
Special Article - Abiotic Stress
Ann Agric Crop Sci. 2021; 6(2): 1072.
Physiological and Molecular Biology of High Temperature Stress in Plants
Ye J*, Zhong T, Yu D and Sun S
National Maize Improvement Center, China Agricultural University, Beijing, PR China
*Corresponding author: Jianrong Ye, National Maize Improvement Center, China Agricultural University, 2 West Yuan Ming Yuan Road, Beijing 100193, PR China
Received: February 17, 2021; Accepted: March 16, 2021; Published: March 23, 2021
Abstract
During the past few years, climate change induced by global warming had caused the appearance of extreme high temperatures worldwide, which had resulted in devastating damage to crop production. High Temperature Stress (HTS) is becoming an increasingly significant problem for agricultural production. Recent studies have elucidated the complex regulatory networks and versatile metabolites involved in HTS tolerance. Here, we provided an overview of current knowledge regarding the adverse effect of HTS on plant growth and development, the impairment of HTS on photosynthesis and membrane system, the role of carbohydrate metabolism, accumulation of osmo-protectants and secondary metabolites, the induced production of Reactive Oxygen Species (ROSs) and ROS detoxification system, and the synthesis of protective proteins like Heat Shock Proteins (HSPs) in HTS tolerance. Furthermore, the role of different phytohormones in plant response to HTS were discussed and epigenetic modifications are reported to be one of the three major signaling pathways associated with HTS response in plants, through the development of a ‘stress memory’ that is generated by hypomethylation to improve the plant’s survival under recurring HTS conditions. These physiological and molecular knowledge underlying plant response to cope with HTS will be helpful for the future directions of breeding crop tolerance to HTS using these factors or other strategies for agricultural applications.
Keywords: High temperature stress; Heat stress; High temperature; Physiological and molecular biology; Plant
Introduction
High Temperature (HT) or extended time of High Temperature Stress (HTS) induces Heat Stress (HS), which is a critical environmental constraint for plant growth and distribution, and a major limiting factor for agricultural productivity [1]. The data from the fifth report of the Intergovernmental Panel on Climate Change predicts an increase of 0.8-4.8 ºC in global mean surface temperature within the twenty-first century [2], thus giving serious alarms due to its strong effect on plant distribution and survival, and biodiversity [3-5]. HS is caused by temperatures far exceeding optimal plant growth conditions that damages cellular components through mechanisms such as membrane fluidization, ROS generation, and protein denaturation [5]. Likewise, crop yield will also suffer greatly from global warming, resulting in the incapability to sustain a growing and more demanding world population [6,7]. The global warming will also have significant impacts on the scientific research to adapt crop species for high tolerance to HTS, which directly affects the yield and quality of crops. Studies show that a 1oC increase of global temperature can lead to a 10-20 % decrease in maize production [8].
When higher plants are exposed to more than 5ºC above their optimal growing conditions, they will show a characteristic set of cellular and metabolic responses required for them to survive under the HT conditions [9]. These induced metabolic and cellular adjustments during an acclimation period enable plants to endure detrimental HTS by reducing the caused damage and help organisms overcome the injuries [8]. Plants have evolved various physiological, cellular and molecular mechanisms to ensure survival under elevated temperatures (Figure 1). The most well-known HTS tolerance mechanisms used in long-term phenological and morphological evolutionary adaptations and short-term stress avoidance and acclimation, are to synthesize osmo-protectants, stress proteins, free-radical scavengers (antioxidants), ion transporters, factors involved in signaling cascades (the production of phytohormones such as abscisic acid, and miRNA) [8]. Reactive Oxygen Species (ROSs) and ROS detoxification related antioxidants, such as ascorbic acid or glutathione and ROS-scavenging enzymes, including Superoxide Dismutase (SOD), Ascorbate Peroxidase (APX), Catalase (CAT), or Glutathione Peroxidase (GPX); protective molecules like Heat Shock Proteins (HSPs) and LEAs, are also involved in plant response to HTS. Furthermore, transcriptional control (accompanied by a decrease in the synthesis of normal proteins and the accelerated transcription and translation of HSPs) are also essential to counteract the adverse effects of HTS [5,10]. The negative effect of HTS on plant growth and development is achieved in a complex manner by disrupting the stability of various proteins, membrane fluidity/permeability, and cytoskeleton structures in cellular level. Thus, various plant physiological processes are negatively affected by HTS, such as photosynthesis, primary and secondary metabolism, lipid and hormonal signaling [5], and temperature becomes a major factor governing the distribution and seasonal behavior of plants.
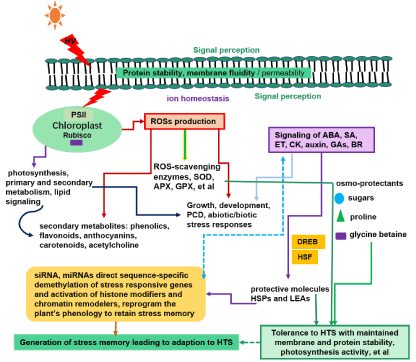
Figure 1: Schematic illustration depicting High Temperature Stress (HTS) induced activation of ROSs, phytohormone and other signals and tolerance response related physiological pathways in plants. HTS affects membrane fluidity, ion homeostasis, phytohormone homeostasis; decreases chlorophyll content, reduces maximum photochemical efficiency of Photosystem II (PSII) and inactivate Rubisco; and finally induces ROSs production. These disturbed signaling pathways reduce photosynthesis, primary and secondary metabolism and induce synthesis of ROS-scavenging enzymes, including Superoxide Dismutase (SOD), Ascorbate Peroxidase (APX), or Glutathione Peroxidase (GPX), et al; osmo-protectants (sugars, proline, glycine betaine ), free-radical scavengers (antioxidants), ion transporters and secondary metabolites such as phenolics including flavonoids, anthocyanins, carotenoids, acetylcholine that play important roles in plant responses to HTS; HTS also modulates the stress responsive TFs such as DREB and HSF and causes an accumulation of HSPs, LEA and other stress responsive genes, all these ultimately contribute to long-term adaptions of plants to HTS. Signaling factors like SiRNA and miRNA are known to involve in plant’s HTS response by directing sequence-specific demethylation of stress responsive genes and activation of DNA demethylases, these histone modifiers and chromatin remodelers can generate an epigenetic memory to improve the plant’s survival under recurring HTS conditions, through the transient activation of repetitive elements or silenced clusters in the centromeric regions. hv, Light.
The Adverse Effects of HTS on Plant Growth and Development
Being sessile, plants are highly sensitive to small variances in environment temperature and will adjust their growth and development accordingly. Generally, the observed effects of HTS on plant growth depending on species and genotype, with remarkable inter- and intra-specific variations. The temperature and duration of HTS also varies with plant tissues and development stages, although HTS affecting to a certain extent all vegetative and reproductive stages [5,11]. The earliest thermomorphogenic effects seen in Arabidopsis seedlings in response to HT are elongation of the hypocotyl and the petiole of rosette leaves and cotyledons. As these elongations and hyponastic growth may operate in concert to sufficiently separate leaves from both the soil and each other to assure optimal transpiration and leaf cooling under well-watered conditions, by moving the sensitive meristematic and photosynthetically active tissues away from heat-absorbing soil [12]. Other HTS-induced physiological injuries, such as scorching of leaves and stems, leaf abscission and senescence, shoot and root growth inhibition or fruit premature abscission, and consequently the resulted reduction in plant productivity, have also been observed [8,11,12]. HTS induces changes in respiration and photosynthesis and thus leads to a shortened life cycle and reduced crop plant productivity, as sexual reproduction and flowering, in particular pollen viability and seed-setting, have been recognized as extremely sensitive to elevated temperature [1,5]. Studies show that HTS during seed development stage may result in quality reductions in starch, protein, and total oil yield in several crop species [13], and also result in reduced germination and loss of vigor, leading to reduced emergence and seedling establishment as continuing HTS can halt further development of the embryo [14,15]. Moreover, HTS cause many temperate cereal crops with a decline in both grain weight and grain number directly proportional with increasing temperatures during flowering and grain filling [16]. It is reported that HT during wheat reproductive development reduces water-use efficiency, and hastens the decline in photosynthesis and leaf area, decreases shoot and grain mass as well as weight and sugar content of kernels, thus alters nutritional quality of flour [1,17].
HTS Impairs Photosynthesis and Disrupts Membrane System
In plants, tissue senescence is one of the typical HTS symptoms, with the feature of membrane damage (including damage of the thylakoid membrane and chlorophyll loss), increased fluidity of membrane lipids, lipid peroxidation, and protein degradation in various metabolic processes [7]. Membranes are the most sensitive targets of HTS, as the membrane system is critical for sensing environmental change, signal transduction and substance metabolism, and the thylakoid membranes are the site for light reactions in photosynthesis. Photosynthetic activity is one of the most heat-sensitive cellular functions, and HTS disrupts thylakoid membranes and decreases photosynthesis. The observed decreased photosynthetic rate under HTS is caused by an interplay between thylakoid membrane damage and thylakoid membrane lipid composition or oxidative damage of cell organelle [18,19]. At cellular level, HTS may affect membrane integrity, fluidity and electron transport rate, damage chloroplasts and mitochondria, decrease Chlorophyll content, reduce maximum photochemical efficiency of Photosystem II (PSII) and inactivate Rubisco [8,11,12]. HTS induced disorganized thylakoids with reduced thickness of grana stacking, decreased size of starch granule and increased numbers of plastoglobules and empty mitochondria were observed in grape leaves [20]. Chlorophyll a:b ratio is suggested to be related to thermotolerance as increased chlorophyll a:b ratio is observed in the tolerant genotypes under HTS [21]. Triacylglycerol levels were increased, polar lipid fatty acyl unsaturation was decreased, while triacylglycerol unsaturation was unchanged under HTS, indicating increases in activities of oxidizing, glycosylating and acylating enzymes under HTS. Thus, the composition of membrane lipid species (polar lipids, triacylglycerol, oxidized lipids and acylated galactolipids), ultrastructure of cell organelles and the related oxidants and antioxidant enzyme activity are the basis of the mechanisms underlying tolerance or susceptibility to HTS. Membrane lipid composition is important for temperature tolerance or susceptibility, as membrane lipid saturation can maintain membrane stability, function and chlorophyll content, and the ability to sustain leaf gas exchange under HTS that are essential for a sustained photosynthetic and respiratory performance and directly correlated with HTS tolerance [22]. Studies reveal that tolerant wheat genotypes are characterized with maintenance of photosynthesis, chlorophyll content, and stomatal conductance under HTS, resulting in maintained yield with higher seed set, grain weight, and extended Grain Filling Duration (GFD) even at elevated temperatures [21]. The decreased seed-set, grain number, grain filling duration, grain filling rate and individual grain weight were observed in wheat under HTS during the reproductive stage [23].
An Appropriate Carbohydrate Content is Beneficial for HTS Tolerance
One of the most important features of HTS during grain filling is inhibition of starch and sucrose synthesis, as HT modifies the activities of carbon metabolism enzymes, starch and sucrose synthesis, by down-regulating specific genes in carbohydrate metabolism [24]. So high carbohydrate availability (glucose and sucrose) during HTS acts as an important physiological trait associated with HTS tolerance. As the principal end product of photosynthesis, sucrose and its cleavage products regulate plant development and response to external stresses through carbon allocation and sugar signaling [25]. Studies show that high cell wall and vacuolar invertases activities and increased sucrose importation contribute to heat tolerance by increasing sink strength and sugar signaling activities [5]. HTS down-regulates sucrose synthase and several cell wall and vacuolar invertases in the developing pollen grains, leading to disrupted sucrose and starch turnover and reduced levels of soluble carbohydrates. Therefore, maintaining an appropriate carbohydrate content under HTS is an important factor in determining pollen quality [26]. In addition, sugars also act as antioxidants and signaling molecule at low concentrations, and becomes a ROS scavenger in high concentrations in plants [27,28].
Osmo-Protectants Accumulation is an Important Adaptive Mechanism for Plants Under HTS
Accumulation of osmo-protectants, such as the primary metabolites including proline, glycine betaine, and soluble sugars like glucose and sucrose, is an important adaptive mechanism for plants under HTS [29], as these osmolyte produced under HTS participate directly in the osmotic adjustment, by increasing protein stability, stabilizing the structure of the membrane bilayer [30,31], regulating osmotic activities and protecting cellular structures from increased temperatures by maintaining the cell water balance, membrane stability and buffering the cellular redox potential [32]. Besides, other osmolytes, such as sugar alcohols (polyols), or tertiary and quaternary ammonium compounds are also reported to accumulate in many plant species under HTS [33]. Transgenic studies have confirmed that enhanced proline production in transformed plants correlates well with a more negative leaf osmotic potential and higher production of protective xanthophyllic pigments under HTS [34]. As the chloroplast stroma and the thylakoid membrane system are considered the primary sites of heat injury [14], photosynthesis and the enzymes of the Calvin–Benson cycle, especially ribulose 1,5-bisphosphate carboxylase (Rubisco) and Rubisco activase, are very sensitive to increased temperature and are severely inhibited even at low levels by HTS. Glycine betaine, produced in chloroplasts, can act as compatible solute and maintain the activity of Rubisco by preventing its thermal inactivation [35]. It is reported that high levels of glycine betaine accumulate in maize and sugarcane under HT, while no glycine betaine is naturally produced in rice, Arabidopsis, and tobacco under stress conditions [36,37].
Secondary Metabolites also Play Important Roles in Plant Responses to HTS
In addition to the above-mentioned primary metabolites (soluble sugars like glucose and sucrose and proline, glycine betaine) and other osmo-protectants that play important roles in plant response to HTS, secondary metabolites such as phenolics including flavonoids, anthocyanins, carotenoids, acetylcholine and plant steroids are also play important roles in plant responses to HTS [38]. Enhanced synthesis of secondary metabolites under HTS can protect plants against oxidative damage. The accumulation of phenolics by activating their biosynthesis as well as inhibiting their oxidation in plant, could be an acclimation mechanism for plant against thermal stress. Accumulation of soluble phenolics, increased Phenylalanine Ammonialyase (PAL) activity, and decreased peroxidase and polyphenol oxidase activity are found in tomato under thermal stress [39]. In addition, anthocyanin plays a role in UV screening, and anthocyanin accumulation under HTS serves to decrease leaf osmotic potential, accompanying an increased uptake and reduced transpiration loss of water [40]. Carotenoids are also known to protect various plant species from several stresses, for example, xanthophylls and some other terpenoids, such as isoprene or tocopherol, can stabilize and photo-protect the lipid phase of the thylakoid membranes, thus enable the leaves to respond quickly to changing environmental conditions, like HTS [41]. The acetylcholine (Ach)-mediated system is reported to have an important role in signal transduction under various stress in plants. ACh, ACh receptor (AChR), and AChE are the three components of this system. Transgenic studies indicate that AChE plays a positive role in maize heat tolerance and native tropical zone plants also show high AChE activity under HTS [42].
HTS Induces Production of ROS and Free-Radical Scavengers (Antioxidants)
Similar to other abiotic stresses, HTS increases production of ROSs, including superoxide radical (O2−) and hydrogen peroxide (H2O2), and increases lipid peroxidation, causes membrane damage, and invokes oxidative stress responses [43,44]. Extended time of HTS also induced cell organelle and membrane damage, reduced antioxidant enzyme activity, and imbalance between ROS and antioxidant defense system. As a symptom of cellular damage, the production of injurious ROSs is also associated with the detrimental effects of heat on chlorophyll and the photosynthetic apparatus, where membrane lipids and pigments peroxidation compromise membrane permeability and function, thus hindering metabolic activities and affecting plant growth and yield [45]. ROS/redox signaling networks in the chloroplast and mitochondria are important for plant adaptation to abiotic stresses, and associate with the complex interplay between organelles homeostasis and different cellular components under stress conditions, by regulating those essential processes like transcription, translation, energy metabolism, and protein phosphorylation [46]. Likewise, ROSs directly cause cellular damage on multiple levels, and results in reduced power and energy production by impairing mitochondrial and chloroplast electron transport chains associated with carbon metabolism. Besides, ROS acts as molecular signals to control gene expression and influence essential processes such as growth and development, Programmed Cell Death (PCD), abiotic stress responses, and pathogen defense [47].
ROS detoxification is important for cellular survival as oxidative damage to membranes could disrupt membrane stability under various stresses, in order to protect themselves from the damaging effects of ROS, plants have to synthesis various antioxidant components, which can be found in almost all cellular compartments [48]. Antioxidants such as ascorbic acid or glutathione and ROS-scavenging enzymes such as SOD, APX, CAT, or GPX are essential for ROS detoxification under stress. Elevated temperatures decrease the activities of antioxidant enzymes like SOD, CAT and Peroxidase (POX), while higher levels of various antioxidants can be found in tolerant plants [44,49]. The expression of APX gene family member is responsive to HTS and regulated by Heat Shock Factor (HSF), therefore links the HTS response with oxidative stress and stress tolerance [50].
The Protection Role of Stress Proteins in HTS Tolerance
At cellular levels, HTS not only directly disrupts ion and osmotic homeostasis, affects photosynthesis, alters enzymatic and non-enzymatic defense systems, but also disturbs the pool of molecular chaperones involved in maintaining protein homeostasis and stabilizing DNA. Chaperons are a specific class of proteins that are capable of assisting other proteins in proper post-translational folding and in maintaining them in a functional state [51]. ROS production induces to the transduction of the heat signal and synthesis of protective molecules, like HSPs. HSPs are the most well-known chaperons that regulate cellular signaling, protein folding, translocation, and degradation under normal growth conditions, while they prevent protein misfolding and aggregation and also protect cellular membranes under HTS, as HTS usually causes the newly synthesized proteins to be misfolded and the existing proteins to be denatured, and HSPs can provide protein thermostability. As important molecular chaperones, the HSPs play an important role in thermotolerance by binding the denatured proteins caused by HTS, and promoting protein disaggregation and maintenance of protein homeostasis. HSPs production is increased when plants experience either abrupt or gradual increases in temperature [52,53]. For instance, HSPs accumulation is reported to associate with heat tolerance in rice, a higher accumulation of HSPs is revealed in the most tolerant rice genotype, compared to the heat sensitive rice genotype, suggesting higher HSPs accumulation might confer greater heat tolerance in rice [54]. HSP101 is reported to involve in heat tolerance responses of reproductive tissues through stabilizing Rubisco isoforms and improving photosynthesis and protect reproductive development. Heat Shock Factors (HSFs) are activators for the transcription of HSPs, their regulation may be achieved by a single “master switch” HSF or by working cooperatively with several other HSFs, depending on the plant species, resulting in the considerable variation in expression pattern of HSP gene in different species and even among genotypes within one species [55]. HSFA3 is an important HTS-responsive Transcription Factor (TF), whose expression is positively regulated by Dehydration-Responsive Element Binding Protein2A (DREB2A). HSFA3 interacts with Nuclear Factor Y (NF-YA2, NF-YB3) before its binding to DNA polymerase II and to regulate the HTS-induced overexpression of HSPs, thereby maintaining/restoring protein homeostasis under HTS [56].
Late Embryogenesis Abundant (LEA) proteins, ubiquitin, and dehydrins have been also reported to associate with protection from heat and drought stress. For example, LEA proteins can prevent aggregation of the citrate synthase (involved in ATP production) from desiccating conditions like heat and drought stress [57]. Similarly, ubiquitin and conjugated-ubiquitin synthesis at the beginning of HTS is also associated with heat tolerance in mesquite and soybean [58].
Different Roles of Phytohormones in HTS
Several studies revealed that the key defense-related phytohormones like ABA, Salicylic Acid (SA), and Ethylene (ET) increase their levels under HTS, while the levels of the growth-related phytohormones like Cytokinin (CK), auxin, and Gibberellic Acids (GAs) decrease, these fluctuations ultimately cause plant senescence under HTS [59,60]. The increased ABA and ET levels and reduced levels and transport of AUXs are known to cause the premature abscission of reproductive organs, which is an important effect of HTS [61]. It is generally accepted that the ability to synthesize ABA under HTS is critical for the higher heat tolerance of plant cells [62] and ABA induction is an important component of thermotolerance and it can be used in survival under heat and desiccation stress in the field [63]. ABA plays a role in the thermotolerance response as ABA-deficient and -insensitive mutants are sensitive to HTS, and ABA has been noted to induce thermotolerance in maize [64]. SA synthesis and SA signaling also involve in heat response, as Arabidopsis SA signaling defective mutant was revealed to increase basal heat tolerance with SA pretreatment, whereas the transgenic plants without SA accumulation show up to 40% reduction in tolerance to heat [65]. SA has also been reported to induce heat tolerance in tomato by upregulating the synthesis of total phenolics and the activities of EDS5 (enhanced disease susceptibility 5) and PAL genes.
An altered AUX biosynthesis in developing anthers is suggested to associate with pollen sterility, as auxin signaling was reported to be repressed by HTS in an anther-specific manner in barley and Arabidopsis, thus leading to abortion of pollen development, and auxin promotes fertility under HTS, as applying exogenous auxin could entirely restore pollen development under HTS [66]. In addition to auxin, both Brassinosteroids (BR) and Gibberellins (GA) contribute to high-temperature-induced hypocotyl elongation, auxin interacts synergistically with BRs, and a highly active BR pathway may enable seedlings to be sensitive to the temperature-induced increase in auxin levels [67]. In tomato and Arabidopsis, brassinosteroids also confer tolerance to HTS by inducing the biosynthesis of major HSPs [68,69], and epibrassinolide treatment results in higher HSPs synthesis and rapid resumption of protein synthesis during and following the application of HTS in Arabidopsis and rapeseed [70]. Similarly, CKs are known to have the potential to reduce oxidative stress in plants. In order to increase thermotolerance and keep yield stability, it is important for the crop systems to maintain high levels of CKs in the kernels during HTS, as CK content variation could reduce kernel filling in cereals [71]. Foliar application of seaweed extract-based CK could increase leaf CK content and delay senescence of Agrostis sp. under heat and drought stress [72].
Both Gibberellin (GA) biosynthesis and signal are required but not sufficient for the thermomorphogenesis, the major GA biosynthesis genes AtGA20ox1 and AtGA3ox1 were up-regulated, whereas the prominent catabolism gene AtGA2ox1 was down-regulated in Arabidopsis seedlings subjected to elevated temperatures [67]. Phytochrome Interacting Factors (PIFs), which is a class of bHLH transcription factor, have been reported to involve in HTS signaling mechanisms. PIFs play a regulatory role in photomorphogenesis, skotomorphogenesis, and regulation of hormone levels, like AUX, GA and ABA [8,73]. PIF4 has emerged as a critical player in regulating phytohormone levels and their activity. GA pathway is more active under HTS, putatively due to the release of DELLA-dependent PIF4 sequestering induced by the increased GA levels, as GA presence results in degradation of growth-repressive DELLA proteins, thus releases DELLA-mediated repression of BZR1 and ARF6 to allow BAP-module function and subsequent induction of hypocotyl elongation [74].
Epigenetic Modifications in Response to HTS
Epigenetic modifications (such as DNA and histone methylation) play critical role in the transcriptional and post-transcriptional regulation of gene expression and plant growth and development in response to dynamic environments [75]. Epigenetic modifications have been reported to involve in response to abiotic stresses in crop species, as plants have to activate or suppress a subset of heat-regulated genes for sustaining and adaptation to HTS [53]. The methylation, acetylation, phosphorylation, ribosylation, and ubiquitination of histone are the most frequently occurred posttranslational modification that influence the gene expression. The well-known major epigenetic marker of open chromatin organization and gene activation are H3K4me2 and H3K9ac [76]. Recently, small RNA-mediated epigenetic modification of histone proteins [56] has been reported to be one of the three major signaling pathways associated with HTS response in plants, in addition to Ca2+-dependent signaling that leads to the activation of Dehydration-Responsive Element-Binding Proteins (DREBs) and HSPs, and the HTS-induced transcriptional regulation of HSP families and their activator, HSF [77]. Small interfering RNAs (siRNA) and micro RNAs (miRNAs) are known to involve in plant’s HTS response by directing sequence-specific demethylation of stress responsive genes and activation of DNA demethylases, these histone modifiers and chromatin remodelers can reprogram the plant’s phenology to retain stress memory [78]. HTS could induce the transient activation of repetitive elements or silenced gene clusters close to the centromeric regions, the transient loss of epigenetic gene silencing [79,80], thus overcome gene silencing mechanisms associated with transcriptional repression by hetero-chromatinization of repetitive DNA regions in plants [81].
Therefore, epigenetic modifications are suggested to involve in the development of a ‘stress memory’ to regulate plant’s innate immunity under HS. HSFA2 involves in modulating histone/chromatin modification by recruiting histone methyltransferase to HS loci, resulting in hypomethylation of histone H3 lysine 4 (H3K4) and Histone H2 variant (H2A.Z). H2A.Z nucleosomes wrap DNA more tightly, which represses the ability of RNA polymerase (Pol) II to transcribe genes in response to temperature. The hypomethylation of H2A.Z removes the repression effect and initiation the transcription of temperature-responsive genes. Variations in histone modifications and a dramatic reduction in the number of nucleosomes associated with DNA, and reduction of nucleosome density throughout the genome could be observed in plant under HTS [82]. Thus, an epigenetic memory could be generated by hypomethylation to improve the plant’s survival under recurring HTS conditions, through the transient activation of repetitive elements or silenced clusters in the centromeric regions [83]. These epigenetic marks could actively reprogram the plants’ physiology to the basal level, once the stress is relieved.
Thermomorphogenesis induced by auxin is useful to facilitate cooling under unfavorable HT for many plants. The chromatin-modifying enzyme Histone Deacetylase 9 (HDA9) is stabilized in response to HT and mediates histone deacetylation at the YUCCA8 locus, YUCCA8 is a rate-limiting enzyme for auxin biosynthesis. HDA9 induces net eviction of the H2A.Z histone variant from nucleosomes associated with YUCCA8, allowing binding and transcriptional activation by PIF4 and auxin accumulation [84]. In maize seedlings, HTS is observed to induce increased electrolyte leakage and hydrolytic activity of the plasma membrane and ROS, decondensed ribosomal DNA (rDNA) chromatin and the accompanied genome-wide increase of histone H3K4me2 and H3K9ac level and increase of HSF and rRNA gene expression [85]. Moreover, HT could result in transcriptional repression of genes associated with cell growth, such as histones and DNA polymerases and deregulation of DNA methylation and transposon activation [86].
References
- Hedhly A, Hormaza JI, Herrero M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009; 14: 30-36.
- IPCC. Climate Change 2013: The Physical Science Basis. editors. In: Stocker TF, et al. Cambridge Univ. Press. 2013.
- Thuiller W, Lavorel S, Araujo MB, Sykes MT, Prentice IC. Climate change threats to plant diversity in Europe. Proc. Natl Acad. Sci. USA. 2005; 102: 8245-8250.
- Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, Mathesius U, et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010; 15: 684-692.
- Bita C, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress tolerant crops. Front. Plant Sci. 2013; 4: 273-281.
- Ovaskainen O, Skorokhodova S, Yakovleva M, Sukhov A, Kutenkov A, Kutenkova N, et al. Community-level phenological response to climate change. Proc. Natl Acad. Sci. USA. 2013; 110: 13434-13439.
- Challinor A, Watson J, Lobell D, Howden S, Smith D, Chhetri N. A meta-analysis of crop yield under climate change and adaptation. Nature Clim. Change. 2014; 4: 287-291.
- Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, van Zanten M. Molecular and genetic control of plant thermomorphogenesis. Nat Plants. 2016; 6: 15190.
- Saidi Y, Finka A, Goloubinoff P. Heat perception and signaling in plants: a tortuous path to thermotolerance. New Phytol. 2011; 190: 556-565.
- Wahid A, Shabbir A. Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycine betaine. Plant Growth Regul. 2005; 46: 133-141.
- Barnabás B, Jager K, Feher A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008; 31: 11-38.
- Crawford AJ, McLachlan DH, Hetherington AM, Franklin KA. High temperature exposure increases plant cooling capacity. Curr. Biol. 2012; 22: R396-R397.
- Banowetz G, Ammar K, Chen D. Temperature effects on cytokinin accumulation and kernel mass in a dwarf wheat. Ann. Bot. 1999; 83: 303-307.
- Akman Z. Comparison of high temperature tolerance in maize, rice and sorghum seeds by plant growth regulators. J. Anim. Vet. Adv. 2009; 8: 358-361.
- Ren C, Bilyeu KD, Beuselinck P. Composition, vigor, and proteome of mature soybean seeds developed under high temperature. Crop Sci. 2009; 49: 1010-1022.
- Mahmood S, Wahid A, Javed F, Basra SMA. Heat stress effects on forage quality characteristics of maize (Zea mays) cultivars. Int. J. Agric. Biol. 2010; 12: 701-706.
- Shah N, Paulsen G. Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil. 2003; 257: 219-226.
- Ristic Z, Bukovnik U, Prasad PVV. Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress. Crop Sci. 2007; 47: 2067-2073.
- Prasad PV, Pisipati SR, Mutava RN, Tuinstra MR. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008; 48: 1911-1917.
- Salem-Fnayou AB, Bouamama B, Ghorbel A, Mliki A. Investigations on the leaf anatomy and ultrastructure of grapevine (Vitis vinifera) under heat stress. Microsc Res Tech. 2011; 74: 756-762.
- Yang J, Sears R, Gill B and Paulsen G. Quantitative and molecular characterization of heat tolerance in hexaploid wheat. Euphytica. 2002; 126: 275-282.
- Djanaguiraman M, Boyle DL, Welti R, Jagadish S, Prasad P. Decreased Photosynthetic Rate Under High Temperature in Wheat Is Due to Lipid Desaturation, Oxidation, Acylation, and Damage of Organelles. BMC Plant Biol. 2018; 18: 55.
- Prasad PV, Pisipati SR, Ristic Z, Bukovnik U, Fritz AK. Impact of nighttime temperature on physiology and growth of spring wheat. Crop Sci. 2008; 48: 2372-2380.
- Ruan YL, Jin Y, Yang YJ, Li GJ, Boyer JS. Sugar input, metabolism, and signaling mediated by invertase: roles in development, yield potential, and response to drought and heat. Mol. Plant. 2010; 3: 942-955.
- Roitsch T and González MC. Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 2004; 9: 606-613.
- Firon N, Shaked R, Peet M, Pharr D, Zamski E, Rosenfeld K. Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Sci. Hortic. 2006; 109: 212-217.
- Sugio A, Dreos R, Aparicio F, Maule AJ. The cytosolic protein response as a subcomponent of the wider heat shock response in Arabidopsis. Plant Cell. 2009; 21: 642-654.
- Lang-Mladek C, Popova O, Kiok K, Berlinger M, Rakic B, Aufsatz W. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis. Mol. Plant. 2010; 3: 594-602.
- Sakamoto A and Murata N. Genetic engineering of glycine betaine synthesis in plants: current status and implications for enhancement of stress tolerance. J Exp Bot. 2020; 51: 81-88.
- Sung DY, Kaplan F, Lee KJ and Guy CL. Acquired tolerance to temperature extremes. Trends Plant Sci. 2003; 8: 179-187.
- Mirzaei M, Pascovici D, Atwell BJ and Haynes PA. Differential regulation of aquaporins, small GTPases and V-ATPases proteins in rice leaves subjected to drought stress and recovery. Proteomics. 2012; 12: 864-877.
- Farooq M, Basra S, Wahid A, Cheema Z, Cheema M, Khaliq A. Physiological role of exogenously applied glycinebetaine to improve drought tolerance in fine grain aromatic rice (Oryzasativa L.). J Agron Crop Sci. 2008; 194: 325-333.
- Sairam R, Tyagi A. Physiology and molecular biology of salinity stress tolerance in plants. Curr. Sci. 2004; 86: 407-421.
- Dobra J, Motyka V, Dobrev P, Malbeck J, Prasil IT, Haisel D. Comparison of hormonal responses to heat, drought and combined stress in tobacco oplants with elevated proline content. J Plant Physiol. 2010; 167: 1360-1370.
- Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P. Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res. 2008; 98: 541-550.
- Quan R, Shang M, Zhang H, Zhao Y, Zhang J. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J. 2004; 2: 477-486.
- Wahid A, Close T. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007; 51: 104-109.
- Wahid A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007; 120: 219-228.
- Rivero RM, Ruiz JM, Garcia PC, López-Lefebre LR, Sánchez E, RomeroL. Resistance to cold and heatstress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001; 160: 315-321.
- Wahid A, Gelani S, Ashraf M, Foolad M. Heat tolerance in plants: an overview. Env iron. Exp. Bot. 2007; 61: 199-223.
- Camejo D, Jiménez A, Alarcón J, Torres W, Gómez J, Sevilla F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant Biol. 2006; 33: 177-187.
- Yamamoto K, Sakamoto H, Momonoki YS. Maize acetylcholinesterase is a positive regulator of heat tolerance in plants. J. Plant Physiol. 2011; 168: 1987-1992.
- Potters G, Pasternak TP, Guisez Y, Jansen MAK. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ. 2009; 32: 158-169.
- Djanaguiraman M, Prasad PV, Seppanen M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem. 2010; 48: 999-1007.
- Xu S, Li J, Zhang X, Wei H, Cui L. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites and ultra- structure of chloroplasts in two cool- season turfgrass species under heat stress. Environ. Exp. Bot. 2006; 56: 274-285.
- Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K. ROS signaling: the new wave? Trends Plant Sci. 2011; 16: 300-309.
- Gechev TS, VanBreusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioes-says 2006; 28: 1091-1101.
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006; 141: 391-396.
- Mittler R, Vanderauwera S, Gollery M, Breusegem FV. Reactive oxygen gene network of plants. Trends Plant Sci. 2004; 9: 490-498.
- Panchuk II, Volkov RA, Schoffl F. Heat stress and heat shock transcription factor- dependent expression and activity of ascorbate peroxidase in Arabidopsis. Plant Physiol. 2002; 129: 838-853.
- Königshofer H, Tromballa HW, Loppert HG. Early events in signaling high-temperature stress in tobacco BY2 cells involve alterations in membrane fluidity and enhanced hydrogen peroxide production. Plant Cell Environ. 2008; 31: 1771-1780.
- Ahuja I, deVos RCH, Bones AM, Hall RD. Plant molecular stress responses face climate change. Trends Plant Sci. 2010; 15: 664-674.
- Scharf KD, Berberich T, Ebersberger I, Nover L. The plant heat stress transcription factor (Hsf) family: structure, function and evo- lution. Biochim. Biophys. Acta. 2012; 1819: 104-119.
- Jagadish SV, Muthurajan R, Oane R, Wheeler TR, Heuer S, Bennett J. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 2010; 61: 143-156.
- Banti V, Mafessoni F, Loreti E, Alpi A, Perata P. The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol. 2010; 152: 1471-1483.
- Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017; 22: 53-65.
- Willits DH, Peet MM. The effect of night temperature on greenhouse grown tomato yields in warm climates. Agric. For. Meteorol. 1998; 92: 191-202.
- Huang B, Xu C. Identification and characterization of proteins associated with plant tolerance to heat stress. J. Integr. Plant Biol. 2008; 50: 1230-1237.
- Larkindale , Huang B. Thermotolerance and antioxidant systems in Agrostis stolonifera: involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J. Plant Physiol. 2004; 161: 405-413.
- Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling path ways in the acquisition of thermotolerance. Plant Physiol. 2005; 138: 882-897.
- Binder BM and Patterson SE. Ethylene-dependent and -independent regulation of abscission. Stewart Postharvest Review. 2009; 5: 1-10.
- Ding W, Song L, Wang X, Bi Y. Effect of abscisic acid on heat stress tolerance in the calli from two ecotypes of Phragmites communis. Biol. plant. 2010; 54: 607-613.
- Maestri E, Klueva N, Perrotta C, Gulli M, Nguyen HT, Marmiroli N. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol. Biol. 2002; 48: 667-681.
- Musatenko L, Vedenicheva N, Vasyuk V, Generalova V, Martyn G, Sytnik K. Phytohormones in seedlings of maize hybrids differing in their tolerance to high temperatures. Russ. J. Plant Physiol. 2003; 50: 444-448.
- Clarke SM, Mur LA, Wood JE, Scott IM. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J. 2004; 38: 432-447.
- Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N. Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci USA. 2010; 107: 8569-8574.
- Stavang JA, Gallego-Bartolomé J, Gómez MD, Yoshida S, Asami T, Olsen JE, et al. Hormonal regulation of temperature-induced growth in Arabidopsis. Plant J. 2009; 60: 589-601.
- Ogweno JO, Song XS, Shi K, Hu WH, Mao WH, Zhou YH. Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicone sculentum. J Plant Growth Regul. 2008; 27: 49-57.
- Bajguz A and Hayat S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009; 47: 1-8.
- Kagale S, Divi UK, Krochko JE, Keller WA and Krishna P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta. 2007; 225: 353-364.
- Hsu SF, Lai HC, Jinn TL. Cytosol-localized heat shock factor-binding protein, At HSBP, functions as a negative regulator of heat shock response by translocation to the nucleus and is required for seed development in Arabidopsis. Plant Physiol. 2010; 153: 773-784.
- Zhang X and Ervin E. Impact of sea weed extract-based cytokinins and zeatin riboside on creeping bent grass heat tolerance. Crop Sci. 2008; 48: 364-370.
- Oh E, Zhu JY, Wang ZY. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nature Cell Biol. 2012; 14: 802-809.
- Bai M, Shang J, Oh E, Fan M, Bai Y, Zentella R, et al. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nature Cell Biol. 2012; 14: 810-817.
- Ramirez-Prado JS, Abulfaraj AA, Rayapuram N, Benhamed M, Hirt H. Plant immunity: from signaling to epigenetic control of defense. Trends Plant Sci. 2018; 23: 833-844.
- Kim JM, To TK, Ishida J, Matsui A, Kimura H, Seki M. Transition of chromatin status during the process of recovery from drought stress in Arabidopsis thaliana. Plant Cell Physiol. 2012; 53: 847-856.
- Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017; 90: 856-867.
- Suzuki N, Katano K. Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front Plant Sci. 2018; 9: 490-498.
- Lang-Mladek C, Popova O, Kiok K, Berlinger M, Rakic B, Aufsatz W. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in Arabidopsis. Mol. Plant. 2010; 3: 594-602.
- Pecinka A, Dinh HQ, Baubec T, Rosa M, Lettner N and Scheid OM. Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell. 2010; 22: 3118-3129.
- Khraiwesh B, Zhu JK, Zhu J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim. Biophys. Acta. 2012; 1819: 137-148.
- Goraya GK, Kaur B, Asthir B, Bala S, Kaur G, Farooq M. Rapid injuries of high temperature in plants. J Plant Biol. 2017; 60: 298-305.
- Ahammed GJ, Li X, Zhou J, Zhou YH, Yu JQ. Role of hormones in plant adaptation to heat stress. In: Ahammed GJ, Yu JQ (eds) Plant Hormones Under Challenging Environmental Factors. Springer, Dordrecht. 2006: 1-21.
- Van der Woude LC, Perrella G, Snoek BL, van Hoogdalem M, Novák O, van Verk MC, et al. HISTONE DEACETYLASE 9 stimulates auxin-dependent thermomorphogenesis in Arabidopsis thaliana by mediating H2A. Z depletion. Proc. Natl. Acad. Sci. U.S.A. 2019; 101: 8245-8250.
- Hou H, Zhao L, Zheng X, Gautam M, Yue M, Hou J, et al. Dynamic Changes in Histone Modification Are Associated With Upregulation of Hsf and rRNA Genes During Heat Stress in Maize Seedlings. Protoplasma. 2019; 256: 1245-1256.
- Smith KT and Workman JL. Chromatin Proteins: key responders to stress. PLoS Biol. 2012; 10: e1001371.