
Research Article
Ann Agric Crop Sci. 2022; 7(3): 1116.
Nitric Oxide Modulates Glycine Max L. Growth and Physio-Molecular Responses during Flooding Stress
Imran M1, Sang-Mo Kang1, Khan AL2, Khan MA1, Shahzad R3, Bilal S4, Khan M1, Kim YH1, Yun BW1 and Lee IJ1*
1School of Applied Biosciences, Kyungpook National University, Republic of Korea
2Department of Engineering Technology, University of Houston, USA
3Department of Horticulture, The University of Haripur, Pakistan
4Natural & Medical Sciences Research Center, University of Nizwa, Nizwa Oman
*Corresponding author: In-Jung Lee, School of Applied Biosciences, Kyungpook National University, Daegu 41566, Republic of Korea
Received: June 10, 2022; Accepted: July 18, 2022; Published: July 25, 2022
Abstract
Long-term exposure to flooding creates hypoxic conditions, affecting plant growth and development. Nitric oxide is a stress signaling and evading molecule involved in many physiological and biochemical response in plants stress tolerance, however, its potential role in response to flooding stress is not fully understood. We illuminate the role of NO in regulation of stress-related biochemical and genetic influence in soybean plants after 3, and 7days of flooding. Exogenous nitric oxide donor (SNP) application improve plants growth and development, and chlorophyll content, which may correlate to increase in the antioxidant enzyme activity such as peroxidase (POD), catalase (CAT), superoxide dismutase [1] and reduced glutathione (GSH), and protect plants from oxidative damage through scavenge the H2O2 accumulation, reduced the MDA level and increased the proline accumulation and further improved the photosynthesis and stomata conductance. Furthermore SNP treatments reduced the ABA content and down regulate the relative expression of ABAreceptors ABAR1, ABAR2 and ABA biosynthesis gene NCED3 after 3, and 7days of flooding stress. In the case of endogenous NO signaling, GSNOR and NR expression was enhanced by SNP treatments and improve the cellular SNO level, showing a positive effects on flooding stress tolerance. These positive effects of NO on stress tolerance were completely reversed by NO scavenger cPTIO, and NO inhibitor L-NAME treatments by enhance the ABA accumulation, relative expression of ABAR1, ABAR2 and NCED3, and cause reduction in antioxidant activity and increase the H2O2 content and MDA level. NO treatments improved plant tolerance to flooding stress and improved various biochemical and transcriptional programs that are ameliorative to plant growth during long-term flooding stress. The potential benefit of SNP or related NO sources during flooding can be attributed to its antagonistic effects on ABA biosynthesis, which in turn regulated flooding tolerance.
Keywords: Soybeans; Nitric Oxide; Flooding Stress; Gene Expressions; Abscisic Acid; NO Scavengers; Stress Tolerance
Background
Agricultural farmers are faced with the challenge of ensuring food availability for an additional 2. 3 billion people in the coming decade. Recently, emphasis has been placed upon the efficient use of limited natural resources and adaptation of crops to climate change. Global environmental changes, such as devastating flooding, hurricanes, and tropical cyclones have resulted in the destruction of crops and economic losses [2]. Plants face many abiotic stresses such as flooding, drought, heat, salinity, depletion of the ozone layer, UVradiation, herbicides, and metal toxicity, as well as biotic stresses such as pathogens, microbes, and insects that threaten crop production [3]. Extreme conditions and global climate change are resulting in more adverse environmental conditions, threatening food security [4].
Flooding is a major environmental factor that limits crop production and development [5]. Flooding is detrimental to terrestrial plants, reducing growth and resulting in premature death. Inter specific variation has an impact on species distribution in floodprone eco systems worldwide [3]. Flood stress negatively influences the physiological processes of plants, often leading to poor hormonal balance, reduced nutrient uptake, and decreased photo synthesis, resulting in stunted growth and reduced yield [6]. Flooding can result in total submergence, which creates hypoxic conditions for nonphotosynthetic organs. Excess water imposes pressure on the plants, limiting the availability of oxygen, carbon dioxide, and light, and reducing photo synthetic rates causing leaf chlorosis [7]. In response to environmental changes, plants develop complex mechanisms involving physio-chemical changes, hormonal modulation, and regulatory genes, which arbitrate the transduction signals under stress-inducing conditions [8]. Plants respond to stresses by producing molecules such as reactive nitrogen intermediates, which subsequently regulate many biochemical and physiological processes.
Nitric Oxide (NO) is an important plant signaling molecule that gains much attention due to its functional role in many physiological, environmental, and developmental responses [9]. Nitric oxide is a very reactive species in the presence of oxygen it form other oxides such as N2O3, N2O4 and NO2, which react with thiols and cellular amines or hydrolyze to NO2 and NO3 [10]. NO can improve plant tolerance toward both biotic and abiotic stresses [11] and playsan important role in plant defense and stress resistance [12]. In soybean nodules, flooding with copper containing nitrite reductase down-regulates the expression of nitrogenase, suggesting that the oxygen carrier leg hemoglobin plays a major role in detoxifying NO and NO2 in response to flooding conditions [13]. In response to abiotic stress NO was reported to reduce the destructive effects of herbicides, drought, and heavy metal in plants [14]. Furthermore to evaluate the protective effects of NO sources on plants under abiotic stress was reported by many researchers through the activities of antioxidant enzyme and ROS scavenging activity [15]. Eventually nitric oxide educe the ROS level during the stress and limited the oxidative damage to plants cell, similarly NO application strongly enhance the growth along with maintain the PS II activity and increased the antioxidant, and increase the gene expression related to stress tolerance [16].
In response to abiotic stressors, NO performs antioxidant activities and acts as a signal to activate Reactive Oxygen Species (ROS). NO reacts with glutathione (GSH) to ensure the accumulation of S-nitrosoglutathione (GSNO3), which performs an endogenous trans nitrosylation function. GSNO3 is more stable than NO and acts as a physiological NO donor and long-distance NO transporter [17]. Sodium Nitroprusside (SNP) is an NO donor that alters NO bioregulatory functions; SNP also executes protective and toxic functions and can act as a signaling compound depending on its concentration. In addition, SNP regulates the concentration, time of production, and development of endogenous proline and polyamine metabolites [18].
In this study, we determined the interaction between exogenous sources of NO (SNP) at the physio-chemical and transcription levels under flooding stress. We subjected soybean (Glycine max L.) to 3 and 7 days of flooding stress to investigate the function and effects of exogenous NO donor (SNP) on the physio-hormonal (abscisic acid) involvement and its effects on antioxidant defense system and ROS scavenging activity in soybeans plants during flooding. Additionally, we examined the role of abscisic acid receptors and promoters during exogenous NO treatments and flooding stress.
Material and Methods
Plant Growth Conditions
Soybean seeds (Glycine max L.) were provided by the Soybean Genetic Resource Center, Kyungpook National University Daegu, and Republic of Korea. The seeds were sterilized using 2.5% sodium hypochlorite for 10min and then washed three times with double distilled water. The seeds were placed in plastic trays filled with horticulture soil containing 10–15% peatmoss, 45–50% cocopeat, 6–8% zeolite, ~0.205 mg/g NO3, 35–40% perlite, ~0.35 mg/g P, ~0.1 mg/g K, and~0.09 mg/g NH+ [19]. The trays were kept in growth chambers under a fixed day and night cycle of 14 h at 27°C and 10 hat 24°C. Relative humidity was maintained at 60% to 70% and the plants were exposed to light at an intensity of 1000μEm-2s-1 from sodium lamps. At the VC stage (unifoliate leaves are fully developed), equallysized seedlings were transferred to a plastic pots filled with the same horticulture substrate.
Nitric Oxide Treatment and Flooding Stress
The experiment was comprised of two time periods (3, and 7 days) and 5 treatments with 8 plants for each treatment: (1) Control, (2) control with flood (CWF), (3) 100μMSNP previously reported by [20], (4) 150μML-NAME, (5) and 150μM cPTIO. At the VC stage plants were pre-treated with 50ml (Twice a day) of each 100μMSNP, 150μML-NAME, and 150μM cPTIO for 3 day and then subjected to flooding stress for 3 and 7 days. For the flooding stress pots were kept in (24×17 cm) 4 plants in each box. During the flooding treatment, the water level was maintained 5-6cm above the soil surface. Chlorophyll content was measured using SPAD (Minolta Chlorophyll Meter SPAD- 502, Japan), upon the completion of each stress period and the plants were harvested. The root and shoot lengths and fresh weights were measured, and then samples were frozen with liquid nitrogen and stored at -80°Cuntil further analysis.
Determination of Antioxidant Enzymatic Activity
Catalase activity was determine using a previously described method of [20,21], by calculation of H2O2 absorption reduction at 240 nm. The reaction buffer contained 15mM H2O2 and 50mM potassium phosphate buffer at a pH of 7.0. Then, 100μl of the enzyme extract was added to the reaction mixture to initiate the reaction. The H2O2 level in the reaction mixture was measured after 1 min using the extinction coefficient of 40 mM-1 cm-1, which indicated CAT enzyme activity.
Superoxide dismutase [1] activity were measures using the method of [22,23], which consisted of evaluating the SOD inhibitory ability to photochemically decrease nitroblue tetrazolium (NBT). SOD activity units were determined as the amount of enzyme required to cause 50% inhibition of the reduction of NBT, as monitored at 560 nm. POD activities were determined using the guaiacol method [24], which was performed by adding 0.1 ml of the supernatant to the reaction mixture containing 1.0 ml of 2% H2O2, 2.9 ml of 50 mM phosphate buffer (pH 5.5) and 1.0 ml of 50 mM guaiacol. Phosphate buffer was used as control without enzyme. Absorbance was read at 470 nm for 3 min, and POD activity was calculated as unit change per minute. For determining the reduction of GSH content, a previously described detailed method [25] was used.
Determination of H2O2 and MDA
Hydrogen peroxide (H2O2) content was measured following the detail method of [26]. Briefly, 0.1 g of leaf sample was ground and extracted using 5 ml of 0.1% TCA and centrifuged at 12,000×g for 15 min. Next, 0.5 ml of the supernatant was collected, and 1 ml of 1 M potassium iodide and 0.5 ml of 10mM phosphate buffer (pH 7.0) were added, and the absorbance was detected at 390 nm. The H2O2 content was estimated using the extinction coefficient (ε) 0.28 mM cm-1 and expressed as μM g-1 DW. Lipid peroxidation in leaves was determined by measuring the levels of MDA as described by [20]. Briefly, 0.1g of fresh plant tissue was ground with 10 ml of 5% TCA and centrifuged at 4000x g for 10 min at 4°C. The resulting supernatant was suspended with 4 ml of TBA, heated at 90°C for 25 min and then immediately cooled down at 4°C. The sample was centrifuged, and the supernatant was read at wavelengths of 532 and 600 nm. The MDA content was calculated as MDA (u mol. G-1 FW).
Determination of Stomata Conductance and Photosynthesis Rate
To determine the physiological traits such as stomata conductance and photosynthesis rate were followed by the previous method of [27]. For the stomata conductance and photosynthesis data were collected for the 2nd trifoliate leaf of soybean plant using and advance portable photosynthesis system (L Cpro T, ADC Bio Scientific Ltd., Hoddesdon, Herts EN11 0NT, UK).
Quantification of Endogenous Abscisic Acid
Endogenous ABA was extracted and quantified following the method of [28]. In brief, abscisic acid was extracted from 0.3g of freeze-dried aerial plant parts and a chromatograph was run using a Me-[2H6]-ABA standard. The fraction was then methylated using diazomethane for detection and was further quantified using gas chromatography mass spectrometry (GCMS; 6890N network gas chromatograph) (Supplementary Table 2). Thermo-Quest software (Manchester, UK) was used to amplify and monitor signal ions (m/z 162 and 190 for Me-ABA and m/z 166 and 194 for Me-[2H6]-ABA).
Endogenous Nitric Oxide Quantification
Nitric oxide was quantified using 100 mg of fresh plant sample, ground in liquid nitrogen using a chilled mortar and pestle. The samples were mixed with 1ml of extraction buffer (1X PBS, pH 7.4) and centrifuged at 12,000 rpm for 10 min, and then the supernatants were collected. Next, 100μl of extract was injected into the Nitric Oxide Analyzer (NOA280i, GE Water & Process Technologies, Germany) containing reducing buffer (CuCl/cysteine with water) to determine the S-Nitrosothiol (SNO) content. NO production values were recorded and the standard curve was plotted using the OD595 values for each standard against its concentration (μg/ml). The standard curve was used to determine the total SNO levels in each sample [20].
RNA Isolation and Quantitative Real-Time PCR
RNA was extracted from the fresh leaves using the method of [29], with slight modifications. Briefly, 0.1g of the leaf samples was ground in liquid nitrogen using a pre-chilled mortar and pestle. Ground samples were transferred to RNA free E-tubes with an extraction buffer (0.25 M NaCl, 0.05 M Tris-HCl (pH 7.5), 20m MEDTA, 1% w/v sodium do decyl sulfate, and 4% w/v PVP [19]. The verified and purified RNA was used to synthesize cDNA using a DiaStar™ RT kit (SolGent, Korea) according to the manufacturer’s standard protocol. Quantitative real-time PCR (qRT-PCR) was used for the transcript accumulation to investigate the response of soybean to flooding stress. The detailed list of genes and relative primers are shown in (Supplementary Table. 1). We used a 2×RT- PCRkit (BioFACTTM, Korea) with 10nM specific gene primer and 100ng template cDNA with a final volume of 20μl. The reaction was carried out according to the manufacturer’s protocol using Eco™ Real-Time PCR (Illumina™) with a template control [30] as the negative control.
3 Days
Treatment
SL
RL
FW
DW
CC (SPAD)
Control
14.3±0.5b
14.9±1.08b
2.2±0.05b
1.5±0.7b
37.3.5±0.8a
CWF
10.1±0.7c
7.8±1.1d
1.3±0.05c
0.73±0.06c
31.4±3.2cd
SNP
15.8±0.5a
16.5±0.9a
2.9±0.07a
2.2±0.05a
37.08±2.5a
L-NAME
9.1±0.7d
6.8±1.7f
1.0±0.05d
0.64±0.05d
31.1±3.06d
cPTIO
10.5±0.9c
7.7±0.9e
1.3±0.06c
0.69±0.1cd
32.05±2.8c
7 Days
Control
16.4 ±0.05b
14.8±0.6b
2.6±0.05b
1.8±0.05c
40.2±0.8a
CWF
13.9±0.6c
12.1±1.1c
1.6±0.06c
0.95±0.1d
29.7±0.8d
SNP
17.5±0.5a
16.4±0.9a
3.4±0.5a
2.6±0.05a
39.4±3.3ab
L-NAME
12.5±0.04d
10.1±0.7d
1.3±0.05cd
0.71±0.07e
29.2±2.1d
cPTIO
13.5±0.06c
9.2±1.2e
1.3±0.05cd
0.97 ±0.05d
30.4±2.5c
SL=Shoot length (cm), RL=Root length (cm), FW=Fresh weight (g), DW=Dried weight (g), CC=Chlorophyll contents (SPAD). Each value represents the mean ± SD of 3 replicates from 2 independent experiment. Values followed by different letters are significantly different at P<0.05.
Table 1: Effect of exogenous nitric oxide on growth attributes 3 and 7 days after flooding stress.
Statistical Analysis
The experimental treatments were performed independently in triplicate. Duncan’s multiple range test (DMRT) was used to determine the mean values with a significance level of P<0.05. Statistical Analysis System (SAS 9.1) was used for the DMRT analysis. For the graphical representation, we used GraphPad Prism software (version 6.0, San Diego, CA, USA).
Results
Phenotypic Variation in Response to Long-Term Flooding Stress
Plant growth attributes shoot length, (RL) root length, [31] fresh weight, dry weight, and (CC) chlorophyll content were measured. The treatments included control (Cont), Control With Flood (CWF), NO-donor sodium nitroprusside (SNP), NO-scavenger (cPTIO), and NO-inhibitor L-NAME, over two time periods 3, and 7 Days- After-Stress (DAS). The results of the current study show that the application of Exo-NO donor SNP improves plant growth and development. Three days after the flooding stress, the shoot and root lengths of plants treated with SNP significantly improved compared to the control with flooding, where as the shoot and root lengths significantly decreased in the NO-inhibitor (L-NAME) and NOscavenger (cPTIO) treatments. Contrarily, plants treated with SNP had higher fresh and dry weights compared to control and CWF (Control With Flooding Stress) 3-DAS (Figure 1; Table 1). Seven DAS, SNP-treated plants displayed an increase in shoot and root length, biomass, and chlorophyll content, which was completely reversed by the cPTIO and L-NAME treatments.
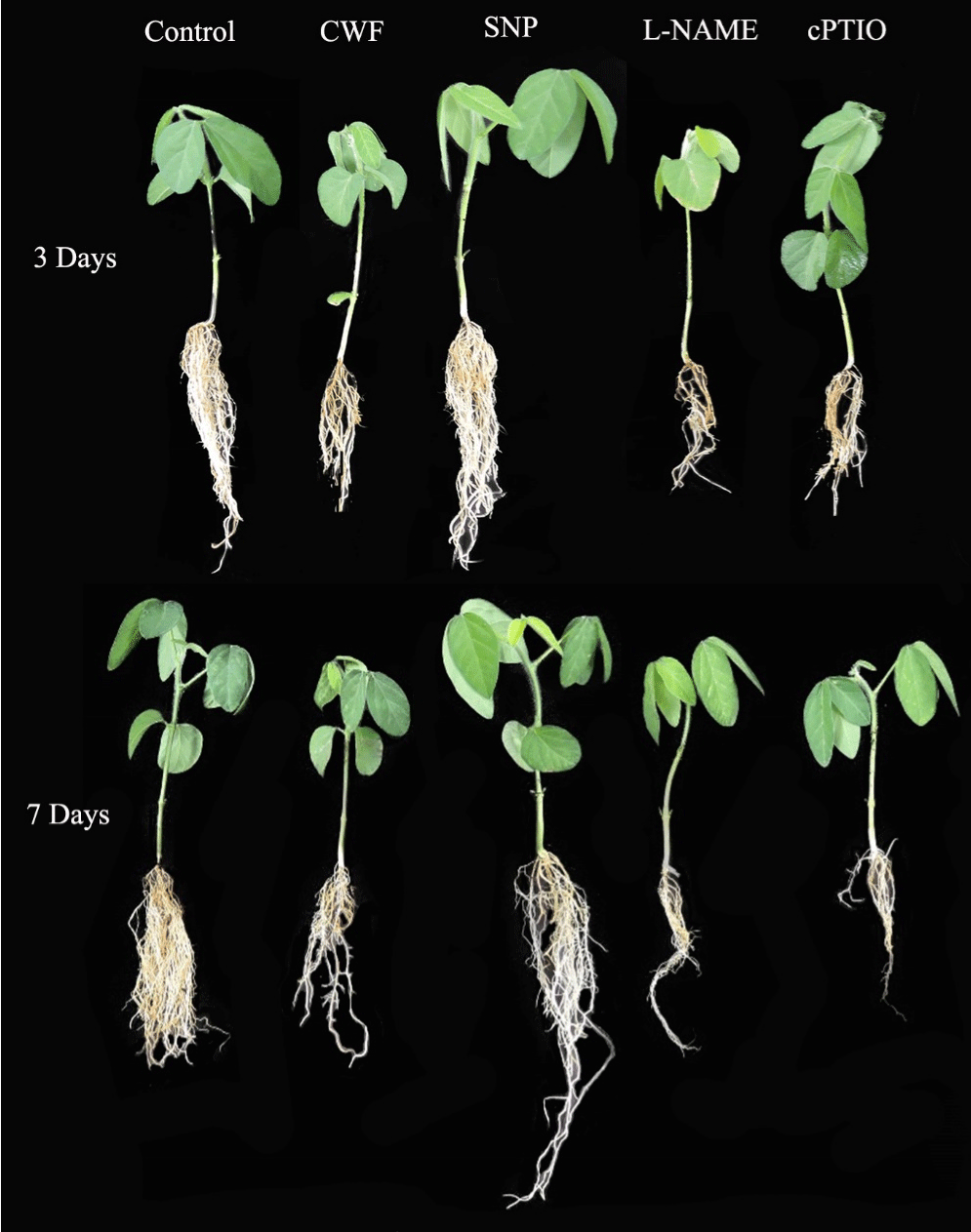
Figure 1: Effects of nitric oxide donor SNP application on the phenotypical
visualization of soybean plants growth under flooding stress.
Effects of Exogenous NO Application on H2O2, MDA and Proline Content
The excess amount of H2O2 induced the lipid peroxidation is an important factor of flooding induced program cell death. In the present study to evaluate the effects of flooding on the soybean plants and the role of nitric oxide on the H2O2 and MDA accumulation. As show in (Figure 2A) flooding stress cause significant increase in H2O2 (73%) after 3 days and (97.6%) after 7 days compared to control plants. However the plants treated with exogenous SNP significantly inhibit the excessive production of H2O2 by 39.8% after 3 days and 33.4% after 7 days compared to plants treated with flooding, while after 7 days of flooding further increase was observed in the H2O2 then 3 days after stress, where SNP treated plants decrease H2O2 level. Similar in the case of MDA content, that flooding stress cause significant increase (68.5%) in MDA content compared to control plants (without flooding stress). While the SNP pre-treatment reduced the MDA level by 27.7% compared to CWF treated plants. These effects of SNP treatment was significantly reverse by the pretreatment of cPTIO and L-NAME, by enhance the level of MDA after 3 and 7 days of flooding stress (Figure 2B). Moreover the proline content was slightly increase by 15.3% at first 3 DAS and was reduced by 32.5% after 7 DAS compared to control plants. However the SNP treatment significantly increases the proline content by (35.9%) after 3 DAS (Days After Stress) and (27.8%) after 7 DAS compared to CWF treated plants (Figure 3).
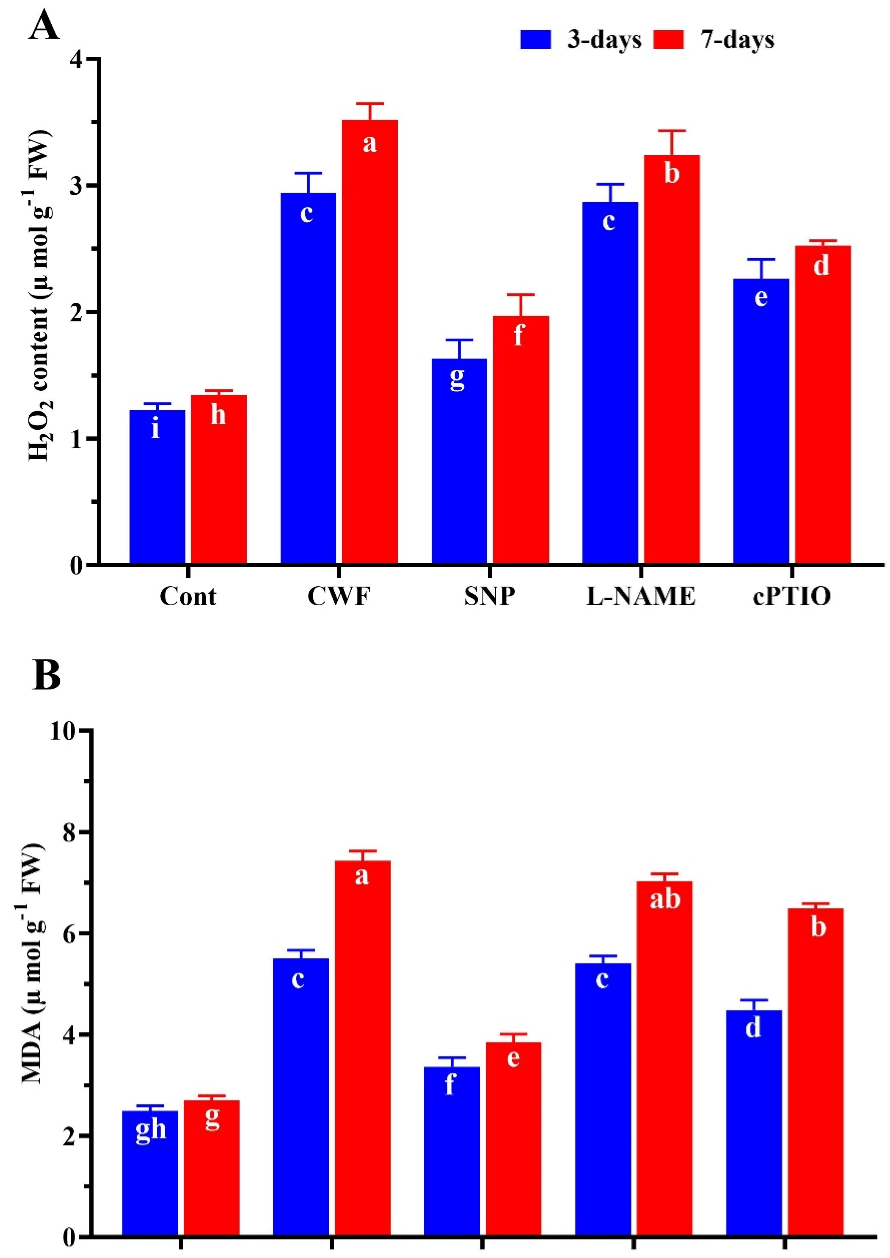
Figure 2: The inhibitor effects of nitric oxide source on the (A) Hydrogen
peroxide (H2O2), (B) MDA level in soybean plants after 3 and 7 days of
flooding stress. Data represent the mean of three replicates, while the error
bars indicate the standard error. The differences between the mean values
were determined using Duncan’s multiple range test (DMRT) at P < 0.05.
Different letter(s) indicate values that are significantly different.
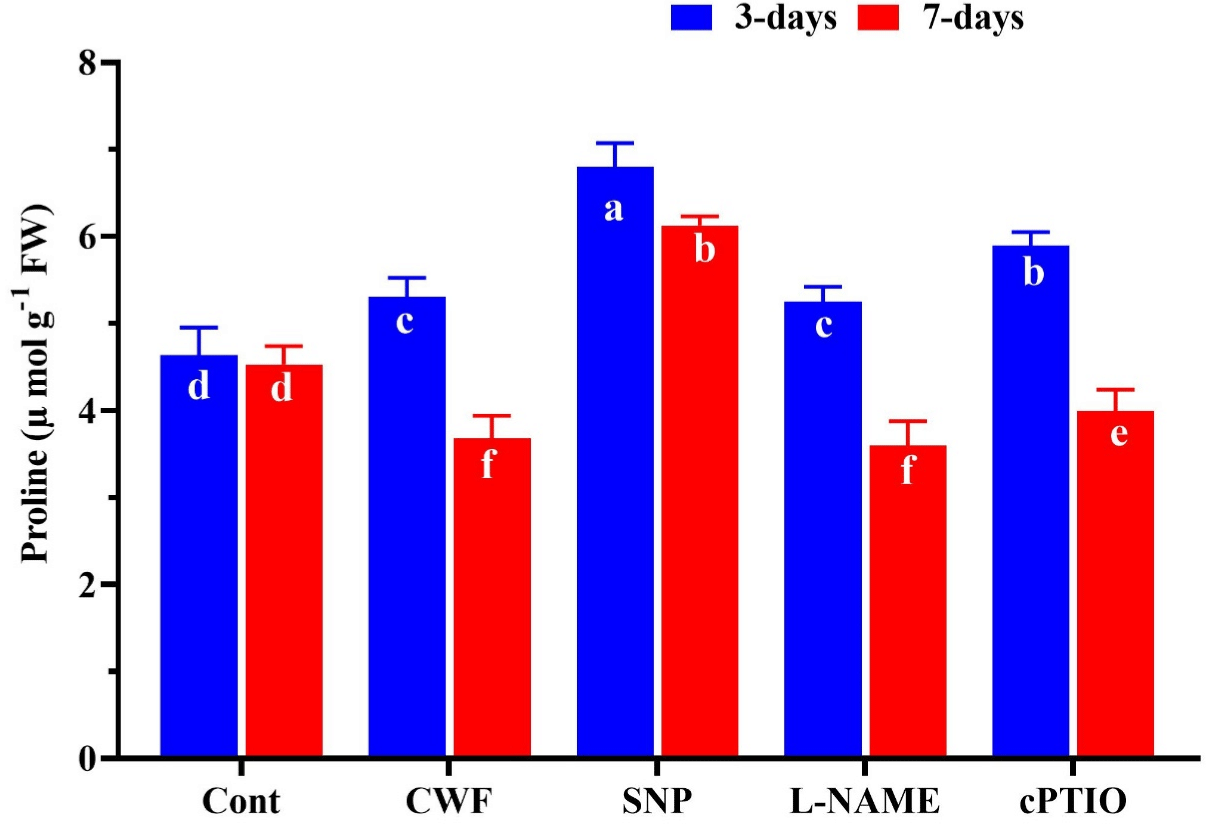
Figure 3: The effects of nitric oxide source on the proline accumulation in
soybean plants after 3 and 7 days of flooding stress. Data represent the
mean of three replicates, while the error bars indicate the standard error.
The differences between the mean values were determined using Duncan’s
multiple range test (DMRT) at P < 0.05. Different letter(s) indicate values that
are significantly different.
Exogenous Nitric Oxide Enhance the Antioxidant Activity Under Flooding Stress
In the present study, the effect of flooding on the soybean plants and the role of nitric oxide involvement in the activation of antioxidant activity was validating. As show in (Figure 4A-B) 3 days after flooding stress a slight increase was found in POD (11.5%) and CAT (12.7%) activities compared to control plants. Where plant treated with SNP further increase in the POD (20.5%) and CAT (26.2%) compared to CWF plants. However 7 DAS after flooding stress a significantly decrease was found in POD (17.2%) and CAT (19.4%) when compared to control plants, while the NO donor SNP significantly increase the POD (49.3%) and CAT (31.6%) compared to plants treated with CWF. The effect of SNP on the POD and CAT activity was significantly reverse by the cPTIO and L-NAME treatment. Similar in the case of GSH content, after 3 and 7 days of flooding stress GSH content was a significantly reduced by 18.6% and 23.4% compared to control plants (no stress), where the plant treated with SNP improve the GSH content by 16.5 and 19.3% compare to CWF treated plants. on the other hand, SOD was found to increase by 48.4% and 28.6% during 3 and 7 days of flooding compare to control plants (no stress), where SNP treated plant cause further more increased in SOD level by 24.7% and 19.8% compare to CWF treated plant (Figure 4C-D). These positive effects of NO donor on the regulation of antioxidant were significantly reduced by the cPTIO and L-NAME treatment.
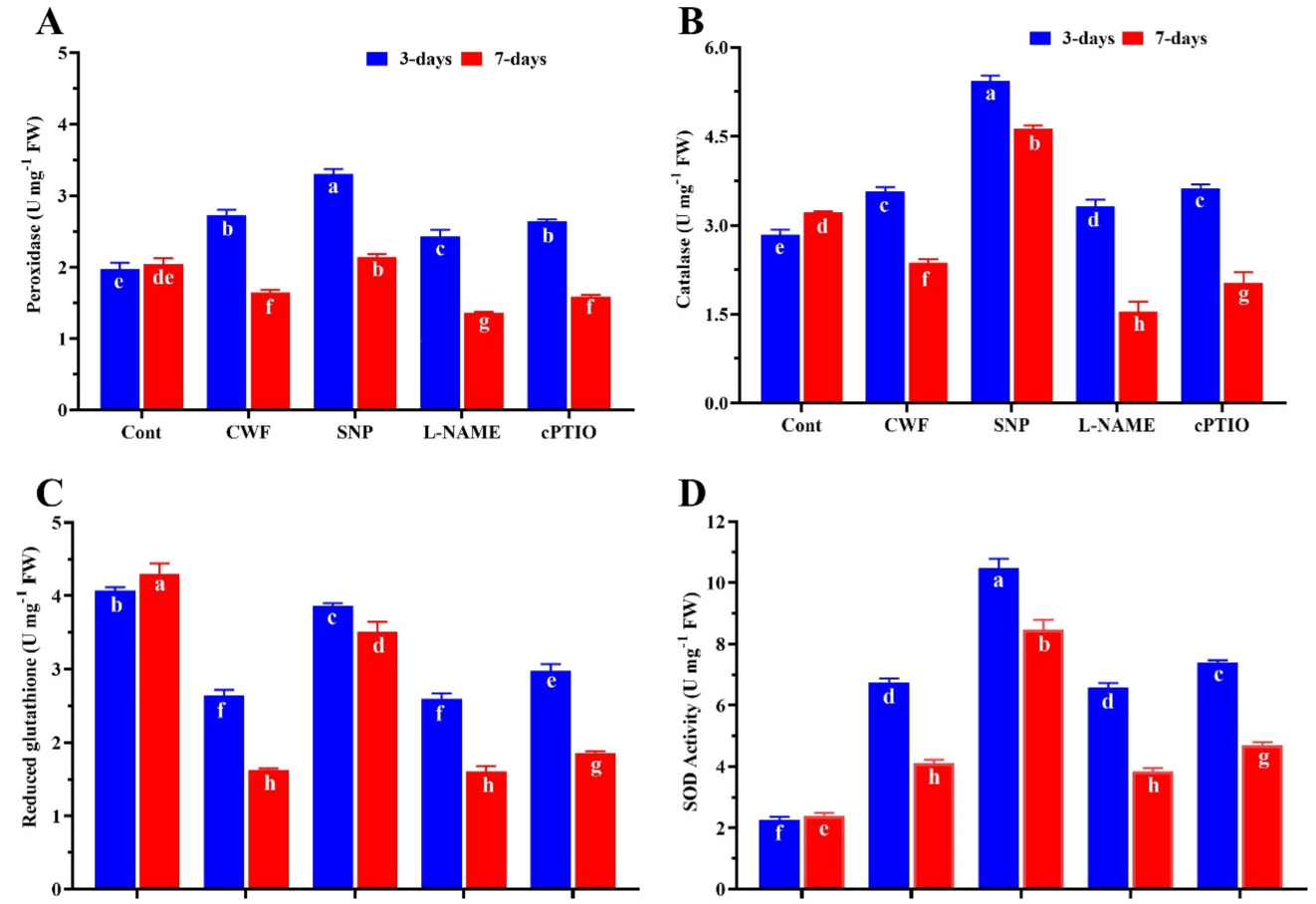
Figure 4: Nitric oxide source regulates antioxidant enzyme activity (A) Peroxidase (POD), (B) Catalase (CAT), (C) Reduced glutathione, (D) Superoxide dismutase
levels insoybean plants after 3 and 7 days of flooding stress. Data represent the mean of three replicates, while the error bars indicate the standard error.
The differences between the mean values were determined using Duncan’s multiple range test (DMRT) at P < 0.05. Different letter(s) indicate values that are
significantly different.
Exogenous Nitric Oxide Application Improve Photosynthesis and Stomata Conductance under Flooding Stress
The results of present study shows that after 3 and 7 days of flooding stress a significantly reeducation was found in net photosynthesis (36.1% and 39.7%) and stomata conductance (26.7% and 31.2%) compare to control plants (no stress). However, the plants treated with SNP improved the net photosynthesis (19.1% and 15.7%) and stomata conductance (17.4% and 15.8%) compare to CWF treated plants. furthermore the plants treated with cPTIO and L-NAME reverse these effects of NO and reduced net photosynthesis and stomata conductance, showing a non-significant difference to CWF treated plants (Figure 5A-B).
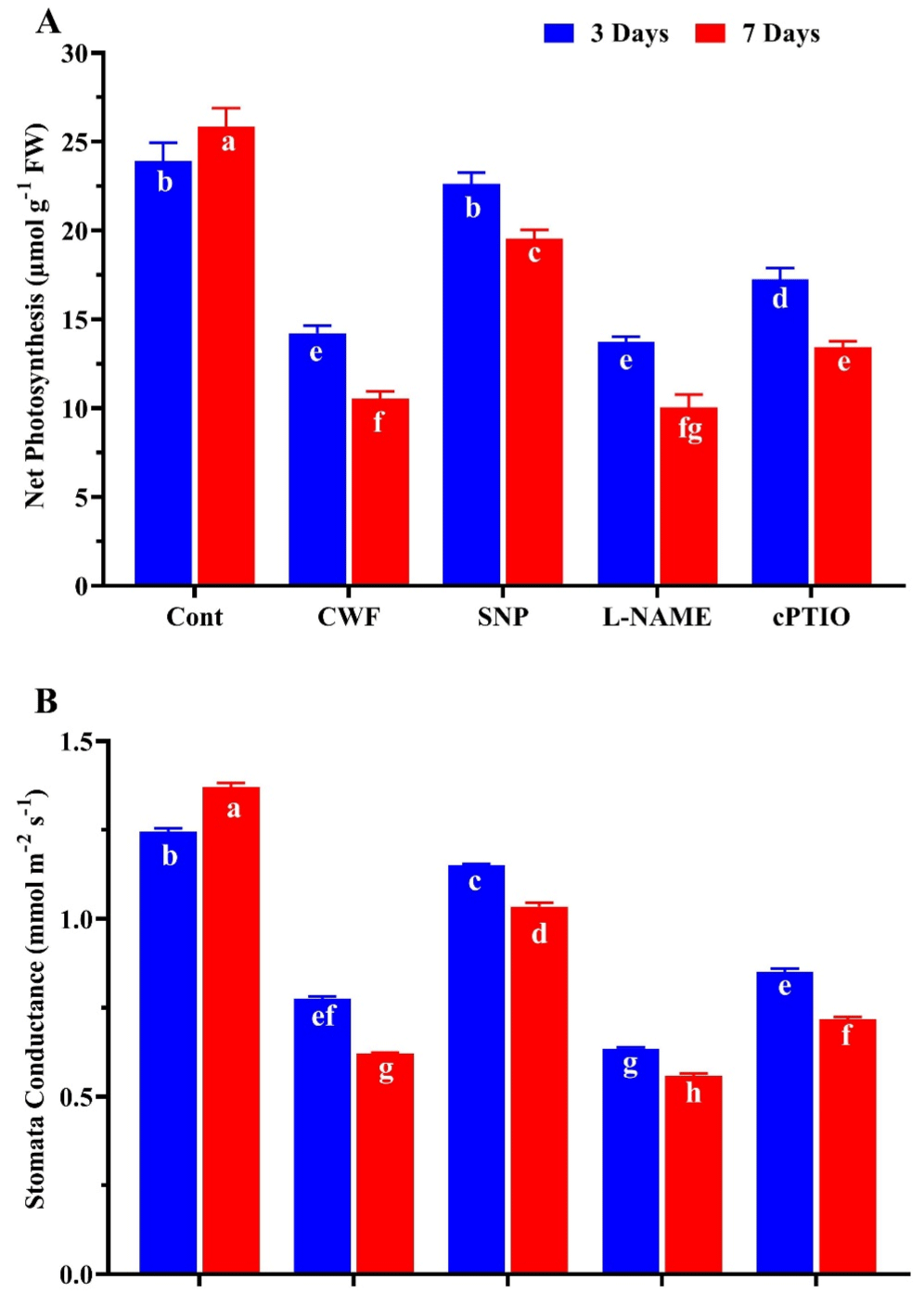
Figure 5: The effects of nitric oxide donor SNP on the (A) net photosynthesis
and (B) stomata conductance in soybean plants after 3 and 7 days of flooding
stress. Data represent the mean of three replicates, while the error bars
indicate the standard error. The differences between the mean values were
determined using Duncan’s multiple range test (DMRT) at P < 0.05. Different
letter(s) indicate values that are significantly different.
Endogenous Phytohormone ABA Content during Flooding Stress
We also evaluate the effects of NO application on ABA accumulation in soybean plants under flooding stress. The results show that after 3 and 7 of flooding stress ABA level was significantly increased by 61.8% and 92.3% compared to control plants. On the other hand plants treated with SNP significantly reduced the ABA content by (35.9%) after 3 DAS (Days After Stress) and (27.8%) after 7 DAS. The L-NAME and cPTIO treated plants up-regulated the ABA accumulation by inhibiting and scavenging the NO production compared to other treatments (Figure 6A). This was also confirm through expression level of ABA biosynthesis gene NCED3 and abscisic acid-responsive protein [32] gene. As shown in (Figure 6B) after 3 and 7 days of flooding stress a significant up-regulation was found in the relative expression of NCED3 by 29.4% and 32.6% compare to control plants (no stress), while the plants treated with NO donor SNP significantly reduced this up-regulation in NCED3 by 24.5% and 21.3% compared to CWF treated plants. Furthermore, there lative expression of the ABAR1 and ABAR2 was significantly upregulated in all treatments compared to the control. When comparing the controls, ABAR1 and ABAR2 expression were significantly increased by 62.1% and 72.4% after 3 and 68.4% and 76.3% after 7DAS, respectively, in CWF treated plants compared to control plants (without flooding stress). In contrast, ABAR1 and ABAR2 expression level significantly decreased in plants treated with SNP by 32.3% and 35.2% at 3 DAS and 34.6% and 29.2% 7DAS compared to the CWF, while the cPTIO and L-NAME treated plants significantly reverse the decrease in ABAR1 and ABAR2 relative expression cause by SNP treatment (Figure 6C-D).
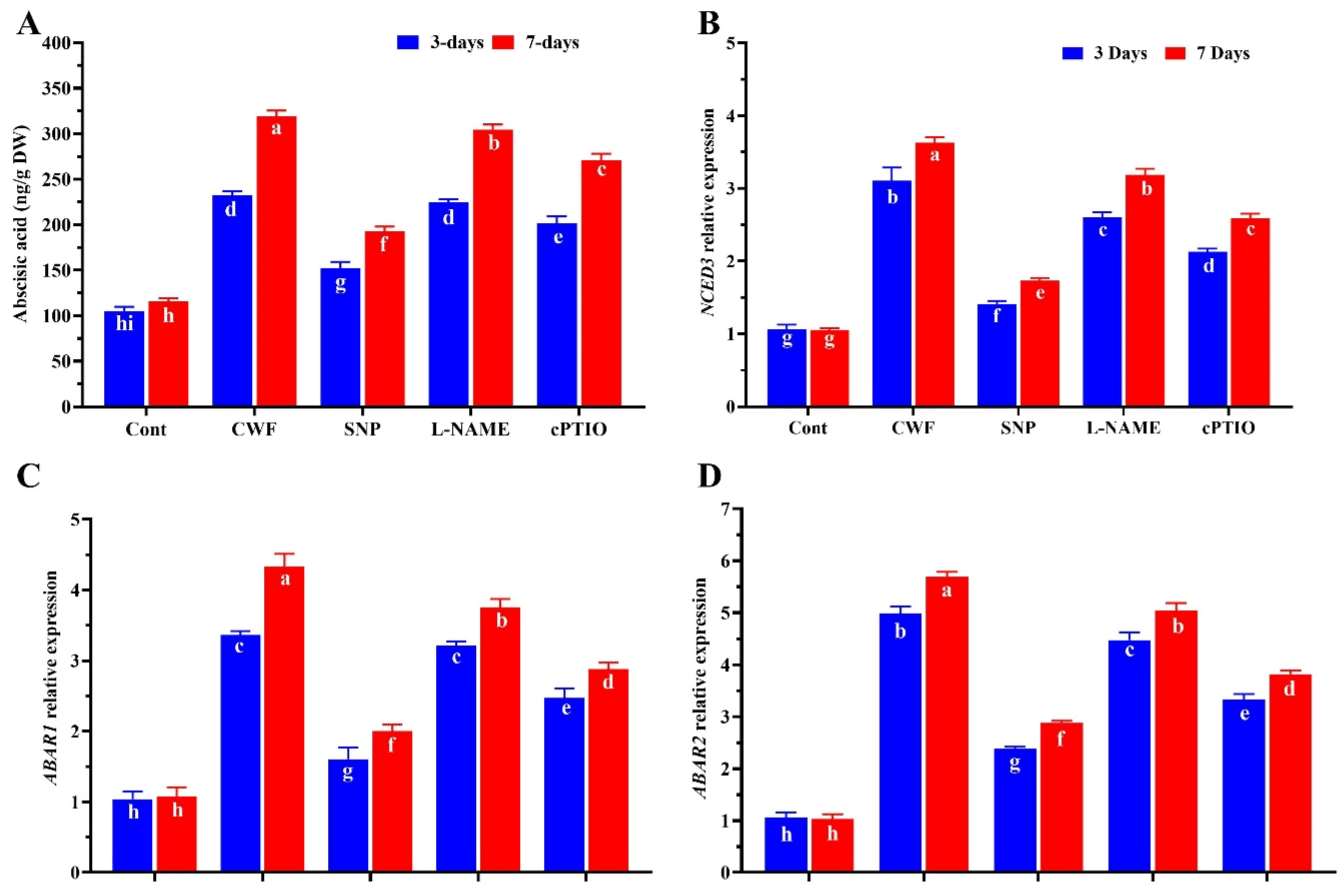
Figure 6: The effects of nitric oxide donor SNP on the (A) ABA accumulation, (B) ABA biosynthesis gene NCED3, and (C and D) ABAR1 and ABAR2 relative
expression in Glycine max (soybean) 3 and 7 days after flooding stress. Data represent the mean of three replicates, while the error bars indicate the standard
error. The differences between the mean values were determined using Duncan’s multiple range test (DMRT) at P < 0.05. Different letter(s) indicate values that
are significantly different.
Endogenous NO Level during Flooding Stress
Plants regulate endogenous NO production by controlling its biosynthesis and scavenging. To clarify the effects of NO in plants under environmental stress, exogenous application of SNP, cPTIO, and enzyme inhibitors showed that NO levels varied significantly in all treatments during the stress condition. When comparing the controls (non-flooded and flooded), the plants treated with flooding slightly improved NO levels by 14.4% after 3 days and was then significantly reduced by 19.7% after 7 days. However the endogenous NO level was significantly enhanced by 21.5% and 24.2% after 3 and 7 days in plants treated with SNP compared to CWF plants. On the other hand this increase in endogenous SNO was reversed by the L-NAME and cPTIO treatments (Figure 7A). It was further confirm through the relative expression of NR and GSNOR. The results show that gmNR and gmGSNOR relative expression is significantly enhanced in CWF treated plants at the first 3 days after flooding stress, where this up regulation was decreased at 7 days after stress, where the plants treated with SNP further enhance the gmNR and gmGSNOR expression by 19.1% and 27.5% after 3 days and 19.6% and 23.8% compared to the CWF after 3 and 7DAS. (Figure 7B-C). Where this expression in gmNR and gmGSNOR was significantly inhibited by L-NAME and cPTIO treatment. Through this catabolic process, GSNOR plays a vital role in the regulation of endogenous S-nitrosothiols, controlling the protein S-nitrosylation-based signaling. Thus, GSNO can serve as a stable NO pool, which can effectively transduce NO signaling [33].
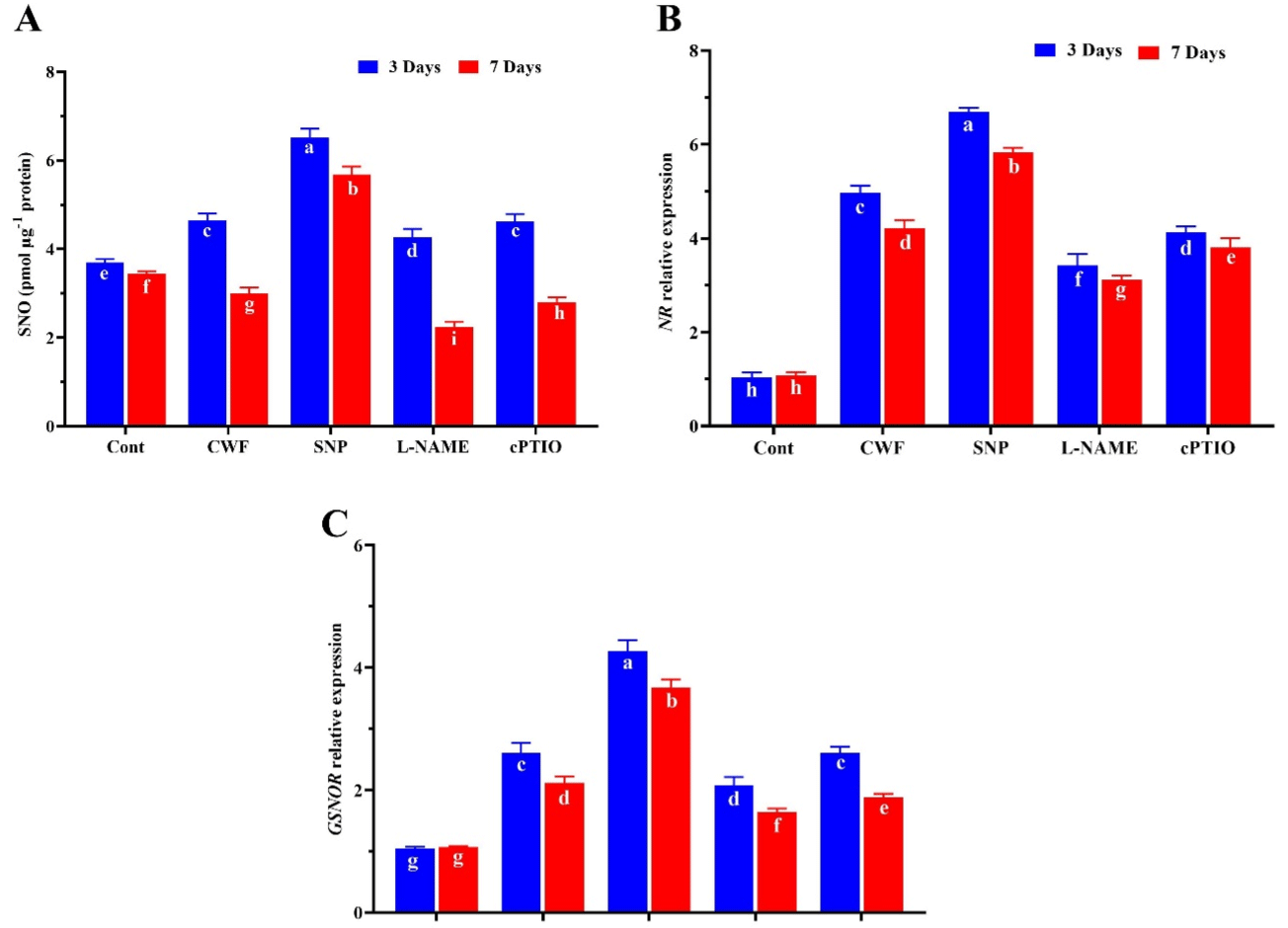
Figure 7: Effects of Nitric oxide application on (A) endogenous Cellular SNO levels and Transcription accumulation of genes involved in nitrogen metabolism and
expression levels of (B) GmNR and (C) Gm GSNOR in Soybean plants, 3 and 7 days after flooding stress. Data represent the mean of three replicates, while the
error bars represent standard error. Different letter(s) indicate significant differences between the mean values, determined using Duncan’s multiple range test
(DMRT) at P < 0.05.
Discussion
Studies have shown that SNP application can enhance photosynthesis and related enzyme activity during metal and flooding stress [34]. The results of this study also show that exogenous NO sources can significantly increase photosynthetic activity in plants 3 and 7, DA Sunder flooding stress and increasing shoot and root length, chlorophyll content, and biomass (Table 1). These increases were much more pronounced in SNP treatments compared to the other treatments. A similar conclusion was drawn by [35]; previously, it was shown that NO induces stomatal closure to help plants adapt to drought stress [36], which could also be true for plants that exhibited reduced photosynthetic rates after flooding. This may correlate with the plants defensive mechanism consists of antioxidant enzyme including SOD, POD, CAT, GSH, and APX [37]. In plants cell maintaining the ROS accumulation enable the proper redox biological reaction and regulate many processes that is essential for plant growth and development [38], however the excess amount of these ROS is also harmful for the steady growth of plants and cause cellular damage and increase the program cell death [39]. Similarly the current result also show that the flooding stress cause increase in H2O2 and MDA level, where the SNP treatment reverse these effect and reduce the over-generation of H2O2 and decrease the MDA level (Figure 2). Nitric oxide play vital role to maintain the ROS at steady level by increasing the antioxidant level under stress condition. Similarly [40] reported that the exogenous nitric oxide application enhance the plants growth and development and inhibit the overproduction of ROS, reduced the MDA content and increase the level of antioxidant enzyme activity in Arachishypogaea L. plants under aluminum stress. Furthermore proline is considered to stabilize the cellular homostasis and protect protein integrity and trigger gene expression [41]. Proline can also protect the ROS scavenging enzyme and activate the detoxification pathway, and increased the activity of GSH-ASC enzyme was reported in tobacco plants against the salt stress [42]. Similarly [43] reported the positive effect of NO treatment on the proline activity under cold stress in banana plants.
Endogenous ABA is involved in a signaling avalanche related to plant defense and stress modulation in many cropplants [44]. In addition, ABA tends to be reduced during flooding stress (contrary to drought stress) by stomatal closure [45] causing suberin deposition in cell walls and the formation of aerenchyma cells [46,47]. ABA levels might also correspond to the stomatal closure and photosynthetic activity in soybean plants. The results of this study show that SNP significantly reduced ABA accumulation in plants; however, ABA accumulation increased with flooding stress intensity. This was also correlated with the photosynthetic rate, as high and low photosynthetic activity was observed in soybeans, which in turn controlled stomatal closure [48]. Soybeans tend to control internal metabolism due to a lowered potential to counter the stress. ABA accumulation is also correlated with the transcript analysis of NCED3 and ABAR related genes during flooding stress. ABAR and TOC1 are responsible for the circadian expression during abiotic stress, where they express reciprocal regulation under stress conditions [49]. The current results show that exogenous application of NO down-regulates NCED3, ABAR1 and ABAR2 expression during the initial three days of flooding; however, there was as light down-regulation after 7DAS. In contrast, ABAR2 was significantly down-regulated in SNPtreated plants exposed to flooding stress 3 and 7, DAS (Figure 6). The evening-phased core clock component TOC1 binds to the promoter of ABAR and controls its circadian expression. TOC1 is induced by ABA, which advances the phase of TOC1binding, modulating ABAR circadian expression. This demonstrates that the reciprocal regulation between TOC1 and ABAR is important for sensitized ABA [49,50]. This suggests that exogenous nitric oxide regulates plant responses by influencing ABA, thereby phase-shifting transcriptional regulation by ABAR1, ABAR2, and TOC1 during long-term flooding stress [48]. Similarly [20,23] reported the ABA content down-regulation in its biosynthesis gene NCED3 by nitric oxide application while upregulation of it two catabolic gene CYP707A1 and CYP707A2 under drought stress in soybean plants.
Nitric oxide is a major plant signaling regulator in response to potential stress conditions [51,52]. A major pathway of NO bioactivity is through the addition of an NO molecule to a protein cysteine thiol, which forms S-nitrosothiol [17,53]. The current results show a significant increase in endogenous NO production in plants treated with an NO donor during the stress condition, which was completely reversed by the application of cPTIO and L-NAME (Figure 7). This suggests that exogenous NO sources can overcome flooding stressinduced NO production during the stress. Manipulating or regulating total cellular NO or SNO levels may lead to different outcomes, as shown by [53]. This also suggests that there is an effect of exogenous NO on root length; nevertheless, the endogenous NO levels significantly varied between roots and shoots during flooding. NO also plays a key role in root architecture by forming a complex with the signaling pathway of auxin [54].Increases in NO levels in the shoots of plants may indicate the transducing NO bioactivity in plants after long-term flooding stress. GSNOR predominantly regulates S-nitrosylation viatrans nitrosylation [55], which transfers the nitric oxide from GSNO to cysteine residue. In the current study, this effect was coupled with transcript accumulation and the reduction of GSNOR in response to flooding. In terms of NR, similarly reduced transcript accumulation was observed in soybean plants (Figure 7). In previous studies, Arabidopsis thaliana showed increased NO accumulation in NOX mutants [56]. This is similar to the ABA results, suggesting that NR determines the NO production in plants and is critical to ABAinduced stomatal closure [52,57,58]. The plants showed minimal gene expression of GSNOR and NR, suggesting its contribution toward the loss of potential function against prolonged flooding stress, a similar conclusion was drawn by [52] for mutant Arabidopsis.
Conclusion
The study concludes that exogenous NO application extends greater tolerance on plant growth and physiology to avert some of the adverse impacts of flooding stress. The current study reveals a wide array of modulation in the biochemical and transcriptional signaling programs that can be alternatively beneficial for plant growth during long-term flooding stress. The potential benefit of SNP or related NO sources during flooding can be attributed to its antagonistic effects on ABA biosynthesis, and enhance the antioxidant activities and scavenge the over generation of ROS, which further maintain the membrane integrity and help to regulated flooding tolerance (Figure 8). Although plenty of work has been done to understand the role of SNO during innate immunity in plants against Abiotic stress resistance, very little is known about the comprehensive regulations of SNO synthesis and transcript accumulation under flooding stress. Some of the important inter-junctions related to the role of NO in a wide array of plant stress signaling needs more attention.
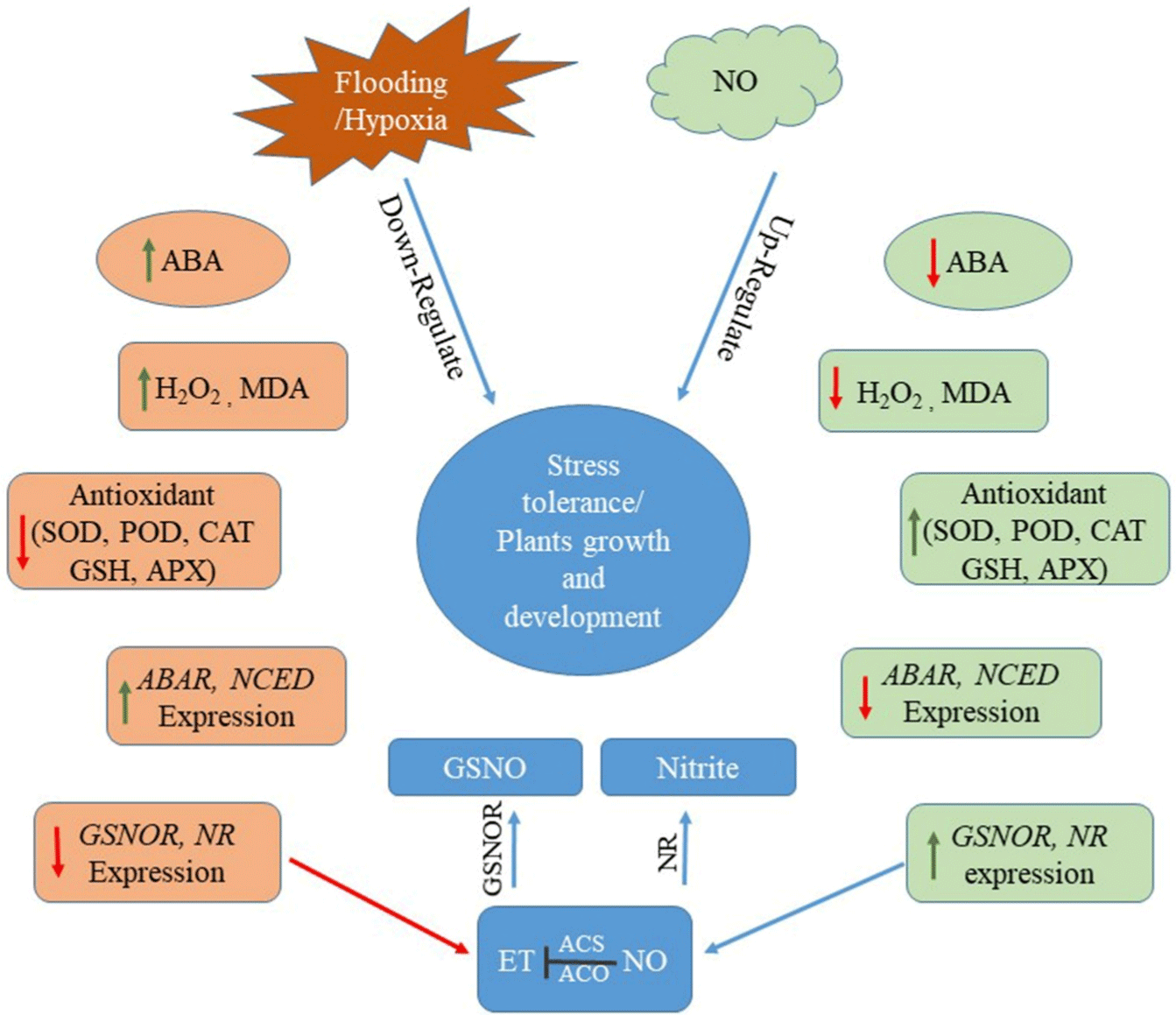
Figure 8: Schematic representation of nitric oxide (NO) interplay during flooding stress responses in soybean plants. (ABA) abscisic acid, (H2O2) hydrogen peroxide,
(NR) nitrate reductase, [1] Superoxide dismutase, (POD) peroxidase, (CAT) catalase, (GSH) Reduced glutathione, (ACS) 1-aminocyclopropane-1-carboxylic acid
synthase, (ACO) aminocyclopropane-1-carboxylic acid oxidase. The red arrow represents the down-regulation and green arrow represents the up-regulation.
Acknowledgment
We are thankful to Prof. Lee In-Jung for the all technical support, laboratory and his supervision.
Author Contribution
MI preform the experimental and original draft writing, ALK, RS, and YHK help in review and drafting manuscript, SB, SMK and MAK help in biochemical analysis, MK and BWY help in SNO quantification, IJL support funding and laboratory.
Funding
This research was supported by Basic Science Research Program the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1L1A1A01065443).
References
- Tunc-Ozdemir M, Miller G, Song L, Kim J, Sodek A, Koussevitzky S, et al. Thiamin Confers Enhanced Tolerance to Oxidative Stress in Arabidopsis1 [W][OA]. Plant Physiology. 2009; 151: 421-432.
- Anand A, Khetarpal S. Impact of climate change on agricultural productivity. Plant biology and biotechnology. 2015: 729-55.
- Ahmad P, Prasad MNV. Environmental adaptations and stress tolerance of plants in the era of climate change. Springer Science & Business Media; 2011.
- Mittler R, Blumwald E. Genetic engineering for modern agriculture: challenges and perspectives. Annual review of plant biology. 2010; 61: 443-462.
- Nanjo Y, Jang H, Kim H, Hiraga S, Woo S, Komatsu S. Analyses of flooding tolerance of soybean varieties at emergence and varietal differences in their proteomes. Phytochemistry. 2014; 106: 25-36.
- Valliyodan B, Ye H, Song L, Murphy M, Shannon JG, Nguyen HT. Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. Journal Of Experimental Botany. 2017; 68: erw433.
- Wu C, Chen P, Hummer W, Zeng A, Klepadlo M. Effect of flood stress on soybean seed germination in the field. American Journal of Plant Sciences. 2017; 8: 53.
- Burniston MT, Wilson DJ. Radioiodine Ablation Outcomes After Imaging with 123I or 131I: Is No News Good News?. Journal of Nuclear Medicine. 2008; 49: 166.1-166.
- Oz MT, Eyidogan F, Yucel M, Öktem HA. Functional role of nitric oxide under abiotic stress conditions.
- Environmental Science, Biology. 2015: 21-41.
- Arasimowicz M, Floryszak-Wieczorek J. Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant science. 2007; 172: 876-87.
- Vital SA, Fowler RW, Virgen A, Gossett DR, Banks SW, Rodriguez J. Opposing roles for superoxide and nitric oxide in the NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue. Environmental and Experimental Botany. 2008; 62: 60-8.
- Yu M, Lamattina L, Spoel SH, Loake GJ. Nitric oxide function in plant biology: a redox cue in deconvolution. The New phytologist. 2014; 202: 1142-1156.
- Sánchez J, Talamillo A, Lopitz-Otsoa F, Pérez C, Hjerpe R, Sutherland JD, et al. Sumoylation Modulates the Activity of Spalt-like Proteins during Wing Development in Drosophila. The Journal of Biological Chemistry. 2010; 285: 25841-25849.
- Yadav A, Mathan J, Bhati KK, Singh A. Nitric oxide signaling and abiotic stress tolerance in plants. Nitric Oxide in Plant Biology: Elsevier; 2022: 373- 90.
- Verma N, Tiwari S, Singh VP, Prasad SM. Nitric oxide in plants: an ancient molecule with new tasks. Plant Growth Regulation. 2020; 90: 1-13.
- Fan HF, Du CX, Ding L, Xu YL. Exogenous nitric oxide promotes waterlogging tolerance as related to the activities of antioxidant enzymes in cucumber seedlings. Russian journal of plant physiology. 2014; 61: 366-73.
- Malik SI, Hussain A, Yun B, Spoel SH, Loake GJ. GSNOR-mediated denitrosylation in the plant defence response. Plant science : an international journal of experimental plant biology. 2011; 181: 540-544.
- Filippou P, Tanou G, Molassiotis A, Fotopoulos V. Plant acclimation to environmental stress using priming agents. Plant acclimation to environmental stress: Springer. 2013: 1-27.
- Imran M, Khan AL, Shahzad R, Khan MA, Bilal S, Khan A, et al. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants. 2021; 13.
- Imran M, Shazad R, Bilal S, Imran QM, Khan M, Kang S-M, et al. Exogenous Melatonin mediates the regulation of endogenous nitric oxide in Glycine max L. to reduce effects of drought stress. Environmental and Experimental Botany. 2021; 188: 104511.
- Halo BA, Khan AL, Waqas M, Al-Harrasi A, Hussain J, Ali L, et al. Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. Journal of Plant Interactions. 2015; 10: 117-25.
- Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant physiology. 1977; 59: 309-314.
- Imran M, Khan MA, Shahzad R, Bilal S, Khan M, Yun B, et al. Melatonin Ameliorates Thermotolerance in Soybean Seedling through Balancing Redox Homeostasis and Modulating Antioxidant Defense, Phytohormones and Polyamines Biosynthesis. Molecules. 2021; 26: 5116.
- Zhang J, Kirkham MB. Drought-Stress-Induced Changes in Activities of Superoxide Dismutase, Catalase, and Peroxidase in Wheat Species. Plant and Cell Physiology. 1994; 35: 785-91.
- Ellman M. A spectrophotometric method for determination of reduced glutathione in tissues. Anal Biochem. 1959; 74: 214-26.
- Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants Protective role of exogenous polyamines. Plant Science. 2000; 151: 59-66.
- Mutava RN, Prince SJK, Syed NH, Song L, Valliyodan B, Chen W, et al. Understanding abiotic stress tolerance mechanisms in soybean: a comparative evaluation of soybean response to drought and flooding stress. Plant physiology and biochemistry: PPB. 2015; 86: 109-120.
- Qi, Rose, Abrams, Taylor, Cutler. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis in brassica napus embryos. Plant physiology. 1998; 117: 979-987.
- Chan C-X, Teo S-S, Ho C-L, Othman RY, Phang S-M. Optimisation of RNA extraction from Gracilaria changii (Gracilariales, Rhodophyta). Journal of Applied Phycology. 2004; 16: 297-301.
- Cawley S, Bekiranov S, Ng HH, Kapranov P, Sekinger EA, Kampa D, et al. Unbiased Mapping of Transcription Factor Binding Sites along Human Chromosomes 21 and 22 Points to Widespread Regulation of Noncoding RNAs. Cell. 2004; 116: 499-509.
- Khan AL, Numan M, Bilal S, Asaf S, Crafword K, Imran M, et al. Mangrove’s rhizospheric engineering with bacterial inoculation improve degradation of diesel contamination. Journal of Hazardous Materials. 2022; 423: 127046.
- Singh PK, Indoliya Y, Chauhan AS, Singh SP, Singh AP, Dwivedi S, et al. Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Scientific Reports. 2017; 7.
- Lima B, Forrester MT, Hess DT, Stamler JS. S-Nitrosylation in Cardiovascular Signaling. Circulation Research. 2010; 106: 633-646.
- Shao H, Chu L, Jaleel CA, Zhao C. Water-deficit stress-induced anatomical changes in higher plants. Comptes rendus biologies. 2008; 331: 215-225.
- Wang T, Yang W, Xie Y, Shi D, Ma Y, Sun X. Effects of exogenous nitric oxide on the photosynthetic characteristics of bamboo (Indocalamus barbatus McClure) seedlings under acid rain stress. Plant Growth Regulation. 2017; 82: 69-78.
- Zhang L, Zhao L. Production of nitric oxide under ultraviolet-B irradiation is mediated by hydrogen peroxide through activation of nitric oxide synthase. Journal of Plant Biology. 2008; 51: 395-400.
- Kaur N, Kaur J, Grewal SK, Singh I. Effect of Heat Stress on Antioxidative defense system and its amelioration by heat acclimation and salicylic acid pre-treatments in three pigeonpea genotypes. Indian Journal of Agricultural Biochemistry. 2019; 32: 106-10.
- Mittler R. ROS Are Good. Trends in plant science. 2017; 22: 11-19.
- Raja V, Majeed U, Kang H, Andrabi KI, John R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environmental and Experimental Botany. 2017; 137: 142-57.
- He H, Oo TL, Huang W, He L, Gu M. Nitric oxide acts as an antioxidant and inhibits programmed cell death induced by aluminum in the root tips of peanut (Arachis hypogaea L.). Scientific Reports. 2019; 9.
- Souri Z, Karimi N, Farooq MA, da Silva Lobato AK. Improved physiological defense responses by application of sodium nitroprusside in Isatis cappadocica Desv. under cadmium stress. Physiologia Plantarum. 2021; 173: 100-15.
- Hoque MA, Banu MNA, Okuma E, Amako K, Nakamura Y, Shimoishi Y, et al. Exogenous proline and glycinebetaine increase NaCl-induced ascorbateglutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. Journal of plant physiology. 2007; 164: 1457-1468.
- Wang Y, Luo Z, Du R, Liu Y, Ying T, Mao L. Effect of nitric oxide on antioxidative response and proline metabolism in banana during cold storage. Journal of agricultural and food chemistry. 2013; 61: 8880-8887.
- Yin X, Hiraga S, Hajika M, Nishimura M, Komatsu S. Transcriptomic analysis reveals the flooding tolerant mechanism in flooding tolerant line and abscisic acid treated soybean. Plant Molecular Biology. 2016; 93: 479-496.
- Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, Matsui M, et al. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proceedings of the National Academy of Sciences. 2012; 109: 9653-9658.
- Kim Y, Hwang S, Waqas M, Khan AL, Lee J, Lee J, et al. Comparative analysis of endogenous hormones level in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Frontiers in Plant Science. 2015; 6.
- Shimamura S, Yoshioka T, Yamamoto R, Hiraga S, Nakamura T, Shimada S, et al. Role of abscisic acid in flood-induced secondary aerenchyma formation in soybean (Glycine max) hypocotyls. Plant Production Science. 2014; 17: 131-7.
- Arbona V, Zandalinas SI, Manzi M, González-Guzmán M, Rodriguez PL, Gómez-Cadenas A. Depletion of abscisic acid levels in roots of flooded Carrizo citrange (Poncirus trifoliata L. Raf. × Citrus sinensis L. Osb.) plants is a stress-specific response associated to the differential expression of PYR/ PYL/RCAR receptors. Plant Molecular Biology. 2017; 93: 623-640.
- Syed NH, Prince SJ, Mutava RN, Patil G, Li S, Chen W, et al. Core clock, SUB1, and ABAR genes mediate flooding and drought responses via alternative splicing in soybean. Journal of experimental botany. 2015; 66: 7129-7149.
- Legnaioli T, Cuevas J, Mas P. TOC1 functions as a molecular switch connecting the circadian clock with plant responses to drought. The EMBO Journal. 2009; 28: 3745-3757.
- Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2003; 218: 900-905.
- Yun B, Skelly MJ, Yin M, Yu M, Mun B, Lee S, et al. Nitric oxide and S-nitrosoglutathione function additively during plant immunity. The New phytologist. 2016; 211: 516-526.
- Feechan A, Kwon E, Yun B, Wang Y, Pallas JA, Loake GJ. A central role for S-nitrosothiols in plant disease resistance. Proceedings of the National Academy of Sciences of the United States of America. 2005; 102: 8054-8059.
- Sun H, Feng F, Liu J, Zhao Q. The Interaction between Auxin and Nitric Oxide Regulates Root Growth in Response to Iron Deficiency in Rice. Frontiers in Plant Science. 2017; 8.
- Foster MW. Methodologies for the characterization, identification and quantification of S-nitrosylated proteins. Biochimica et biophysica acta. 2012; 1820: 675-683.
- Dall’Osto L, Piques M, Ronzani M, Molesini B, Alboresi A, Cazzaniga S, et al. The Arabidopsis nox Mutant Lacking Carotene Hydroxylase Activity Reveals a Critical Role for Xanthophylls in Photosystem I Biogenesis[C][W]. Plant Cell. 2013; 25: 591-608.
- Kwon E, Feechan A, Yun B, Hwang B, Pallas JA, Kang J, et al. AtGSNOR1 function is required for multiple developmental programs in Arabidopsis. Planta. 2012; 236: 887-900.
- Zhao C, Cai S, Wang Y, Chen Z. Loss of nitrate reductases NIA1 and NIA2 impairs stomatal closure by altering genes of core ABA signaling components in Arabidopsis. Plant Signaling & Behavior. 2016; 11: e1183088.