1.16b
36.66±1.30de
0.055
Moderately Resistant (MR)
3.
DGGV-7×V-02-802
135.33±1.3b
67.05±1.11b
29.51±0.09f
26.82±1.40b
90.66±1.16a
0.061
Moderately Susceptible (MS)
4.
DGGV-7×V-02-709
43.00±1.32g
88.38±0.52a
33.55±0.18d
26.12±1.19b
37.66±1.30d
0.058
Moderately Resistant (MR)
5.
V-02-802×DGGV-2
48.00±1.04fg
62.79±2.06b
33.53±0.07cd
13.49±1.72de
30.33±2.33ef
0.043
Resistant (R)
6.
V-02-802×DGGV-7
121.66±2.20c
22.79±0.88e
34.76±0.11b
25.18±1.33bc
27.66±0.92f
0.039
Resistant (R)
7.
V-02-709×DGGV-2
66.00±0.76e
55.35±1.59cd
34.02±0.14bc
24.44±1.92bc
34.66±1.20def
0.051
ModeratelyResistant (MR)
8.
V-02-709×DGGV-7
133.33±3.3bc
61.31±0.68bc
29.02±0.17f
44.25±1.03a
81.66±1.87b
0.061
ModeratelySusceptible (MS)
9.
V-02-802 (Check-1)
10.66±0.83h
37.50±1.60d
39.48±0.12a
6.25±0.60f
4.00±0.28g
0.039
Resistant (R)
10.
V-02-709 (Check-2)
8.00±0.57h
66.66±2.42bcd
39.05±0.11a
8.84±0.68ef
5.33±0.33g
0.046
Resistant (R)
11.
DGGV-2 (Check-3)
181.66± 2.20a
49.57±0.46d
23.15±0.11g
47.70±0.76a
90.00±0.57a
0.073
Susceptible (S)
12.
DGGV-7 (Check-4)
180.33±3.9a
51.24±1.29d
22.34±0.07h
50.87±0.87a
92.00±0.50a
0.076
Susceptible (S)
F test (5%)
Sig
Sig
Sig
Sig
Sig
-
-
CD (0.05)
11.96
9.9
0.76
6.99
7.25
-
-
Table 3: Performance of mungbean breeding lines (F3 generation) to Callosobruchusmaculatusthrough Force choice test (rabi-2017).
*All values represent mean of three replications, values represent Mean±Standard error.
*Means in a column followed by different letter(s) are significantly different at P=0.05.
Sl. No.
Treatments
Percent Disease Incidence (PDI %)
Disease Score
Reaction group
After 7th week of sowing
After 8th week of sowing
After 9thweek of sowing
Mean
1
DGGV-2×V-02-709
96.95
99.50
100
99.81
5
HS
2
DGGV-2×V-02-802
98.80
99.26
100
99.35
5
HS
3
DGGV-7×V-02-709
74.94
85.47
89.94
83.45
5
HS
4
DGGV-7×V-02-802
15.68
34.52
35.66
28.62
3
MS
5
V-02-802×DGGV-2
6.91
7.66
8.24
7.60
1
R
6
V-02-802×DGGV-7
95.23
96.89
100
97.35
5
HS
7
V-02-709×DGGV-2
62.62
74.22
82.29
73.04
5
HS
8
V-02-709×DGGV-7
95.92
97.20
100
97.70
5
HS
Where, R: Resistant; MR: Moderately Resistant; MS: Moderately Susceptible; S: Susceptible; HS: Highly Susceptible.
Table 4: Percent Disease Incidence (PDI) for mungbean breeding lines (F4 generation) to Mungbean Yellow Mosaic Virus (MVMV) under field condition (summer-2018).
Sl. No.
Treatments
Days to 50% flowering (days)
Plant height (cm)
Number of branches per plant
Number of clusters per plant
Number of pods per cluster
Number of pods per plant
Pod length (cm)
Number of seeds per pod
100 seed weight (g)
Seed yield per plant (g)
1.
DGGV-2×V-02-709
40.35
46.54
5.52
6.35
4.22
4.52
8.64
12.3
3.8
8.55
2.
DGGV-2×V-02-802
41.23
47.35
4.66
4.95
4.22
12.35
9.32
14.25
5.1
8.32
3.
DGGV-7×V-02-709
40.52
49.66
4.68
7.54
7.54
15.45
8.45
12.36
3.7
7.56
4.
DGGV-7×V-02-802
40.42
51.22
4.65
7.66
7.66
17.25
9.32
13.25
4.1
8.55
5.
V-02-802×DGGV-2
42.25
49.66
4.98
6.35
4.62
20.32
7.45
11.22
4.2
7.34
6.
V-02-802×DGGV-7
41.32
58.63
3.85
6.55
4.32
16.25
8.28
13.62
4.5
8.62
7.
V-02-709×DGGV-2
41.48
64.32
4.55
5.21
6.35
20.33
8.32
13.55
4.8
9.65
8.
V-02-709×DGGV-7
40.82
63.45
4.88
5.23
4.22
19.32
8.42
13.56
5.8
10.54
Table 5: Mean estimates of yield and attributing traits in advanced breeding lines of Mungbean in F4-generation (summer-2018).
The biological parameters like oviposition preference, percent adult emergence, Mean Developmental Period (MDP), percent adult emergence, percent infestation, and Susceptibility index were the most consistent indicators for screening resistance reaction of green gram genotypes to the damage of C. maculatus. The cross derivatives V-02-802×DGGV-2 and V-02-802×DGGV-7 were found to be resistant with a longer mean developmental period (33.53 and 34.76 days), low infestation (30.33 and 27.66%) and low susceptibility index (0.043 and 0.039) when compared to the susceptible one with a mean developmental period (29.51 days), highest infestation (90.66%) and highest susceptibility index (0.061) among the breeding lines. The results of Divya et al. [29]; Sekar and Nalini [28] were comparable with the present study. When DGGV-2 was used as a female parent in the crosses viz., DGGV-2×V-02-802 and DGGV-2×V-02-709 it was revealed moderately resistant reaction with susceptibility index 0.056 and 0.055 respectively. The cross derivative DGGV-2×V-02-709 (SI of 0.055) and its reciprocal cross V-02-709×DGGV-2 (SI of 0.051) were found to be moderately resistant. The above findings were more elaborately mentioned by Majhi and Mogali [23]. Considering all the studied biological parameters, breeding lines of eight crosses were categorized as resistant (2 genotypes), moderately resistant (2 genotypes), moderately susceptible (3 genotypes) and susceptible (1 genotype). The findings from the above study specify that the observed resistance may be due to antixenosis or antixenosis effect of the host. The physical seed traits including seed color, seed shape and size, seed volume and seed coat hardness were varied from genotypes to genotype which shows preferential vitiation by the bruchid. Physical barriers such as thickened plant epidermal layers, waxy deposition on leaves, stems or pods and increased trichomes density prevent the insect to oviposit on the surface, ingest or consume the seed. The resistant breeding lines were attributed to reduced egg viability and egg-to-adult survival rate. Therefore, it is necessary to establish which traits or combinations thereof contributing to the resistance reaction.
Symptomatology and Screening for MYMV resistance
MYMV is the most severe threat in mungbean due to its persistent nature of transmission, the rapid development of the viral recombinant strains and the presence of a wide host range for the vector Bemisia tabaci possess a serious constraint to the mungbean production in India. The field was regularly monitored to observe the development symptoms of MYMV under natural conditions. After the first and second weeks of sowing, there was no symptom of MYMV. The symptoms started appearing on the third week onwards in some susceptible lines as yellowing or chlorotic like appearance. Then it was spread very quickly and subsequently, the leaves became necrotic, shortening of internodes and severe stunting of plants. The infected plants bear few flowers and fewer number pods with deformed shapes containing small, and shriveled seeds (Figure 2b,c,d,e). The difference in the level of resistance was observed in different breeding lines based on the visual symptoms in response to MYMV infection. The present results also in accordance with the observations recorded by Akhtar et al., [30]; Subedi et al., [31]; Darshan et al. [32].
However, the disease incidence was recorded on the 7th, 8th and 9th weeks after sowing to determine the Percent Disease Incidence (PDI) based on the disease scoring scale given by Basir et al., [25]. The disease score varied between the observation taken on the 7th week and 8th week after sowing. But, the disease score did not vary that much between the 8th and 9th week after sowing (Table 4). On the basis of Percent Disease Incidence (PDI), the mungbean breeding lines were classified into six groups based on the classification of Basir et al. [25]. The disease symptoms were observed very early (after 3 weeks of sowing) in most of the breeding lines from viz; DGGV-2×V-02-709, DGGV-2×V-02-802, DGGV-7×V-02-709, V-02-802×DGGV-7, V-02-709×DGGV-2, V-02-709×DGGV-7. The coincidence of temperature, relative humidity and increased whitefly population creates a conducive environment for the rapid spread of the disease. The percent disease incidence varied from 7.6 to 99.1 percent in summer 2018 under field conditions. More than 90 percent of disease incidence was recorded in the breeding lines viz; DGGV-2×V-02-709 (99.81%), DGGV-2×V-02-802 (99.35%) and V-02-802×DGGV-7 (97.35%) and they were classified as a highly susceptible category as per PDI categorization. Moderate susceptibility was observed for only one cross derivative, DGGV-7×V-02-802 with a disease incidence score of 28.62 percent, while the cross derivative of V-02- 802×DGGV-2 was observed to be resistant with a disease incidence score 7.60 percent (Figure 3a,b,c). The earlier findings by Mohan et al., [33]; Deepa et al., [34]; Nair et al., [35] and Darshan et al., [32] were also shown the similar conclusions. None of the progeny lines from the present study were found to be highly resistant to MYMV with the available climatic condition. This indicates that the resistance genes were contributed by some modifier genes of the male parent.
Yield potential of advanced breeding lines of mungbean
The mean estimates of the yield and yield attributing traits were recorded to select the high yielding lines. The highest average seed yield per plant (10.54g) was observed in the breeding lines of V-02-709×DGGV-7 and second highest yield (9.65g) in V-02- 709×DGGV-2 (Table 5). The seed yield per plant was at par in the cross derivatives of V-02-802×DGGV-7 (8.62g), DGGV-2×V-02-709 (8.55g) and DGGV-7×V-02-802 (8.55g). When the 100 seed weight per plant was compared with other lines, the highest (5.8g) observed in the breeding lines of V-02-709×DGGV-7. Long pod size with a more average number of seeds per pod was observed in the cross derivatives of DGGV-2×V-02-802 (14.25), V-02-802×DGGV-7 (13.62) and V-02-709×DGGV-7 (13.56). The average number of pods per plant was more in the case of V-02-709×DGGV-2 (20.33) and V-02-802×DGGV-2 (20.32). The plant height is ranged between 46.54cm (DGGV-2×V-02-709) to 64.32cm (V-02-709×DGGV-2). The observations recorded earlier by Aparna et al., [36], Das and Barua [37], Vir and Singh [38], Parihar et al., [39], Sandhya and Saravanan [40]; and Majhi et al., [41] for the traits seed yield per plant, 100 seed weight, number of pods and number of branches per plant are similar as per the present findings. The average number of pods per cluster was recorded 7.66 and 7.54 in breeding lines of DGGV- 7×V-02-802 and DGGV-7×V-02-709 respectively. The experimental findings of Majhi et al., [42] were similar to the results recorded in the present studies. Very less difference (40.35 to 42.25 days) in days to 50% percent flowering was observed among the breeding lines. There is a positive correlation was observed among the yield attributing traits which are indirectly contributed to yield. These findings were in accordance with the result of Parihar et al. [39]; Sandhya et al., [40] and Pavan et al., [43]. When the number of branches is more than, the number of clusters and the number of pods per plant was higher. This indicates that selection of the promising lines with more number of branches will give a higher yield as compared to the plants with less number of secondary branches. Therefore, progeny lines with more branches, more pods per cluster, large pod size, more seeds per pod and large bold seed size are the best selection criteria for the development of high yielding lines.
Sl. No.
Pedigree
Plant Code No.
Days to 50% flowering (days)
Days to maturity (days)
Number of pods per plant
100 seed weight (g)
Seed yield/Plant (g)
Reaction to Bruchid
Reaction to MYMV
1.
DGGV-7×V-02-802
4S-3
40
75
42
4.1
17.3
MS
MS
4S-15
40
75
25
4.1
15.2
MS
MS
4S-16
40
75
36
4.1
16.2
MS
MS
4S-17
40
75
44
4.1
18.2
MS
MS
2.
V-02-802×DGGV-2
5S-2
42
75
30
4.2
10
R
R
5S-10
42
75
35
4.2
10.7
R
R
5S-14
42
75
25
4.2
9.5
R
R
5S-19
42
75
29
4.2
9.2
R
R
3.
V-02-802×DGGV-7
6S-5
41
75
32
4.5
14
R
HS
6S-6
41
75
20
4.5
10.2
R
HS
6S-8
41
75
36
4.5
14.2
R
HS
4.
V-02-709×DGGV-2
7S-4
41
75
25
4.5
13.6
MR
HS
7S-11
41
75
23
4.5
11.8
MR
HS
7S-18
41
75
23
4.5
11.6
MR
HS
5.
V-02-709×DGGV-7
8S-2
40
75
38
5.8
20
MS
HS
8S-12
40
75
33
5.8
16.5
MS
HS
8S-13
40
75
25
5.8
15.2
MS
HS
8S-16
40
75
24
5.8
15
MS
HS
Table 6: Selected promising breeding lines of mungbean and their yield attributes along with combined resistance to Bruchid and MYMV.
Selection of Promising Breeding lines with combined resistance to MYMV and Bruchid
There are some promising breeding lines were selected from cross derivatives of DGGV-7×V-02-802, V-02-802×DGGV-2, V-02- 802×DGGV-7, V-02-709×DGGV-2, and V-02-709×DGGV-7 (Table 6). The individual progenies were selected with a code number based on their overall performance on yield attributing traits like days to 50% flowering, days to maturity, number of pods per plant, 100 seed weight and seed yield per plant along with tolerance to bruchid and MYMV disease. Early days to 50% flowering were observed in progeny lines of DGGV-7×V-02-802 (40 days) and V-02-709×DGGV-7 (40 days), whereas the progeny lines of V-02-802×DGGV-2 has taken 42 days to 50% flowering. But, all the progeny lines from all crosses were matured at the same time i.e. 75 days has taken to maturity. The number of pods per plant ranged from 20 to 44. The highest number of pods per plant was reported in progeny lines of DGGV-7×V-02-802 plant code no. 4S-17 (44 pods). The progeny lines from the cross V-02-802×DGGV-2 were recorded an overally higher number of pods; plant code no. 5S-2 (30 pods), 5S-10 (35 pods), 5S-19 (29 pods). 100 seed weight is recorded highest 5.8g in the progeny lines of the cross V-02-709×DGGV-7, but the lines are highly susceptible to MYMV and moderately susceptible to bruchid. The progeny lines of the cross V-02-802×DGGV-2 shown resistance to both MYMV and bruchid with yield per plant is 10.0g (plant code no. 5S-2) and 10.7g (plant code no. 5S-10). The result indicated that there may be some modifier genes in the male parent which is contributing to combined resistance. There may be some conserved gene blocks present in the rare site of the parental lines for resistance reactions, which are not expressed normally. But, when they are crossed with their favorable counter parents, they express the resistance character. Thus, it needs a few more generations of stringent selection to identify the true resistant advanced breeding lines. Therefore, the breeding lines which are resistant to both bruchid and MYMV need further attention to develop superior genotypes [44-70].
Conclusion
The lack of a sufficient number of highly resistant donor lines from the available germplasm of mungbean increases the interest for extensive research for exploring new sources of resistance/tolerance to bruchid and MYMV. This facilitates to develop tolerant genotypes through interspecific hybridization. The wild relative, Vigna trilobata possesses resistance to MYMV, but the success of crossing of this species with Vigna radiata is difficult. In the endeavor of the present investigation, the derivative breeding lines of V-02-802×DGGV-2 were found to be resistant to both C. maculatus and MYMV disease. The selected progeny lines with plant code no. 5S-2, 5S-10, 5S-14, and 5S-19 were selected for further molecular characterization to confirm the true resistance. Other than combined resistance, some promising lines were selected for high yielding and early maturity from the cross derivative DGGV-7×V-02-802 (plant code no. 4S-3, 4S-15, 4S-16, and 4S-17) with moderate susceptibility to C. maculatus and MYMV. The breeding line (plant code no. 7S-4, 7S-11, 7S-18) from the cross V-02-802×DGGV-7 were selected for resistance to bruchid with a reasonable yield level. The advancement in molecular breeding will facilitate the conventional breeding to harness the potential from interspecific Vigna gene pools through genomics, transcriptomics, proteomics, and plant innate immunity approaches to identifying and tagging candidate genes for bruchid and MYMV resistance, as well as for determining their precise introgression in mungbean to develop advance breeding materials with high yielding and multipleresistant to biotic and abiotic stresses. Hence, the above findings need the molecular conformity to develop favorable resistant genotypes to compensate for the yield loss by maintaining good seed quality.
Acknowledgement
The authors are thankful to the Department of Genetics and Plant Breeding, College of Agriculture, University of Agricultural Sciences, Karnataka, India for support of research materials and land; and to ICAR-New Delhi for providing the research contingency during the research period.
References
- Pratap A, Gupta DS, Singh BB, Kumar S. IPM 205-7 (IC0589309-IC0589310; INGR11043- INGR11044), a Mung bean (Vigna radiata (L.) Wilczek) Germplasm with Super Early Maturity. Indian J Plant Genet Resour. 2012; 26: 89-90.
- Gowda CL, Laxmipathi, Sushil KC, Pooran MG, Sameer Kumar CV, Aravind KJ. Pulses research and development strategies for India. GRSV Consulting Services. ICRISAT, IIPR, CAZRI. India. 2015.
- Karthikeyan AS, Shobhana VG, Sudha M, Raveendran M, Senthil N, Pandiyan M, et al. Mungbean Yellow Mosaic Virus (MYMV): a threats to green gram (Vigna radiata) production in Asia. Int J Pest Manag. 2014; 60: 314-324.
- Fernandez GCJ, Talekar NS. Genetics and breeding for bruchid resistance in Asiatic Vigna species. Fujii K, Gatehouse AMR, Johnson CD, Mitchell R, Yoshida T, editors. In: Bruchids and legumes: economics, ecology and coevolution. Nethelands: Kluwer. 1990; 209-217.
- Southgate BJ. The importance of the bruchidae as pests of grain legumes, their distribution and control. Singh SR, van Emden HF, Taylor TA, editors. In: Pests of Grain Legumes: Ecology and Control. London: Academic. 1978; 219-29.
- Liu MS, Tony CK, Chia-Yun K, Dung-Chi W, Kuan-Yi L, Wu-Jui L, et al. Genomic and transcriptomic comparison of nucleotide variations for insights into bruchid resistance of mungbean (Vigna radiata [L.] R. Wilczek). BMC Plant Biol. 2016; 16(46): 1-16.
- Fujii K, Miyazaki S. Infestation resistance of wild legumes (Vigna sublobata) to azuki bean weevil., Callosobruchus chinensis (L.) (Coleoptera: Bruchidae) and its relationship with cytogenetic classification. Appl Entmol Zoo. 1987; 22: 229-230.
- Talekar NS, Lin CL. Characterization of Callosobruchus chinensis (Coleoptera: Bruchidae) resistance in mungbean. J Econ Entomol. 1992; 85: 1150-1153.
- Somta C, Somta P, Tomooka N, Ooi PAC, Vaughan DA and Srinives P. Characterization of new sources of mungbean (Vigna radiata L. Wilczek) resistance to bruchids., Callosobruchus spp (Coleoptera: Bruchidae). J Stored Prod Res. 2008; 44: 316-321.
- Nariani TK. Yellow mosaic of mungbean. Indian phytopathol. 1960; 13: 24-29.
- Nene YL. Viral diseases of some warm weather pulse crops in India. Plant Disease Reporter. 1973; 57: 463-467.
- Naimuddin MA. Major viral diseases of pulses and their management, Indian Institute of Pulses Research, Kanpur, India. 2001; 1-22.
- Borah BK, Dasgupta I. Begomovirus research in India: A critical appraisal and the way ahead. J Biosci. 2012; 37: 791-806.
- Naimuddin MA, Singh NP. Yellow mosaic of mungbean and urdbean: Current status and future strategies. J Food Leg. 2016; 29: 77-93.
- Singh CM, Singh P, Pratap A, Pandey R, Purwar S, Douglas CA, et al. Breeding for enhancing Legumovirus resistance in mungbean: current understanding and future directions. Agronomy. 2019; 9: 622.
- Pandiyan M, Sivakumar C, Krishnaveni A, Paramasivam V, Karthikeyan A, Senthil N, et al. Development of Mungbean Yellow Mosaic Virus resistant genotypes in mungbean through Interspecific Crosses Of Wild Vigna Species. J Plant Sci Curr Res. 2020; 4: 1-8.
- Koona P, Dom S. Extracts from Tephrosia vogelii for the protection of stored legume seeds against damage by three bruchid species. Ann Appl Biol. 2005; 147: 43-48.
- Swella GB, Mushobozy DMK. Evaluation of the efficacy of protectants against cowpea bruchids (Callosobruchus maculatus F.) on cowpea seeds (Vigna unguiculata L. Walp.). Plant Prot Sci. 2007; 43: 68-72.
- AVRDC. Diseases and insect pests of mungbean and blackgram: A bibliography, Shanhua, Taiwan, Asian Vegetable Research and Development Centre. 1999; 1998: 254.
- Ponnusamy D, Pratap A, Singh SK, Gupta S. Evaluation of screening methods for bruchid beetle (Callosobruchus chinensis) resistance in green gram and black gram genotypes and influence of seed physical characteristics on its infestation. Int J Plant Res. 2014; 27: 60-69.
- Soumia PS, Chitra S, Dikshit HK and Pandi GGP. Screening for resistance against pulse beetle, Callosobruchus analis (F.) in Greengram (Vigna radiata L. Wilczek) accessions. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences. 2015; 87: 551-558.
- Howe RW. A parameter for expressing the suitability of an environment for insect development. J Stored Prod Res. 1971; 7: 63-65.
- Majhi PK, Mogali SC. Characterization and Selection of Bruchid [Callosobruchus maculatus (F.)] Tolerant Greengram [Vigna radiata (L.) Wilczek] Genotype. Indian J Agric Res. 2020a; 1-10.
- Mogali S, Vijayakumar AG, Yamanura and Jaggal L. Yield performance and MYMV resistance in green gram [Vigna radiata (L.) Wilczek]. Int J Plant Res. 2017; 30: 1-5.
- Basir M, Ahmad Z, Mansoor S. Occurence and distribution of viral diseases of mungbean and mashbean in Punjab. Pak J Bot. 2005; 38: 1341-1351.
- Duraimurugan P, Aditya P, Singh SK, Sanjeev G. Evaluation of screening methods for bruchid beetle (Callosobruchus chinensis) resistance in greengram (Vigna radiata) and blackgram (Vigna mungo) genotypes and influence of seed physical characteristics on its infestation. Int J Plant Res. 2014; 27: 60-67.
- Chakraborty S, Mondal P, Senapati SK. Evaluation of relative susceptibility of Callosobruchus chinensis Linn. On five different stored pulse seeds. Asian J Plant Sci Res. 2015; 5: 9-15.
- Sekar S and Nalini R. Screening of mungbean genotypes against pulse beetle Callosobruchus chinensis and evaluating the biochemical basis of resistance. Int J Chem Stud. 2017; 5: 1296-1301.
- Divya BT, Krishnayya PV and Madhumathi T. Develop-mental response of Callosobruchus maculatus F. and C. chinensis L. On different pulse host-grains. Chemi Sci Rev Let. 2017; 6: 786-792.
- Akhtar KP, Dickinson M, Hodgetts J, Abbas G, Asghar MJ, Shah TM, et al. The phytoplasma disease mung bean phyllody is now present in Pakistan. Plant Pathol. 2010; 59: 399.
- Subedi S, Neupane S, Ghimire TN. Screening of mungbean and black gram genotypes as sources of genetic resistance against Mungbean Yellow Mosaic Disease. Nepal J Agric Sci. 2016; 14: 148-155.
- Darshan TD, Ravindrababu Y, Nalin KP. Screening of Mungbean [Vigna radiate (L.) Wilczek] genotypes for resistance against Mungbean Yellow Mosaic Virus (MYMV) under field condition. Int J Curr Microbiol Appl Sci. 2018; 7: 3472-3483.
- Mohan S, Sheeba A, Murugan E, Ibrahim SM. Screening of mungbean germplasm for resistance to mungbean yellow mosaic virus under natural condition. Indian J Sci Technol. 2014; 7: 891-896.
- Deepa H, Govindappa MR, Kenganal M, Kulkarni SA, Biradar SA. Screening of greengram genotypes against mungbean yellow mosaic virus diseases under field condition. Int J Pure Appl Biosci. 2017; 5: 1049-1056.
- Nair RM, Götz M, Winter S, Giri RR, Boddepalli VN, Sirari A, et al. Identification of mungbean lineswith tolerance or resistance to yellow mosaic in fields in India where different begomovirus species and different Bemisia tabaci cryptic species predominate. Euro J Plant Pathol. 2017; 149: 349-365.
- Aparna R, Singh SK, Vinay S, Rakesh P. Genetic variability, heritability, genetic advance and path analysis in mungbean [Vigna radiata (L.) Wilczek]. Legume Res. 2015; 38: 157-163.
- Das RT, Barua PK. Association studies for yield and its components in green gram. Int J Agri Environ Biotecnol. 2015; 8: 561-565.
- Vir O, Singh AK. Analysis of morphological characters inter-relationships in the germplasm of mungbean [Vigna radiate (L.) Wilezek] in the hot arid climate. Legume Res. 2016; 39: 14-19.
- Parihar RA, Agrawal P, Sharma DJ, Minz MG. Character association and path analysis studies on seed yield and its yield attributing traits in mungbean [Vigna radiata (L.) Wilczek]. J Pharmacogn Phytochem. 2018; 7: 2148-2150.
- Sandhiya V, Saravanan S. Genetic variability and correlation studies in greengram [Vigna radiata (L.) Wilczek]. Electron J Plant Breed. 2018; 9: 1094-1099.
- Majhi PK, Mogali SC, Abhisheka LS. Enhancement of Genetic Variability for Yield and Component Traits through Recombination followed by Induced Mutagenesis in Greengram [Vigna radiata (L.) Wilczek]. Curr J Appl Sci Technol. 2020b; 39: 3848.
- Majhi PK, Mogali SC, Abhisheka LS. Genetic Variability, Heritability, Genetic Advance and correlation studies for seed yield and yield components in early segregating lines (F3) of Greengram[Vigna radiata (L.) Wilczek]. Int J Chemi Stud. 2020c; 8: 1283-1288.
- Pavan K, Reddy P, Mehta CM. Estimation of variability through genetic parameters and identification of superior pure lines for yield attributing traits in green gram [Vigna radiata (L.). J Pharmacogn Phytochem. 2019; 3: 55-61.
- Akhtar KP, Sarwar G, Abbas G, Asghar MJ, Sarwar N, Shah TM. Screening of mungbean germplasm against mungbean yellow mosaic India virus and its vector Bemisia tabaci. Crop prot. 2011; 30: 1202-1209.
- Akhtar KP, Kitsanachandee R, Srinives P, Abbas G, Asghar MJ, Shah TM, et al. Field evaluation of mungbean recombinant inbred lines against mungbean yellow mosaic disease using new disease scale in Thailand. Plant Pathol J. 2009; 25: 422- 428.
- Ammavassai S, Phogat DS, Solanki IS. Inheritance of resistance to Mungbean Yellow Mosaic Virus (MYMV) in greengram [Vigna radiata (L.) Wilczck]. Indian J Genet Plant Breed. 2004; 64: 146-152.
- Ashraf M, Foolad M. Crop breeding for salt tolerance in the era of molecular markers and marker-assisted selection. Plant Breed. 2013; 132: 10-20.
- Basak J, Kundagrami S, Ghose TK, Pal A. Development of Yellow Mosaic Virus (YMV) Resistance Linked DNA Marker in Vigna mungo from Populations Segregating for YMV-Reaction. Mol Breed. 2004; 14: 375-383.
- CGIAR. Research Program on Grain legumes. 2012; 1-15.
- Dharajiya DT, Khadia SM, Pagi NK, Khatrani TJ, Jasani HV, Khunt AD, et al. Modified method of high quality genomic DNA extraction from mungbean [Vigna radiate (L.) Wilczek] suitable for PCR based amplification. Indian J Sci Technol. 2017; 10: 1-7.
- Haq QMI, Rouhibakhsh A, Ali A, Malathi VG. Infectivity analysis of a blackgram isolate of mungbean yellow mosaic virus and genetic assortment with MYMIV in selective hosts. Virus Gene. 2011; 42; 429-439.
- Hull R. Mathew’s Plant Virology. Fourth Edition. Elsevier Publishers, Andheri East, Mumbai (India). 2004; 180-182.
- International Year of Pulses. What Are Pulses and Why Are They Important?. 2016.
- Jayappa HK, Ramappa, Devamani BD. Management of Mungbean Yellow Mosaic Virus (MYMV) in Mungbean (Vigna radiata L.) J Entomol Zoo Stud. 2017; 5: 596-601.
- Jones DR. Plant viruses transmitted by whiteflies. Europen J Plant Pathol. 2003; 109: 195-219.
- Kang BC, Yeam I, Jahn MM. Genetics of plant virus resistance. Ann Rev Phytopathol. 2005; 43: 581-621.
- Karthikeyan AS, Vanitharani R, Balaji V, Anuradha S, Thillaichidambaram P, Shivaprasad PV, et al. Analysis of an isolate of Mungbean yellow mosaic virus (MYMV) with a highly variable DNA B component. Arch Virol. 2004; 149:1643-1652.
- Khaliq N, Koul V, Shankar U, Ganai SA, Sharma S, Norboo T. Screening of mungbean [Vigna radiata (L.) Wilczek] varieties against whitefly (Bemisia tabaci Genn.) mungbean yellow mosaic virus. Int J Curr Microbiol Appl Sci. 2017; 6: 129-132.
- Khattak GSS, Haq MA, Ashraf M, Srinives P. Combining ability in mungbean [Vigna radiate (L.) Wilczek]. agronomic traits. Korean J Crop Sci. 2001; 46: 420-423.
- Khattak GSS, Haq MA, Ashraf M, Elahi T. Genetics of mungbean yellow mosaic virus (MYMV) in mungbean (Vigna radiata (L.) Wilczek). J Genet Breed. 2000; 54: 237-243.
- Maiti S, Basak J, Kundagrami S, Kundu A, Pal A. Molecular marker-assisted genotyping of Mungbean Yellow Mosaic India Virus resistant germplasms of mungbean and urdbean. Mol Biotechnol. 2011; 47: 95-104.
- Mishra SP. Studies on inheritance of yellow mosaic resistance in mungbean (Vigna radiata (L.) Wilczek. Kanpur University. India. 2003.
- Nishant BA, Singh MN, Srivastava K. Screening mungbean [Vigna radiata (L.) Wilczek] genotypes for mungbean yellow mosaic virus resistance under natural condition. Adv Plant Agric Res. 2017; 7: 1-4.
- Paul PC, Biswas MK, Mandal D and Pal P. Studies on host resistance of Mungbean against Mungbean Yellow Mosaic Virus in the agro-ecological condition of lateritic zone of West Bengal. The Bioscan. 2013; 8: 583-887.
- Reddy KR, Singh DP. Inheritance of Resistance to Mungbean Yellow Mosaic Virus. Madras Agric J. 1995; 88: 199-201.
- Sandhu TS, Brar JS, Sandhu SS, Verma MM. Inheritance of resistance to mungbean yellow mosaic virus in greengram. J Res Punjab Agri Univ. 1985; 22: 607-611.
- Shukla GP, Pandya BP. Resistance to yellow mosaic in greengram. SABRAO J Breed Genet. 1985; 17: 165-171.
- Sudha M, Karthikeyan A, Nagarajan P, Raveendran M, Senthil N, Pandiyan M, et al. Screening of mungbean (Vigna radiata) germplasm for resistance to mungbean yellow mosaic virus using agroinoculation. Canadian J Plant Pathol. 2013; 35: 424-430.
- Suman S, Sharma VK, Kumar H and Shahi VK. Screening of mungbean [Vigna radiate (L.) Wilczek] genotypes for resistance to mungbean yellow mosaic virus (MYMV). Environ Ecol. 2015; 33: 855-859.
- Verma RPS and Singh DP. Inheritance of resistance to mungbean yellow mosaic virus in greengram. Annal Agri Res. 1988; 9: 98-100.