
Special Article: Fruit Science and Viticulture
Ann Agric Crop Sci. 2023; 8(3): 1137.
Transcriptome Comparison of Strawberry Ripen Fruit Harvested/Collected from Plants Grown under Three Different Temperature Treatments
Bao YX²; Jia YD²; Liu XJ²; Zhu YR²; Tong YN¹*; Wang Y²*; Liu LL¹; Yang LF¹; Zuo ZJ³
1Tianjin Academy of Agricultural Sciences, Tianjin 300192, China
2Nankai university, Tianjin 300350, China
3Zhejiang Provincial Key Laboratory of Forest Aromatic Plants-based Healthcare Functions, Zhejiang A&F University, Hangzhou 311300, China
*Corresponding author: Tong YN Tianjin Academy of Agricultural Sciences, No.268 Baidi Road, Nankai District, Tianjin 300192, China. Tel: +86 13802002722; Fax: +86022-82939032 Wang Y, Nankai University, No.94 Weijin Road, Nankai District, Tianjin 300350, China. Tel: +86 13821811751 Email: tongyana03@163.com; wangyong@nankai.edu.cn
Received: June 13, 2023 Accepted: July 13, 2023 Published: July 20, 2023
Abstract
In this study, the runner plants of strawberry cv. Ssanta was used as the material, and three different daily temperature combinations were designed, which were CK (22°C/22°C), DHT (day high temperature, 30°C/22°C) and NLT (night low temperature, 22°C/8°C), respectively. The results showed that DHT accelerated fruit ripening process, increased the single fruit weight and the horizontal and vertical diameter, increased the sucrose, SSC content of the fruit, reduced the TA content, and improved the fruit quality. The results of qRT-PCR showed that after DHT, the expression of sucrose metabolism related genes (Fa SPS, Fa SUT2 and Fa SUT3) was significantly increased, but the expression of citrate synthesis related genes (Fa PDHE, Fa PEPC, Fa PK, Fa CS) was not affected, while NLT treatment showed the opposite trend. In order to further understand the effect of temperature on gene expression in strawberry fruit, transcriptome sequencing and differential expression analysis were carried out. The results showed that temperature treatment had an effect on multiple metabolic pathways in the fruit, including TCA cycle, starch and sucrose metabolism, fatty acid metabolism, amino acid biosynthesis, etc.
Keywords: Cultivate strawberry; Temperature control; Sugar acid metabolism; Transcriptome analysis
Abbreviations: CK: Suitable for Plant Growth; DHT: Day High Temperature; NLT: Night Low Temperature; SSC: Soluble Solids Content; TA: Titratable Acidity; DEGs: Differentially Expressed Genes; CS: Citrate Synthase; PEPC: Phosphoenolpyruvate Carboxylase; Aco: Acetyl-CoA; PDHE: Pyruvate Dehydrogenase
Introduction
Strawberry (Fragaria × ananassa Duch.) is cultivated and consumed worldwide. Most commercially important cultivars of strawberry are octaploids (2n=8x=56) [1] and there are two flowering types: one is an annual Short Day and Low Temperature-type (SDLT) (seasonal strawberry), and the other is the perpetual (everbearing) day-neutral or Long Day and High Temperature-type (LDHT) [2]. The SDLT type is also known as single-cropping or June-bearing type [3]. Because of a pronounced interaction of photoperiod and temperature, floral initiation also takes place in most SDLT cultivars under long day conditions if the temperature is low. Therefore, temperature is considered to be one of the most important factors that influence the strawberry production and fruit quality [4,5,22].
For quality fruit production, strawberry requires cool and moist climate [6] with 25/12° C (day/night) optimum temperature [7]. However, it is also reported that a DIF (difference between daytime temperature and night temperature) of 12°C (31°C/19°C, day/night temperature) was most suitable for the growth, especially for the sugar content accumulation of strawberry cultivar Hong Yan [22]. The importance of DIF has been emphasized also for other fruit production [8,9].
In the present study, we grew strawberry plants after blooming at continuous 22°C (without a DIF) as the reference (CK), and compared with those grown at a DIF of 8°C (30°C/22°C, day/night temperature, as DHT treatment) and with those grown at a DIF of 14°C (22°C/8°C, day/night temperature, as NLT treatment). After measurement of the fruit phenotype, sugar/acid ratio and sucrose content, we detected the expression of genes related to sucrose and titrate metabolism, and then we performed a transcriptome analysis and comparison, in order to explore genes involved in the influence of strawberry fruit quality, which could contribute to the further elucidation of mechanisms under which the fruit quality control by hinger or lower temperature or by temperature difference between daytime and night.
Materials and Methods
Plant Growth and Temperature Treatments
Runner plants of strawberry cv.Ssanta were grown in plastic pots (1.65 L) filled with commercial potting media (Peat: vermiculite: perlite 1:1:1) and kept under ambient environmental conditions in a glasshouse until the appearance of the initial flowers. Irrigation and supplement of liquid fertilizer were regularly carried out during the growing period.
90 healthy plants with uniform size were taken and divided into three groups for the temperature treatments in the condition-controlled growth chambers, which have a photoperiod of 16 hours dark and 8 hours light. Three different daily temperature combinations were designed as CK (22°C/22°C), which was suitable for plant growth, DHT (day high temperature, 30°C/22°C) and NLT (night low temperature, 22°C/8°C), respectively. The plants were irrigated and fertilized regularly to maintain their healthy growth.
The temperature treatments were continued for around 8 weeks. Ripen fruits were harvested on the 30th day of the experiment and every 5 days afterwards and stored at 4°C or at -80°C after deep frizzed in liquid N2.
The 45d harvested fruits were then analyzed for their physical properties, fruit quality parameters and extraction of RNA for RT-PCR essay and transcriptome analysis.
Each laboratory analytical test (described below) was performed with at least three biologic repeats.
Fruit Physical Property and Quality Parameter Determination
The weight of each sample was measured by using an electronic balance with an accuracy of 0.01g. The size of the fruits was measured with the Vernier Caliper. After the fruit was picked, ground into juice, centrifuged, and 300μL supernatant was taken for determination. The SSC content of each fruit was measured three times and the average value was the SSC content of the fruit. The original juice was diluted 50 times, and 300μL was taken for determination. The average value was TA content after repeated determination for three times. Soluble Solids Content (SSC) and content of Titratable Acidity (TA) were determined by using a refractometer (ATAGO, Ai Tan, Japan, PAL-1).
Measurement of Sucrose
Sucrose content of the fresh ripen strawberry fruits was determined by using the testing kit from Plant Sucrose Content Assay Kit (Solarbio, BC2465). The process was performed according to the user´s instruction.
RNA Extraction and Purification
Total RNA was extracted by using the Rapid RNA Extracting Kit from. Fresh harvested ripen fruits were sliced and submerged in liquid N2, then crashed into powder and extracted in the lysing solution containing protease K. Contaminated DNA was removed by passing a specific little column and purified RNA was deposited in alcohol and finally dissolved in RNase-free water. RNA samples were used directly or stored in the fridge, after quality and quantity check.
Real time RT-PCR
Purified total RNA was used to conduct the quantitative analysis of gene expression by using TB Green Premix Ex Taq, with actin gene as the reference. The primer pares designed by using Primer 5 and used in the present study are listed in Table 1.
Primer
Primer Sequences (5'-3')
actin
actin-F
GGTATTGTGAGCAACTGGGATG
actin-R
CAGGGAAAGAACAGCTTGAATG
SPS
qSPS-F
GCTCGGTCGTGACTCTGATACTGG
qSPS-R
TCCTCGAAACCTTCTGCACTTAA
SS
qSS-F
CAGCCTCATCAGATAATCAACGA
qSS-R
ACGGACATATTCCCATACACCAG
InvA
qInvA-F
GGCTTACGGAAAGATAACTGGTG
qInvA-R
TCATTTACGGCAAGCATCTCACG
InvB
qInvB-F
CCTCATTCTCAGCCTCTGTCTATC
qInvB-R
AAATTCCTTACCCACATCGTCTT
InvD
qInvD-F
GGGAAGAGTTGGTCGGAGAAATG
qInvD-R
AGCCGTTAGCATCCATAATAGCA
InvE
qInvE-F
CATTCTTCATACCCTTCAGTTGC
qInvE-R
AGATAATCCACCATAACCCAGAG
FaPK
qFaPK-F
CTGAGGATGTCAAGCCTGGGAGT
qFaPK-R
GGGTTGGGAGATCCACAACGACT
PDHE
qPDHE-F
CTGGCTTGAAGGTAGATGGTATGG
qPDHE-R
AATTGGATCACGCTCCTGTCTCA
PEPC
qPEPC-F
GGCTGCTAACTTGTATTTCTCCC
qPEPC-R
CCAAGAATAACACGATATGGCTC
CS
qCS-F
ATGATGTTGTGCCTCCCATACTGA
qCS- R
ACTGTGAGCCAATCCCGATACTC
ACO
qACO-F
GTGTTGCCTGGAGTTGTTGGATT
qACO-R
TAGTTGCCCTGTCAGCTAAAGAT
SUT1
qSUT1-F
CATCGACATGCCTCTCAC
qSUT1-R
GGTCCACTTACTAGAGAAAC
SUT2
qSUT2-F
CTTCGTTTATCTGGCTTTGTGGC
qSUT2-R
TGAAAGTTCTGCAATGCTCGTGT
SUT3
qSUT3-F
TCACCATCCTCGCACTCACAT
qSU3-R
TTCCCATCCAGTCAGTGTCGAACAG
SUT4
qSUT4-F
CTACTACAGCGACCGTAACACCTCT
qSUT4-R
CCCTACAACAAATACAGCCACAGC
SUT5
qSUT5-F
ATTGCTCTGCTTGCGGTACTCAC
qSUT5-R
TCCACATCGGCTTCTTCAACTCC
SUT6
qSUT6-F
CGGTTATGCTGCTGGATCGTTG
qSUT6-R
CGTTCCTCCTGCTGCTTCTGTT
SUT7
qSUT7-F
GAGTTGAAGAAGCCGATGTGGAT
qSUT7-R
GATGGAGTTTAGCATGAGCCCTAG
Table 1: Primer pares used for qRT-PCR analysis of genes in the study.
cDNA was synthesized using the Prime Script RT reagent kit from Takara, following the User´s instructions. PCR conditions were preheating at 95°C 30°C for 30s followed by 39 cycles of 95°C for 10s, 58°C for 30s, and 68°C for 15s. The relative expression was then calculated based on the expression level of the actin gene.
RNA-seq Analysis
RNA-seq analysis was performed using the same RNA samples as used for RT-PCR measurement and each treatment had three biological replicates. 9 cDNA libraries were constructed for transcriptome sequencing on the BGISEQ-platform by the Huada Gene (Wuhan, China). Raw reads were processed for quality using the SOAP nuke software (BGI, Guangdong, China). Clean reads were obtained by removing lowquality reads containing joint contamination or >5% unknown nucleotides. The clean reads from each RNA-seq library were aligned using HISAT (Hierarchical Indexing for Spliced Alignment of Transcripts) (Kim et al., 2015) to the Fragaria_vesca_subsp_vesca genome, GCF_000184155.1_FraVesHawaii_1.0 (NCBI). The cleaned reads were compared to the reference sequence using Bowtie2 (Langmead and Salzberg, 2012). The gene and transcript expression levels were calculated using the RSEM method (Li and Dewey, 2011). The differentially expressed genes (DEGs) were determined using six samples (3 replicates, 2 treatments) using the DEGseq method [19]
The unigene sequences were BLAST-aligned to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. The DEGs between the treatment pare were functionally classified into GO terms using the Phyper function in R software. The calculated P-values were FDR-corrected to generate Q-values. Go terms with FDR =0.05 were considered significantly enriched.
Results
Phenotype Comparison of Strawberry Fruits Ripened Under Different Temperature Conditions
Vigorously growing strawberry plants with initial flowers were grown in pots placed in the growth chamber as described in “Materials and methods” and three different temperature treatments were carried out for comparison, which are indicated as DHT (Day High Temperature), NLT (Night Low Temperature) and CK (temperature suitable for growth as control).
Compared with CK, no big difference was observed in DHT group for the number of ripen fruit within a period of 45 days, but NLT treatment postponed the mature of strawberry fruits (Supplementary data 1). The average single fruit weight, fruit transverse and longitudinal diameter of the three groups were similar before 40 days. At 40 days and 45 days, the DHT group was significantly higher than CK (Figure 1). The fruit shape index did not change significantly (Figure 1). The average yield of a plant in DHT was significantly higher and in NLT was significantly lower than in CK group (Figure 2). Measurement of indicators of the fruit quality, such as Soluble Solids (SSC), Titratable Acid (TA), Solid acid ratio and content of sucrose all showed that DHT promoted and NLT lowered the fruit quality compared to the CK (Figure 3).
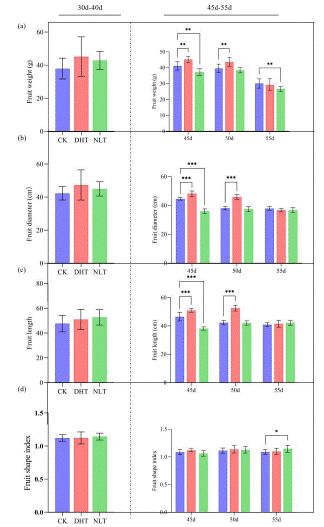
Figure 1: Changes of single fruit weight, transverse and longitudinal diameter and fruit shape index of strawberry fruit after three groups of temperature treatment. (a) After three groups of temperature treatment, strawberry fruits were collected at 30, 35, 40, 45, 50 and 55 days after treatment, and the single fruit weight was measured. (b) After three groups of temperature treatment, the transverse diameter of all mature strawberry fruits was measured; (c) After three groups of temperature treatment, the longitudinal diameter of all mature strawberry fruits was measured; (d) After three sets of temperature treatment, the fruit shape index of all mature strawberry fruits was measured. The mean ± SD was calculated by three independent experiments and three biological replicates. ANOVA test (*P<0.05, **P<0.01, P***<0.001) was used to evaluate the significant difference.
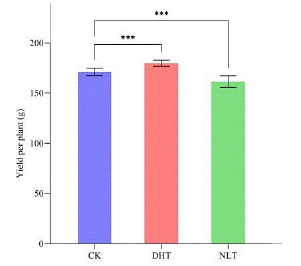
Figure 2: After three groups of temperature treatment, the average yield of single plant was counted at 55 d, and 30 strawberries were counted in each group. The mean ± SD was calculated by 3 independent experiments and 10 biological replicates. ANOVA test (*P<0.05, **P<0.01, P***<0.001) was used to evaluate the significant difference.
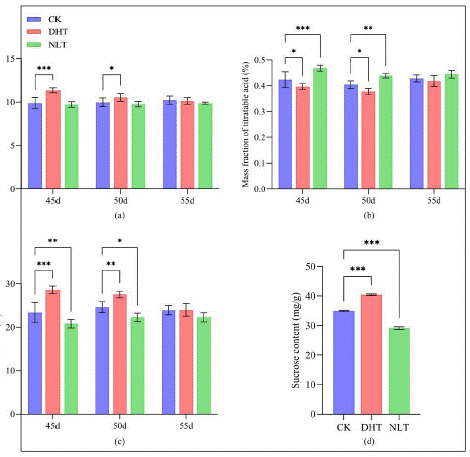
Figure 3: Changes of strawberry fruit quality after three groups of temperature treatment. (a) After three groups of temperature treatment, the strawberry fruit was collected at 40,45,50 and 55 days after treatment ( no significant difference within 30-40 days, regardless of analysis ), and the soluble solids content of the fruit was measured; (b) After three groups of temperature treatment, fruit strawberries were collected on days 40,45,50 and 55d to measure the titratable acid content of the fruit; (c) Strawberries were collected at 40,45,50 and 55 days after three groups of temperature treatment, and the fruit solid-acid ratio (solid-acid ratio = soluble solids content (%) / titratable acid content (%) ) was measured. (d) After three groups of temperature treatment, the sucrose content of the fruit was measured at 55d, and 30 strawberries were counted in each group. The mean ± SD was calculated by three independent experiments and three biological replicates. ANOVA test (*P<0.05, **P<0.01, P***<0.001) was used to evaluate the significant difference.
RT-PCR Detection of the Expression of Genes Involved In Sucrose and Citric Acid Metabolism
After 45-days of experimental treatment, total RNA was extracted and used for measurement of transcript levels by RT-PCR. Strawberry sucrose phosphate synthesis and sucrose synthase are two key enzymes for sucrose biosynthesis; therefore, their encoding genes Fa SPS and Fa SS were chosen for this experiment. Invertases are responsible for sucrose conversion and sucrose degradation pathway genes include acid / neutral invertase gene family (Fa InvA- FaInv F). The results showed that the expression levels of Fa InvB and Fa InvF genes in mature fruits were low or not expressed. The expression levels of 3 encoding genes, Fa InvA, Fa InvD and Fa Inv E were significantly higher than those of the control. Besides, the expression of 4 genes encoding sucrose transport (Fa SUT) were also investigated.
As shown in Figure 4, the expression level of Fa SPS in ripen fruits from plants under DHT treatment was extremely significantly higher than, and that under NLT treatment significantly lower than that under the CK condition (Figure 4a). However, higher expression of Fa SS was detected in samples from CK treatment than that from both of the other treatments (Figure 4b). All the 3 genes encoding invertase had significantly higher expression in samples from NLT treatment than that from the other two treatments (Figure 4c, d and e). The expression level of both Fa SPS and Fa SS in leaves was higher under DHT than other two conditions (Supplementary data 2).
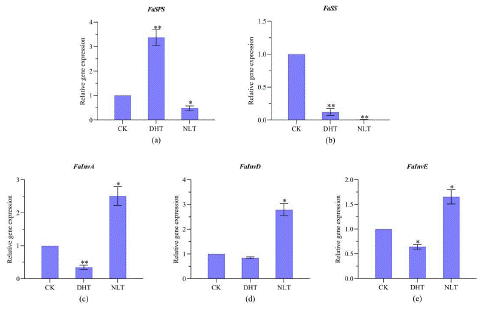
Figure 4: Transcript levels of genes involved in sucrose metabolism in strawberry ripen fruits from plants grown under different temperature treatment. The expression of genes was analyzed by RT-PCR. Abbreviations: FaSPS, FaSS, FaInvA, FaInvD, FaInv E, DHT, NTL and CK. * and ** indicates, respectively, significant difference from CK at P<0.05 and P<0.01 level. Each point represents mean ± SE of 3 biological repeats. Based on one-way ANOVE analysis.
In strawberry, the sucrose transporter gene families have seven members (SUT1-SUT7). By RT-PCR, the expression of Fa SUT1, Fa SUT5 and Fa SUT6 in the ripen fruits of our experiments was almost undetectable. The results of the other SUT genes are shown in Figure 5.
Similar expression pattern of FaSUT2 and FaSUT3 was exhibited (Figure 5a, 6b). The expression of these genes was found significant higher in samples of ripen fruits from plants under DHT than under CK treatment. In contrary, the expression level was lowest in samples of NLT. Either higher day temperature or lower night temperature treatment, both caused the dramatic lowering of the expression of Fa SUT4 and Fa SUT7 in ripen fruits (Figure 5c, 6d).
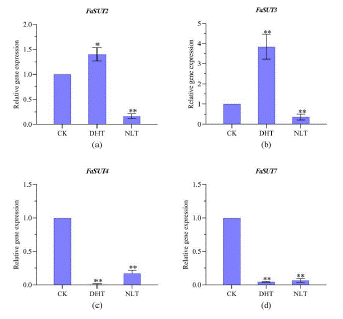
Figure 5: Transcript levels of genes involved in sucrose metabolism in strawberry ripen fruits from plants grown under different temperature treatment. The expression of genes was analyzed by RT-PCR. Abbreviations: Fa SUT2, Fa SUT3, Fa SUT4, FaSUT7, DHT, NTL and CK. * and ** indicates, respectively, significant difference from CK at P 0.05 and P 0.01 level. Each point represents mean ± SE of 3 biological repeats. Based on one-way ANOVE analysis.
The expression levels of Fa SUTs in leaves are shown in Supplementary data 3. In order to detect the expression of FaSUT1 and FaSUT6 genes in leaves, it may be that the gene expression level is low and cell-specific or the gene is not expressed; daytime high temperature treatment significantly increased the expression of Fa SUT3-5 gene and Fa SUT7 gene in leaves, reaching 8.0 times, 10.3 times, 6.7 times and 12 times of the control group, respectively, and had no significant effect on the expression of Fa SUT2 gene. Night low temperature treatment had no significant effect on the expression of Fa SUT2-7 gene, but there was a slight decrease. The results showed that high temperature treatment could significantly increase the expression of sucrose transporter genes and promote the transport of sucrose synthesized by photosynthesis in source leaves to storage and use in sink organs, and promote the accumulation of sucrose in fruits as sink organs. The expression of sucrose transporter genes in the low temperature treatment group was reduced. The simultaneous reduction of multiple genes may lead to the obstruction of the sucrose transport process from leaves to fruits, resulting in a decrease in the sucrose content transported to the fruits, which in turn leads to a decrease in the sucrose content accumulated in the fruits.
The acid type of strawberry fruits belongs to citric acid. The accumulation of citrate during carbohydrate metabolism in the fruit leads to the sauer taste and low commercial value of the fruits. In one hand, in the cell citrate is synthesized from OAA and Acetyl-CoA by the enzyme activity of Citrate Synthase (CS) and the PEP produced through glycolysis pathway is the source for both OAA which is generated through the activity of Phosphoenolpyruvate Carboxylase (PEPC) and Acetyl-CoA, which is generated consequently through the activity of PK (pyruvate kinase) and Pyruvate Dehydrogenase (PDHE), respectively. And on the other hand, citrate is converted to isocitrate by Acetyl-CoA (Aco), which lowers the level of citrate in the cell. All these enzymes are responsible for the accumulation of citrate in the fruit. Therefore, we measured the transcript level of the genes coding for these enzymes involved in citrate biosynthesis and conversion in ripen strawberry fruits collected from plants grown under different temperature treatments (Figure 6). The results show that DHT treatment promoted the expression of Fa ACO in comparation with the other two temperature treatments. NLT treatment, on the other hand, promoted the expression of Fa PK, Fa PEPC, Fa PDHE and Fa CS in comparation with the other two temperature treatments.
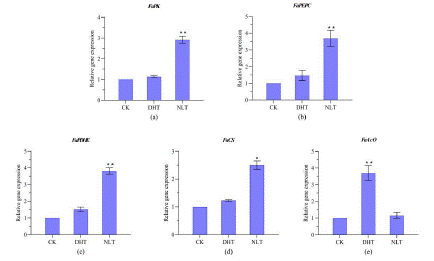
Figure 6: Transcript levels of genes involved in sucrose metabolism in strawberry ripen fruits from plants grown under different temperature treatment. The expression of genes was analyzed by RT-PCR. Abbreviations: FaSPS encoding, FaSS FaInvA, FaInvD, FaInv E, DHT, NTL and CK. * and ** indicates, respectively, significant difference from CK at P 0.05 and P 0.01 level. Each point represents mean ± SE of 3 biological repeats. Based on one-way ANOVE analysis.
Comparison of Rranscriptomes
The above RT-PCR results indicate that the expression of genes related to sucrose biosynthesis, conversion and transport, and citrate biosynthesis and conversion is generally favorable for better taste of the strawberry fruit at the DHT, and worse taste at NLT cultivation conditions. Therefore, a transcriptome analysis and comparison were conducted in the following experiment.
Totally 21173 genes were detected from samples of the three different treatments, each consisted of 3 replicates. Compared with the CK, 210 up-regulate and 56 down-regulated genes were recorded in NLT samples, and 1048 up-regulated and 826 down-regulated genes were gotten, showing much higher influence of gene expression by elevation of temperature during the light period than lowering of temperature during the dark period of the day (Figure 7a). Besides, 93 DEGs (differently expressed genes) were recorded in samples from both DHT and NLT treatment (Figure 7b).
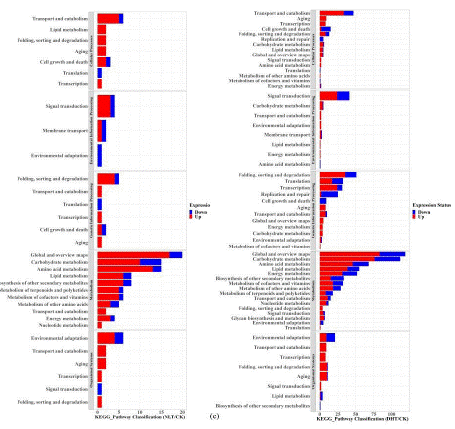
Figure 7: Transcriptome analysis of DEGs in strawberry fruit after three groups of temperature treatment. (a) DEGs between night low temperature treatment (NLT), daytime high temperature treatment (DHT) and control treatment (CK); (b) Venn diagram of strawberry fruit after temperature treatment. These numbers indicate DEGs between DHT treatment, NLT treatment and control (CK). The threshold of DEG significance was determined by | Log2 (FC) | =1 and Q-value =0.001. (c) KEGG metabolic pathway classification of DEGs response to different temperature treatments. The left side represents the KEGG path name. The x-axis represents the number of DEGs. Red and blue stripes represent up-regulated and down-regulated genes, respectively.
Temperature treatment significantly up-regulated and down-regulated genes enriched in 20 KEGG metabolic pathways (Figure 7c). The differential genes of NLT/CK were enriched in 15 genes in the carbohydrate metabolism pathway, of which 10 were up-regulated and 5 were down-regulated. 15 genes were enriched in the amino acid metabolism pathway, of which 13 were up-regulated and 2 were down-regulated. Compared with NLT treatment, the number of DEGs in DHT treatment was significantly increased, and more genes were significantly up-regulated in the enriched KEGG pathway, affecting the metabolism and synthesis of carbohydrates, amino acids, lipids and other secondary metabolites. The carbohydrate metabolism pathway enriched 112 genes, of which 76 were up-regulated and 36 were down-regulated. 68 genes were enriched in the amino acid metabolism pathway, of which 46 were up-regulated and 22 were down-regulated. Lipid metabolism pathway enriched 55 genes, of which 46 were up-regulated and 17 were down-regulated.
GO functional classification is divided into three functional categories: biological process, cellular component and molecular function. As shown in Figure 8, NLT/CK have 232 differentially expressed genes annotated in the GO database. The differentially expressed genes are divided into 17 subcategories in biological processes, of which cellular process and metabolic process are the most enriched, containing 71 and 67 genes, respectively. The differential genes were divided into 10 subclasses in terms of cell components. The differential genes were mainly enriched in membrane, membrane part, cell, cell part and organelle, and the number of genes was 76,70,62,62,40, respectively. The differential genes were divided into 8 subclasses in molecular function, of which the most enriched were catalytic activity and binding, containing 144 and 98 genes, respectively. As shown in Figure 3.18-b, there are 1578 genes annotated in the GO database in the mature strawberry fruit of DHT/CK, and the differential genes are divided into 21 subcategories in the biological process. The two subcategories with the largest number of enriched genes are the same as NLT/CK, containing 535 and 501 genes, respectively. Differential genes were divided into 16 subcategories in terms of cell components, and differential genes were mainly enriched in cells.
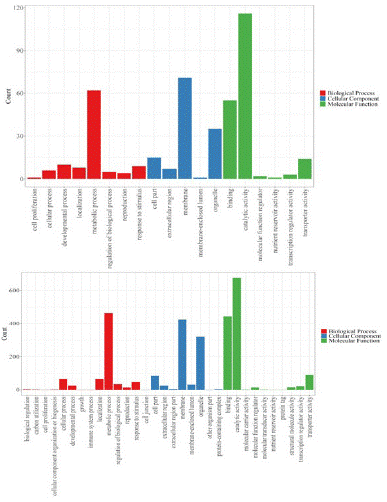
Figure 8: GO enriched genes in strawberry ripen fruits for HDT(A) and NLT (B) treatment. The X-axis and the Y-axis represents the number of DEGs and the GO terms, respectively.
KEGG pathway enrichment analysis was used to analyze the main pathways of differentially expressed genes, and the results were as follows (Figure 9). The differential genes of NLT/CK were enriched in 20 pathways, of which two KEGG pathways had the largest number of DEGs and significant expression, which were Biosynthesis of cofactors and Biosynthesis of amino acids, respectively. The number of genes was 8 and 7 (Supplementary data 4). The pathway with the highest enrichment ratio was the isoflavonoid biosynthesis pathway, which had only one differential gene (Supplementary data 5). Isoflavone is a secondary metabolite with anti-cancer, anti-oxidation and other functions. Glyoxylate and dicarboxylate metabolism and pyruvate metabolism are related to sugar and acid metabolism pathways (Supplementary data 6).
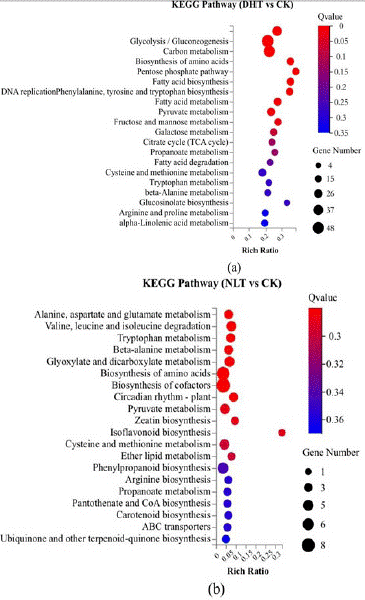
Figure 9: KEGG pathway analysis of DEGs in strawberry ripen fruits for HDT(a) and NLT (b) treatment. The Y axis and the X-axis represents the KEGG pathway name and the ratio of enrichment, respectively. The size of bubbles indicates the number of genes annotated to respective KEGG pathways. The color represents the Q-value, from low (red) to high (blue).
The differential genes of DHT/CK were enriched in 20 pathways, of which 3 KEGG pathways had the largest number of DEGs and significant expression, which were carbon metabolism (Supplementary data 7), Biosynthesis of amino acids (Supplementary data 8)oC Glycolysis/Gluconeogenesis (Supplementary data 9), contained 48, 44 and 29 genes, respectively. The pathway with the highest enrichment ratio was Fatty acid biosynthesis, containing 14 differential genes (Supplementary data 10). Fatty acid is the precursor of fruit aroma synthesis, and its synthesis will affect the aroma of fruit. The results of KEGG pathway enrichment analysis showed that among the DEGs enriched pathways of DHT/CK, the pathways related to glycoacid metabolism were glycolysis, pyruvate metabolism, TCA cycle and fructose and mannose metabolism. The number of enriched genes was 30, 23, 13 and 13, respectively (Supplementary data 11).
The differentially expressed genes in the sucrose metabolism pathway in the fruit were FaSPS, FaSS, FaInvA, FaInvD and FaInvE, respectively. The results of RNA-Seq analysis were consistent with the results of quantitative analysis. The differentially expressed genes in the citric acid metabolism pathway in the fruit were FaPEPC, FaPK, FaCS, and FaACO, respectively. The results of RNA-Seq analysis were consistent with the results of quantitative analysis (Table 2).
Symbol
Gene ID
log2(HT/CK)
Qvalue(HT/CK)
log2(LT/CK)
Qvalue(LT/CK)
KEGG Pathway/GO Desc
SPS
101306372
4.054
0.707100267
-0.792
0.999969338
KEGG:00500///Starch and sucrose metabolism
SS
101292831
-0.394
0.968855986
-1.776
0.999969338
KEGG:00500///Starch and sucrose metabolism
Inv A
101304591
-0.394
0.780042986
0.179
0.15126046
GO:0005987///sucrose catabolic process
Inv D
101296831
-0.337
0.690201289
2.744
0.999969338
GO:0005987///sucrose catabolic process
Inv E
101292085
-0.223
0.989667556
0.010
0.688925527
GO:0005987///sucrose catabolic process
PEPC
101309134
1.763
0.886929538
3.823
0.023405788
KEGG:00620///Pyruvate metabolism
PK
101311878
0.112
0.575580075
1.176
0.990433611
KEGG:00010///Glycolysis / Gluconeogenesis
CS
101307981
0.029
0.861655438
0.640
0.650652583
KEGG:00020///Citrate cycle (TCA cycle)
Aco
101291564
2.784
0.005856655
0.444
0.435145835
KEGG:00020///Citrate cycle (TCA cycle)
Table 2: Comparison of the transcriptome data for genes involved in biosynthesis and metabolism of sucrose and citrate in ripen fruits from three different growing conditions.
Discussion
Temperature plays key roles in fruit production of strawberry. Propriate temperature(s) is (are) necessary for the vigorous growth of plant seedlings [10-12]. Different flowering type will be significantly affected by temperature for flower induction [13,14]. Temperature environment during fruit development and ripening is even important, as it can influence the fruit ripening time [15], fruit weight [16], and fruit yield [17], as well as the quality of fruit, such as [18-20]. High temperature during the day, low temperature during the night and DIF (difference between daytime temperature and night temperature) have all been designed and investigated for the strawberry fruit production, and the results reported are not always in accordance [21,22]. One of the explanations for these differences could be attributed to different genetic background of the cultivars used in individual research [13], perhaps the perpetual (everbearing) day-neutral or Long Day and High Temperature-type (LDHT) is very different from the annual Short Day and Low Temperature-type (SDLT) in response to environment temperature.
In the present study, the SDLT type cultivar “Ssanta” was used and 3 different temperature treatments were designed and investigated. The results demonstrated that cultivation of seedlings after blooming at 30oC during the day and 22oC during the night had the best fruit quality (SSC, TA, solid acid ratio, sucrose content), the largest average fruit size and weight (single fruit weight, fruit transverse and longitudinal diameter and fruit shape index), and the most yield per plant, in comparison with the other two treatments. It was a little bit unexpected that cultivation of seedlings at 22oC during the day and 8oC during the night was the most unfavorable temperature combination for the fruit production of this cultivar found in the present study, especially, with the postpone of the fruit ripening (fruit yield and fruit quality). Relative higher temperature is favorable for photosynthesis and accumulation of carbonhydrates [23,26], and around 30oC during the day has been reported to positive effect on strawberry fruit [28], as well as on other fruit production [23-25]. No fruit deformalty was noticed at the DHT treatment, which indicates that 30oC was not the extream high temperature for the “Ssanta” strawberry cultivar. Low temperature during the night is generally good for strawberry fruit production [27], maybe the DIF is most important [28]. In one hand, low temperature during the night is favorable for the accumulation of sugars, on the other hand it will affect the growth of the seedlings and acid metabolism [23,26]. Therefore, too low temperature, below 8oC during the night, will not be advised for “Ssanta” strawberry cultivar growers.
The quality of the fruit is based on the metabolisms within the cells. Therefore, the results in sucrose content and the ratio of Soluble Solids Content (SSC) to Titratable Acidity (TA) (Figure 3) are well in accordance with the expression of genes involved in their metabolic pathways (Table 2). Similar results have also been reported before [29,30].
Transcriptomic comparison has been used for mining genes involved in strawberry fruit quality control, e.g., for investigation in postharvest of fruits [31], and for the effect of nitrate usage [32]. Transcriptome analysis of strawberry fruits under different temperature treatment is less reported. Based on the above findings by comparison of three temperature combination treatment, the transcriptome sequencing of the samples from these treatments was performed. The comparison results not only confirmed the difference in the expression of genes involved in sugar and citric acid metabolism (Table 2), but also showed differences in GO and KEGG enrichment, which provides clues for further investigation in the mechanisms for fruit quality control by different temperatures, or temperature differences. The KEGG enrichment shows gene expression differences in other metabolic pathways, correlated to strawberry quality, e.g. iso metabolism, which determines the fruit color and responsible for the accumulation of anti-oxidant substances (Supplementary data 12) [33], amino acid metabolism (Supplementary data) (Duan, et al., 2021), ascorbic acid metabolism (Supplementary data 12) (Liu, et al., 2022), lipid metabolism(Supplementary data 12) , which is responsible for (Shen, et al., 2021).
References
- Edger PP, Poorten TJ, VanBuren R, Hardigan MA, Colle M, McKain MR, et al. Origin and evolution of the octoploid strawberry genome. Nat Genet. 2019; 51: 541-7.
- Nakajima R, Otagaki S, Yamada K, Shiratake K, Matsumoto S. Molecular cloning and expression analyses of FaFT, FaTFL, and FaAP1 genes in cultivated strawberry: their correlation to flower bud formation. Biol Plant. 2014; 58: 641-8.
- Sønsteby A, Heide OM. Temperature responses, flowering and fruit yield of the June-bearing strawberry cultivars Florence, Frida and Korona. Sci Hortic. 2008; 119: 49-54.
- Verheul MJ, Sønsteby A, Grimstad SO. Influences of day and night temperatures on flowering of Fragaria ananassa Duch., cvs. Korona and Elsanta, at different photoperiods. Sci Hortic. 2007; 112: 200-6.
- Ledesma NA, Nakata M, Sugiyama N. Effect of high temperature stress on the reproductive growth of strawberry cvs. ‘Nyoho’ and ‘Toyonoka’. Sci Hortic. 2008; 116: 186-93.
- Sinha NK. Strawberries and raspberries. In: Cano MP, editor. Handbook of fruits and fruit processing. Wiley-Blackwell. 2008; 581-9.
- Wang SY, Camp MJ. Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci Hortic. 2000; 85: 183-99.
- Chujo T, Kataoka M, Yamauchi S, Ashizawa M. The effect of temperature on the growth and quality of kaki fruit. II. Day and night temperature treatments during the stage of fruit enlargement [technical bulletin]. 1973; 25: 339-43.
- Li L, Li J, Gao Q, Chen JX. Effects of day and night temperature difference on growth, development, yield and fruit quality of tomatoes. Ying Yong Sheng Tai Xue Bao. 2015; 26: 2700-6.
- Miyazawa Y, Hikosaka S, Goto E, Aoki T. Effects of light conditions and air temperature on the growth of everbearing strawberry during the vegetative stage. Acta Hortic. 2009; 842: 817-20.
- Sønsteby A, Solhaug KA, Heide OM. Functional growth analysis of ’Sonata’ strawberry plants grown under controlled temperature and daylength conditions. Sci Hortic. 2016; 211: 26-33.
- Jo WJ, Shin JH. Effect of root-zone heating using positive temperature coefficient film on growth and quality of strawberry (Fragaria x ananassa) in greenhouses. Hortic Environ Biotechnol. 2022; 63: 89-100.
- Nakajima R, Otagaki S, Yamada K, Shiratake K, Matsumoto S. Molecular cloning and expression analyses of FaFT, FaTFL, and FaAP1 genes in cultivated strawberry: their correlation to flower bud formation. Biol Plant. 2014; 58: 641-8.
- Rivero R, Remberg SF, Heide OM, Sønsteby A. Environmental regulation of dormancy, flowering and runnering in two genetically distant everbearing strawberry cultivars. Sci Hortic. 2021; 290: 110515.
- Miura H, Yoshida M, Yamasaki A. Effect of temperature on the size of strawberry fruit. J Jpn Soc Hortic Sci. 1994; 62: 769-74.
- Balasooriya BLHN, Dassanayake K, Ajlouni S. High temperature effects on strawberry fruit quality and antioxidant contents. Acta Hortic. 2020; 1278: 225-34.
- Sun P, Mantri N, Lou H, Hu Y, Sun D, et al. Effects of elevated CO2 and temperature on yield and fruit quality of strawberry (Fragaria×ananassa Duch.) at two levels of nitrogen application. PLOS ONE. 2017; 7.
- Steyn WJ, Wand SJ, Jacobs G, Rosecrance RC, Roberts SC. Evidence for a photoprotective function of low-temperature-induced anthocyanin accumulation in apple and pear peel. Physiol Plant. 2009; 136: 461-72.
- Yan Y, Lei B, Wang L, Bie Z. Effects of different day and night temperature on the growth and quality of hydroponic lettuce. J Changjiang Veg. 2010; 24: 39-42.
- Yi M, Kong J, Yu Z. Effect of heat treatment on the quality and energy metabolism in ’golden delicious’ apple fruit. J Food Biochem. 2021; 45: e13759.
- Macha MM, Chowdhury AK, Nomura K, Ide M, Yonemoto Y. Effect of temperature regime and soil moisture level on fruit quality of ’summer queen’ passionfruit (Passiflora edulis×P. edulis f. flavicarpa). Jpn J Trop Agric. 2006; 50.
- Wu X, Han W, Yang Z, Zhang Y, Zheng Y. The difference in temperature between day and night affects the strawberry soluble sugar content by influencing the photosynthesis, respiration and sucrose phosphatase synthase. Hortic Sci. 2021; 48: 174-82.
- Kitano M, Araki T, Eguchi H. Temperature dependence of postphloem transport regulated by respiration in tomato fruits. Biotronics. 1998; 27: 33-9.
- Fukuoka N, Masuda D, Kanamori Y. Effect of timing of high temperature treatment around the fruit on sugar accumulation in watermelon (Citrullus lanatus (Thunb.) Matsum. et Nakai). Environ Control Biol. 2008; 46: 241-8.
- Zhang MF, Xu RW, Sun GC, Cheng Y, Wang Z. The effect of short-term temperature pretreatments on sugars, organic acids, and amino acids metabolism in Valencia Orange Fruit. J Food Qual. 2022; 2022: 1-9.
- Rivero R, Sønsteby A, Heide OM, Solhaug KA, Remberg SF. Growth analysis of the everbearing strawberry ’Delizzimo’ under controlled temperature and photoperiod conditions. CABI Agric Biosci. 2022; 3.
- Kumakura H, Shishido Y. The effect of daytime, nighttime, and mean diurnal temperatures on the growth of ’Morioka-16’ strawberry fruit and plants. J Jpn Soc Hortic Sci. 1994; 62.
- Wu Z, Tu M, Yang X, Xu J, Yu Z. Effect of cutting and storage temperature on sucrose and organic acids metabolism in postharvest melon fruit. Postharvest Biol Technol. 2020; 161.
- Beaulieu JC, Lea JM, Eggleston G, Peralta-Inga Z. Sugar and organic acid variations in commercial cantaloupes and their inbred parents. J Am Soc Hortic Sci. 2003; 128: 531-6.
- Matsumoto H, Ikoma Y. Effect of different postharvest temperatures on the accumulation of sugars, organic acids, and amino acids in the juice sacs of Satsuma mandarin (Citrus unshiu Marc.) fruit. J Agric Food Chem. 2012; 60: 9900-9.
- Zheng T, Lv J, Sadeghnezhad E, Cheng J, Jia H. Transcriptomic and metabolomic profiling of strawberry during postharvest cooling and heat storage. Front Plant Sci. 2022; 13: 1009747.
- Hao F, Wu M, Wu S, Ge X, Sun Q, Zhao J, et al. Transcriptome analysis reveals the involvement of nitrate transporters in regulating strawberry fruit development. Sci Hortic. 2022; 296: 110910.
- Abinaya M, Koeun H, Sun Yi L, Hye Eun L, et al. Genome-wide analysis of MYB10 transcription factor in Fragaria and identification of QTLs associated with fruit color in octoploid strawberry. Int J Mol Sci. 2021; 22.
- Wenkai D, Wanlu S, Weida L, Ling Y, Qiuwei L, et al. Integrated metabolomics and transcriptomics reveal the differences in fruit quality of the red and white Fragaria pentaphylla morphs. Food Biosci. 2021; 40.
- Huabo L, Lingzhi W, Yang N, Linlin C, Jing D, et al. Genome-wide analysis of ascorbic acid metabolism related genes in Fragaria × ananassa and its expression pattern analysis in strawberry fruits. Front Plant Sci. 2022; 13.
- Jincheng S, Wanlu S, Junmin L, Hongfei L. Integrated metabolomic and transcriptomic analysis reveals factors underlying differences in fruit quality between Fragaria nilgerrensis and F. pentaphylla. J Sci Food Agric. 2021; 102.