
Review Article
Ann Agric Crop Sci. 2023; 8(4): 1142.
Plant Microbial Interactions in the Rhizosphere: Associations to Plant Growth Promoting Rhizosphere Microorganisms, Genetic Diversity, Competition and Interactions with Host Plants
Zehara Mohammed Damtew*
Ethiopian Institute of Agricultural Research, Debre Zeit Research Center, Addis Ababa, Ethiopia
*Corresponding author: Zehara Mohammed Damtew Ethiopian Institute of Agricultural Research, Debre Zeit Research Center, PO Box 32, Hora road, Debre Zeit, Ethiopia. Email: zeharamicro2015@gmail.com
Received: July 15, 2022 Accepted: December 18, 2023 Published: December 23, 2023
Summary
Microbial interactions are crucial for successful establishment and maintenance of a microbial population. These interactions occur by the environmental recognition followed by transference of molecular and genetic information that include many mechanisms and classes of molecules. Microorganisms are rarely encountered as single species populations in the environment, since studies in different habitats have shown that an enormous richness and abundance variation are usually detected in a small sample. The rhizosphere is known to be a hot spot of microbial activities. Therefore, rhizosphere is an environment with a high microbial diversity. Rhizobacteria as PGPR can play an important role in promoting nutrient acquisition by plants, favoring factors inducing root biomass accumulation and/or hindering those that could have detrimental effects on root system development. This role of PGPR can be achieved via either an indirect (antagonism against pathogens) or direct (e.g., phytoharmones production) mode of action. Plant growth-promoting mechanisms differ between bacterial strains and to a great extent depend on the type of organic compounds released by these strains. For example, plant growth-promoting hormones and other secondary metabolites released by the bacteria can alter plant growth and development. Recently, it has been reported that associations between plant and associated bacteria have reached such levels that the host plant cannot develop properly without their associated bacteria.
Keywords: Interaction; Microorganisms; Phytoharmones; Rhizosphere; Species
Introduction
The positive plant-microbe relationship established in the rhizosphere could, moreover, sustain an additional service, which is phyto-rhizoremediation which constitutes a promising sustainable approach for the in-situ remediation of polluted soils and sediments. It relies on the stimulation by the plant of the degrading microbes in its rhizosphere, in a complex interaction involving roots, root exudates, rhizosphere soils, and microbial communities [56]. Microbial interactions are crucial for a successful establishment and maintenance of a microbial population. These interactions occur by the environmental recognition followed by transference of molecular and genetic information that include many mechanisms and classes of molecules. These mechanisms allow microorganisms to establish in a community, which depending on the multi-trophic interaction could result in high diversity. The result of this multiple interaction is frequently related to pathogenic or beneficial effect to a host. In humans, for example, the microbial community plays an important role in protection against diseases, caused by microbial pathogens or physiological disturbances. Soils microbial communities also play a major role in protecting plants from diseases and abiotic stresses or increasing nutrient uptake [19,56].
The interaction among microbe, plants and environmental factors are determinant for the colonization and development of microorganisms and plants in ecosystem. These interaction can be refelected in different aspect, such as variation in cellular morphology, changes in physicochemical traits, exchange and conversion of metabolite, molecular dialoog, gene transfer [62]. Suggesting that microbial interaction are inherent to the establishment of populations in the environment, which includes soil, sediment, animal, and plants, including also fungi and protozoa cells. The many years of coevolution of the different species lead to adaptation and specialization and resulted in a large variety of relationships that can facilitate cohabitation, such as mutualistic and endosymbiotic relationships, or competitive, antagonistic, pathogenic, and parasitic relationships [18,62].
Many secondary metabolites have been reported to be involved in the microbial interactions. These compounds are usually bioactive and can perform important functions in ecological interactions. A widely studied mechanism of microbial interaction is quorum sensing, which consists of a stimulation response system related to cellular concentration. The production of signaling molecules (auto-inducers) allows cells to communicate and respond to the environment in a coordinated way [2,48]. During interaction with the host cells, plant-associated molecular patterns (PAMP or MAMP microbial-associated molecular pattern) are conserved throughout different microbial taxon increasing the fitness during interaction with plant or animal cells and regulating the microbial interactions with different hosts [2,60]. There are many microbe host interactions, which can be related to beneficial or pathogenic interactions in plants and animals. In these interactions, the microbial cells may be found in biofilm or planktonic state, which result in different genetic and physiological states [9,30].
A fundamental knowledge on plants’ physiological properties and their associated microorganisms in the undisturbed natural environments is necessary to understand the impact of microorganisms on the plant development in general. The existence of positive plant-microbial interactions also in disturbed soils is unquestionable, but the mechanisms are often scarcely known. Microorganisms contribute essentially to the protection of plants against unfavorable soil conditions. In this chapter a selection of possible unfavorable soil properties in disturbed soils will be focused to analyze the possible impact of associated microorganisms on plants growth and vitality. Applicability of microbial inoculum for an improved remediation of such disturbed soils will be presented [1,27].
Literature of Review
Plant Microbe Interactions and the Rhizosphere Diversity
A narrow zone of soil affected by the presence of plant roots is defined as rhizosphere. The rhizosphere is known to be a hot spot of microbial activities. This is caused by an increased nutrient supply for microorganisms; since roots release a multitude of organic compounds (e.g., exudates and mucilage) derived from photosynthesis and other plant processes [10,26]. Therefore, rhizosphere is an environment with a high microbial diversity. An important consequence of the high diversity is an intense microbial activity with feedback effects on root development and plant growth in general. In general, the microbes serve as intermediary between the plant, which requires soluble mineral nutrients, and the soil, which contains the necessary nutrients but often in low concentrations and/or complex and inaccessible forms. Thus, rhizosphere microorganisms provide a critical link between plants and soil [26,36].
The highest portions of microorganisms, which inhabit the rhizosphere, are fungi and bacteria. When considering the rhizosphere effect on their abundance, the fungal abundance is 10–20 times higher and the bacterial abundance 2–20 times higher in the rhizosphere than in the bulk soil [2,42]. Competition for nutrient sources in the rhizosphere is very high. Therefore, different microorganisms have developed distinct strategies, giving rise to a range of antagonistic to synergistic interactions, both among themselves and with the plant [2,47]. A very high diversity of interactions can be assumed on the basis of the tremendous diversity of soil microorganisms and plants. The understanding of fundamentals of these interactions is critical for their use in plant growth promotion and remediation of disturbed soils.
The rhizosphere can be defined as the soil region where processes mediated by microorganisms are specifically influenced by the root system (Figure 1). This area includes the soil connected to the plant roots and often extends a few millimeters off the root surface, being an important environment for the plant and microorganism inter- actions [23,36,56], because plant roots release a wide range of compounds involved in attracting organisms which may be beneficial, neutral or detrimental to plants [4,36,56]. The plant growth-promoting bacteria (or PGPB) belong to a beneficial and heterogeneous group of microorganisms that can be found in the rhizosphere, on the root surface or associated to it, and are capable of enhancing the growth of plants and protecting them from disease and abiotic stresses [16,22,25,56]. The mechanisms by which PGPB stimulate plant growth involve the availability of nutrients originating from genetic processes, such as biological nitrogen fixation and phosphate solubilization, stress alleviation through the modulation of ACC deaminase expression, and production of phytohormones and siderophores, among several others.
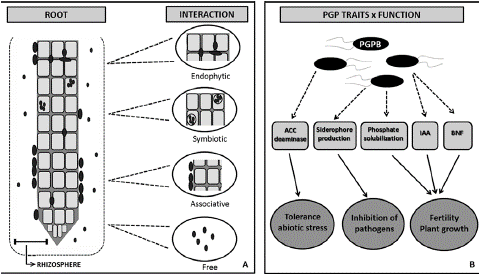
Figure 1: Rhizosphere/bacteria interactions. A) Different types of association between plant roots and beneficial soil bacteria; B) After colonization or association with roots and/or rhizosphere, bacteria can benefit the plant by (i) tolerance toward abiotic stress through action of ACC deaminase; (ii) defense against pathogens by the presence of competitive traits such as siderophore production; (iii) increase of fertility and plant growth through biological nitrogen fixation (BNF), IAA (indole-3-acetic acid) production, and phosphate solubilization around roots. [32,59].
Interactions between plants and bacteria occur through symbiotic, endophytic or associative processes with distinct degrees of proximity with the roots and surrounding soil (Figure 1). Endophytic PGPB are good inoculant candidates, because they colonize roots and create a favorable environment for development and function. Non-symbiotic endophytic relationships occur within the intercellular spaces of plant tissues, which contain high levels of carbohydrates, amino acids, and inorganic nutrients [3,15].
One of the most profound discoveries in biological research of the past decade is the overwhelming importance of host associated microbial communities for the health of multicellular organisms [2,7,20]. Microbes growing in association with either an animal gut or plant roots (in the rhizosphere) can affect the health of their host. Because host genotype can shape the associated microbial communities [2,12,35,43,58]. It is possible that eukaryotic organisms have evolved the ability to cultivate specific beneficial microbiomes. In plants, the microbial composition of the surrounding soil is the largest determinant of the final rhizosphere community, and the effect of host genotype is minor by comparison [2,12,35]. As a result, it is unclear whether host genotype-mediated differences are important for host health, or if it is only random environmental encounters that matter. For a microbiome to be a host genotype-dependent adaptive trait (i.e. under natural selection), we reasoned that the following attributes of host-microbe interactions should be apparent in a single host species: first, populations of the host organism should possess genetic variation in traits that allow them to select environmentally adaptive microbes; and second, differences in microbial communities should differentially affect the fitness of the variant host organisms.
Microorganisms present in the rhizosphere play important roles in ecological fitness of their plant host. Important microbial processes that are expected to occur in the rhizosphere include pathogenesis and its counterpart, plant protection/growth promotion, as well as the production of antibiotics, geochemical cycling of minerals and plant colonization [28,62]. Plant microbe interactions may thus be considered beneficial, neutral, or harmful to the plant, depending on the specific microorganisms and plants involved and on the prevailing environmental conditions [5,62]. Exploring these microorganisms by unraveling their possible relationships with plants has launched a new and fascinating area of investigations in the rhizosphere research.
The rhizosphere microflora includes bacteria, fungi, nematodes, protozoa, algae and soil microarthrops [30,51]. Approximately 80–90% of soil microorganisms are not yet cultured by means of classical methods. Their identification, characterization and the description of their role are therefore particularly difficult. Recently, nucleic acid-based techniques including analysis of DNA and rRNA molecules from soil samples have revealed enormous diversity in the rhizosphere inhabiting microbial flora (Suzuki et al., 2006; Khan et al., 2019). The molecular methods used for soil microbial diversity are covered in the review by Nannipieri and collaborators (2003); (Khan et al., 2019). The number of microbial species present in soil may vary from thousands to millions. Many studies indeed suggest that the Proteobacteria and the Actinobacteria form the most common of the dominant populations (>1%, usually much more) found in the rhizosphere of many different plant species (Singh et al., 2007; Khan et al., 2019). These groups contain many “cultured” members. They are the most studied of the rhizobacteria, and as such, contain the majority of the organisms investigated, both as beneficial microbial inoculants and as pathogens.
The specific content of root exudates may create a niche that influences which microorganisms are to colonize the rhizosphere, thereby altering the composition and diversity of microorganisms colonizing the rhizosphere in a plant specific manner [24,56]. Plant species, plant developmental stage and soil type have thus been indicated as major factors determining the composition of rhizosphere microbial communities [11,56]. That said, the extent to which the above-cited factors contribute to microbial communities is not fully understood and there are several contrasting reports in the literature indicating either plant or soil type as dominant factor [46,56]. Owing to the above statement, it can be generalized that the diversity and predominance of rhizosphere microbial population depend on a number of abiotic and biotic factors prevailing in that particular ecological niche.
Beneficial Interactions Between Plants, Soil and Bacteria
As autotrophic organisms, plants play a major role in sustaining all other life forms. The plant root system is a chemical factory that mediates several interactions of the plant with soil microorganisms. Roots release organic compounds, which act as signaling agents to attract beneficial microbes and to combat pathogenic ones. Generally, these interactions are mutualistic with beneficial microbes, such as rhizobia, mycorrhizal, endophytes, and Plant Growth-Promoting Rhizobacteria (PGPR). However, these plant microbe interactions are not only driven by organic compounds released by the roots but are highly integrated with and influenced by biotic and abiotic factors [2,34,49].
Root released organic compounds also enhance the abundance and diversity of beneficial microorganisms in the rhizosphere and plant environment. In return, plant associated microbes may enhance plant growth and health by several activities such as nitrogen fixation, synthesis of plant hormones and vitamins, the improvement of nutrient uptake, and induction of stress resistance. They also outcompete invading pathogens by different mechanisms such as niche occupation by competition for space, nutrients, and physical niches of the rhizosphere/rhizoplane and endophytic tissues. Some of the beneficial rhizobacteria and endophytic bacteria can secrete not only antibiotics but also lytic enzymes enabling the inhibition of various pathogens [30,50].
Most of the plant associated bacteria are also soil inhabitants [26,52,53]. They may move from the bulk soil to the rhizosphere of the living plant and aggressively colonize the rhizosphere and roots of plants. Some of them can penetrate plant roots, and some strains may move to shoots, leaves, flowers, and even seeds [14,26,54]. However, different plant species host different microbial communities [8,26], which is mostly due to the different composition of root exudates excreted by different plants. Root exudate is a key element of plant homeostasis, in part through playing a key role in communication between aboveground and belowground elements of plants.
Plant growth and productivity depend considerably on the availability of nutrients at the soil root interface, which in turn is influenced by a wide range of factors including the soil type and chemical physical characteristics, plant species and genotype, soil macro- and microorganism communities, and environmental conditions. In this context, biological activities of both roots and microorganisms can play an important role [33,38]. In addition to a brief introduction about the main mechanisms used by plants root for the acquisition of N, P, and Fe, in the following sections, the contribution of microbes to the dynamics of these three nutrients in the rhizosphere is described; moreover, a review of the effects of PGPRs on physiological and molecular mechanisms underlying the root acquisition of nutrients will be presented in the next sections.
Plant Growth Promoting Rhizobacteria
Rhizobacteria as PGPR can play an important role in promoting nutrient acquisition by plants, favoring factors inducing root biomass accumulation and/or hindering those that could have detrimental effects on root system development. This role of PGPR can be achieved via either an indirect (antagonism against pathogens) or direct (e.g., phytoharmones production) mode of action [22,30,32]. Furthermore, microorganisms can also affect plant nutrient acquisition processes by influencing nutrient availability in the rhizosphere and/or functionality of the biochemical mechanisms underlying the nutritional process.
Soil bacteria are very important in biogeochemical cycles and have been used for crop production for decades. “Plant bacterial interactions” in the rhizosphere are the determinants of plant health and soil fertility. Interaction of with host plants is an intricate and interdependent relationship involving not only the two partners but other biotic and abiotic factors of the rhizosphere region (Figure 1) [2,17]. “Plant growth-promoting rhizobacteria” are free-living soil bacteria that can either directly or indirectly facilitate rooting [26,40] and growth of plants [21,32]. In the last ten years, a number of PGPR that have been identified has seen a great boost, mainly because the role of the rhizosphere as an ecological unit has gained importance in the functioning of the biosphere.
Many studies have deciphered the mechanisms of action of PGPR using one individual strain and one host plant. But in reality, as described above, PGPR strains are not acting individually in the rhizosphere but rather as part of bacterial communities, in which cell communication signals may coordinate the activities of all individual strains [1,33]. Indeed, a vast array of PGPR populations dis- playing co-occurring plant beneficial activities and that may share between each other antagonistic or synergistic effects are interacting with a same host plant (Figure 2). When analyzing plant growth-promoting effects, it is thus important to integrate the complexity of the interactions between PGPR populations within the rhizomicrobiome. To do so, functional ecology approaches are needed, in which the relations between the size, diversity and activities of PGPR assemblages in the rhizosphere are taken into account. This is of particular importance when assessing the effect of various environmental factors, including that of plant genotype [2,61].
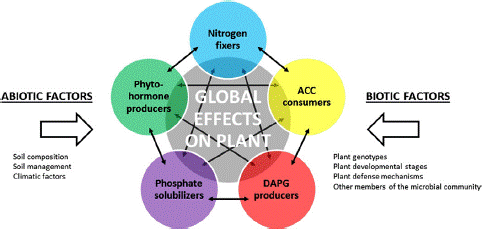
Figure 2: Implementation of plant growth promoting traits in PGPR functional groups. Different colored circles represent selected PGPR functional groups. The gray circle symbolizes the resulting effect of all PGPR functional groups on the plant. Abiotic and biotic factors may influence the activity of each functional group. Solid arrows represent potential interactions (inhibition, signaling, etc.) between members of the functional groups, which may impact on the size, diversity and activity of these groups. [32,33,61].
Figure 3: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
Plant growth-promoting mechanisms differ between bacterial strains and to a great extent depend on the type of organic compounds released by these strains. For example, plant growth-promoting hormones and other secondary metabolites released by the bacteria can alter plant growth and development. Recently, it has been reported that associations between plant and associated bacteria have reached such levels that the host plant cannot develop properly without their associated bacteria [13,62]. In addition to sustainable growth of food and feed crops, bacteria may enhance plant growth and the remediation of organic and inorganic pollutants from the soil and water. The enhanced microbial population in the rhizosphere can mineralize organic contaminants in the soil. In this regard, interactions among plant, soil, and bacteria have received great attention because of the biotechnological potential of microorganisms for improving growth of food and feed crops [30,33].
Besides these PGP activities, PGPR also enhances resistance to drought, salinity, waterlogging and oxidative stress [15,57] and production of water-soluble B group vitamins such as niacin, pantothenic acid, thiamine, riboflavine and biotin [30,39,55]. PGPRs are well known for their role in enhancing the soil fertility and promoting crop productivity by providing essential nutrients [32,64] and growth regulators [56,63]. They also promote the growth of plants by alleviating the stress induced by ethylene- mediated impact of plant by synthesizing ACC deamiase [1,6].
The application of PGPR has also been extended to remediate contaminated soils in association with plants [29,32]. The major applications of PGPR include agriculture, horticulture, forestry and environmental restoration. Thus, it is an important need to enhance the efficiency of insufficient amounts of external inputs by employing the best combinations of beneficial bacteria in sustainable agriculture production systems [15]. PGPR may use one or more of above-mentioned traits in the rhizosphere. Biochemical and molecular approaches are providing new insight into the genetic basis of these traits, the biosynthetic pathway involved, their regulation and importance for biological control in laboratory and field studies [2,33,45].
Conclusion
Microbial interactions allow microorganisms to establish in a community, which depending on the multi-trophic interaction could result in high diversity. Suggesting that microbial interactions are inherent to the establishment of populations in the environment, which includes soil, sediment, animal, and plants, including also fungi and protozoa cells. A fundamental knowledge on plants’ physiological properties and their associated microorganisms in the undisturbed natural environments is necessary to understand the impact of microorganisms on the plant development in general. Furthermore, microorganisms can also affect plant nutrient acquisition processes by influencing nutrient availability in the rhizosphere and/or functionality of the biochemical mechanisms underlying the nutritional process. Generally, existence of positive plant-microbial interactions also in disturbed soils is unquestionable, but the mechanisms are often scarcely known. Microorganisms contribute essentially to the protection of plants against unfavorable soil conditions.
References
- Abd-Alla MH, Nafady NA, Bashandy SR, Hassan AA. Mitigation of effect of salt stress on the nodulation, nitrogen fixation and growth of chickpea (Cicer arietinum L.) by triple microbial inoculation. Rhizosphere. 2019; 10: 100148.
- Adeleke RA, Nunthkumar B, Roopnarain A, Obi L. Applications of plant microbe interactions in agroecosystems. In: Kumar V, Prasad R, Kumar M, Choudhary DK, editors. Microbiome in plant health and disease. Springer Nature Singapore Pte Ltd. 2019; 1-34.
- Bacon CW, Hinton DM. Bacterial endophytes: the endophytic niche, its occupants, and its utility. In: Gnanama- nickam SS (Ed) Plant-Associated Bacteria. Neth: Springer- erlands. 2006; 155-94.
- Badri DV, Vivanco JM. Regulation and function of root exudates. Plant Cell Environ. 2009; 32: 666-81.
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol. 2006; 57: 233-66.
- Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, et al. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem. 2005; 37: 241-50.
- Berendsen RL, Pieterse CM, Bakker PA. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012; 17: 478-86.
- Berg G, Smalla K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol Ecol. 2009; 68: 1-13.
- Braga RM, Dourado MN, Araújo WL. Microbial interactions: ecology in a molecular perspective, Environmental Microbiology. Braz J Microbiol. 2016; 47S: 86-98.
- Brimecombe MJ, FAAM DL, Lynch JM. Rhizodeposition and microbial populations. In: Pinton R, Varanini Z, Nannipieri P, editors. The rhizosphere: biochemistry and organic sub- stances at the soil-plant interface. Boca Raton, London, New York: CRC Press, Taylor & Francis Group. 2007; 73-109.
- Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM. Root exudates regulate soil fungal community composition and diversity. Appl Environ Microbiol. 2008; 74: 738-44.
- Bulgarelli D, Rott M, Schlaeppi K, Ver Loren van Themaat E, Ahmadinejad N, Assenza F, et al. Revealing structure and assembly cues for Arabidopsis root- inhabiting bacterial microbiota. Nature. 2012; 488: 91-5.
- Carlier AL, Eberl L. The eroded genome of a Psychotria leaf symbiont: hypotheses about lifestyle and interactions with its plant host. Environ Microbiol. 2012; 14: 2757-69.
- Compant S, Clément C, Sessitsch A. Plant growth-promoting bacteria in the rhizosphere and endosphere of plants: their role, colonization, mechanisms involved and pros- pects for utilization. Soil Biol Biochem. 2010a; 42: 669-78.
- Dahiya A, Kumar R, Sindhu SS. Microbial endophytes mediated phosphorus solubilization: sustainable approach to improve soil fertility and plant growth. In: Maheshwari DK, editor. Endophytes: mineral nutrients management in the series ‘Sustainable Development and Biodiversity’; 2021. Gewerbestrasse. Switzerland. Springer Nature. 2021; 35-75.
- Dimkpa C, Weinand T, Asch F. Plant-rhizobacteria in- teractions alleviate abiotic stress conditions. Plant Cell & Environment. 2009a; 32: 1682-94.
- Dutta S, Podile AR. Plant growth promoting rhizobacteria (PGPR): the bugs to debug the root zone. Crit Rev Microbiol. 2010; 36: 232-44.
- Faust K, Raes J. Microbial interactions: from networks to models. Nat Rev Microbiol. 2012; 10: 538-50.
- Frey-Klett P, Burlinson P, Deveau A, Barret M, Tarkka M, Sarniguet A. Bacterial–fungal interactions: hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol Mol Biol Rev. 2011; 75: 583-609.
- Gallo RL, Hooper LV. Epithelial antimicrobial defense of the skin and intestine. Nat Rev Immunol. 2012; 12: 503-16.
- Glick BR. Enhancement of plant growth by free living bacteria. Can J Microbiol. 1995; 41: 109-17.
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012; 2012: 963401.
- Gray EJ, Smith DL. Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem. 2005; 37: 395-412.
- Grayston SJ, Wang SQ, Campbell CD, Edwards AC. Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem. 1998; 30: 369-78.
- Grover M, Ali SZ, Sandhya V, Rasul A, Venkateswarlu B. Role of microorganisms in adaptation of agriculture crops to abiotic stresses. World J Microbiol Biotechnol. 2011; 27: 1231-40.
- Gupta R, Anand G, Gaur R, Yadav D. Plant–microbiome interactions for sustainable agriculture: a review. Physiol Mol Biol Plants. 2021; 27: 165-79.
- Hrynkiewicz K, Baum B. The potential of rhizosphere microorganisms to promote the plant growth in disturbed soils. In: Malik A, Grohmann E, editors. Environmental protection strategies for Sustainable 35 development, strategies for sustainability. 2011; 35-64.
- Kent AD, Triplett EW. Microbial communities and their interactions in soil and rhizosphere ecosystems. Annu Rev Microbiol. 2002; 56: 211-36.
- Khan AA, Jilani G, Akhtar MS, Muhammad S, Naqvi S, Rasheed M. Phosphorus solubilizing bacteria: occurrence, mechanisms and their role in crop production. J Agric Biol Sci. 2009; 1: 48-58.
- Khan MN, Ijaz M, Ali Q, Ul-Allah S, Sattar A, Ahmad S. Biological nitrogen fixation in nutrient management. In: Hasanuzzaman M, editor. Agronomic crops. Dhaka, Bangladesh: Sher-e-Bangla Agricultural University. 2019; 127-48.
- Kumar P, Dubey RC. Plant growth promoting rhizobacteria for biocontrol of phytopathogens and yield enhancement of Phaseolus vulgaris L. Journal of current perspectives in applied. Microbiology. 2012; 1: 1-38.
- Kumar A, Meena VS, Roy P, Vandana, Kumari R. Role of rhizobia for sustainable agriculture: lab to land. In: Kumar A, Meena VS, editors. Plant growth promoting rhizobacteria for agricultural sustainability; 2019; 129-49.
- Kumar S, Diksha SSS, Kumar R. Biofertilizers: an ecofriendly technology for nutrient recycling and environmental sustainability. Curr Res Microb Sci. 2022; 3: 100094.
- Lichtenthaler HK. The stress concept in plants: an introduction. Ann N Y Acad Sci. 1998; 851: 187-98.
- Lundberg DS, Lebeis SL, Paredes SH, Yourstone S, Gehring J, Malfatti S, et al. Defining the core Arabidopsis thaliana root microbiome. Nature. 2012; 488: 86-90.
- Lynch JM. Introduction: some cosequences of microbial rhizosphere competence for plant and soil. In: Lynch JM, editor. The rhizosphere. Chichester, UK: John Wiley & Sons. 1990; 1.
- Lynch JM. The rhizosphere. Wiley-Interscience, Chiches- ter. 1990; 458.
- Marschner P, Crowley D, Rengel Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis model and research methods. Soil Biol Biochem. 2011; 43: 883-94.
- Martinez-Toledo MV, Rodelas B, Salmeron V, Pozo C, Gonzalez-Lopez J. Production of pantothenic acid and thiamine by Azotobacter vinelandii in a chemically defined medium and a dialysed soil medium. Biol Fertil Soils. 1996; 22: 131-5.
- Mayak S, Tirosh T, Glick BR. Effect of wild type and mutant plant growth promoting rhizobacteria. 1999.
- Mitter B, Brader G, Afzal M, Compant S, Naveed M, Trognitz F, et al. Advances in Elucidating Beneficial Interactions Between Plants, Soil, and Bacteria. Advances in Agronomy. 2013; 121: 381-445.
- Morgan JAW, Bending GD, White PJ. Biological costs and benefits to plant–microbe interactions in the rhizosphere. J Exp Bot. 2005; 56: 1729-39.
- Mukherjee S, Zheng H, Derebe MG, Callenberg KM, Partch CL, Rollins D, et al. Antibacterial membrane attack by a pore-forming intestinal C-type lectin. Nature. 2014; 505: 103-7.
- Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G. Microbial diversity and soil functions. Eur J Soil Sci. 2003; 54: 655-70.
- Nelson LM. Plant Growth Promoting Rhizobacteria (PGPR): Prospects for new inoculants. Online Crop Management. 2004.
- Nunan N, Daniell TJ, Singh BK, Papert A, McNicol JW, Prosser JI. Links between plant and rhizoplane bacterial communities in grassland soils, characterized using molecular techniques. Appl Environ Microbiol. 2005; 71: 6784-92.
- Perotto S, Bonfante P. Bacterial associations with mycorrhizal fungi: close and distant friends in the rhizosphere. Trends Microbiol. 1997; 5: 496-501.
- Phelan VV, Liu W, Pogliano K, Dorrestein PC. Microbial metabolic exchange—the chemotype-to-phenotype link. Nat Chem Biol. 2012; 8: 26-35.
- Phillips DA, Fox TCK, Bhuvaneswari MD TV, Teuber LR. Microbial products trigger amino acid exudation from plant roots. Plant Physiol. 2004; 136: 2887-94.
- Pleban S, Chernin L, Chet I. Chitinolytic activity of an endophytic strain of Bacillus cereus. Lett Appl Microbiol. 1997; 25: 284-8.
- Raaijmakers JM, Weller DM. Exploiting genotypic diversity of 2,4-diacetylphloroglucinol- producing Pseudomonas spp.: characterization of superior root-colonizing P. fluorescens strain Q8r1-96. Appl Environ Microbiol. 2001; 67: 2545-54.
- Rasche F, dl H, Poll V, Kandeler C, Gerzabek E, van Elsas MH, et al. Rhizosphere bacteria affected by transgenic potatoes with antibacterial activities in comparison to effects of soil, wildtype potatoes, vegetation stage and pathogen expo- sure. FEMS Microbiol Ecol. 2006a; 56: 219-35.
- Rasche F, Velvis H, Zachow CB, van Elsas G, Sessitsch A. Impact of transgenic potatoes expressing antibacterial agents on bacterial endophytes is comparable to effects of wild type potatoes and changing environmental conditions. J Appl Ecol. 2006b; 43: 555-66.
- Reinhold-Hurek B, Hurek T. Living inside plants: bacterial endophytes. Curr Opin Plant Biol. 2011; 14: 435-43.
- Revillas JJ, Rodelas B, Pozo C, Martínez-Toledo MV, González-López J. Production of B- group vitamins by two Azotobacter strains with phenolic compounds as sole carbon source under diazotrophic and adiazotrophic conditions. J Appl Microbiol. 2000; 89: 486-93.
- Riva V, Terzaghi E, Vergani L, Mapelli F, Zanardini E, Morosini C, et al. Exploitation of rhizosphere microbiome services. In: Reinhardt D, Sharma AK, editors. Methods in rhizosphere biology research. Springer Nature Singapore. 2019; 105-32.
- Saleem M, Arshad M, Hussain S, Bhatti AS. Perspective of Plant Growth Promoting Rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol. 2007; 34: 635-48.
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010; 11: 76-83.
- de Souza, Ambrosin RA, Luciane MP, Passaglia LMP. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet Mol Biol. 2015; 38: 401-19.
- Stuart LM, Paquette N, Boyer L. Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nat Rev Immunol. 2013; 13: 199-206.
- Vacheron J, Desbrosses G, Bouffaud ML, Touraine B, Loccoz YM, Muller D, et al. Plant growth promoting rhizobacteria and root system functioning. Functional and ecological roles of the plant PGPR cooperation; Reviewed article. 2013; 4: 10-9.
- Wang ET, Tian CF, Chen WF, Young JPW, Chen WX. Ecology and evolution of rhizobia: springer Singapore. 1st ed. 2019; 1-13.
- Wani PA, Khan MS, Zaidi A. Cadmium, chromium and copper in greengram plants. Agron Sustain Dev. 2007a; 27: 145-53.
- Zaidi A, Khan MS. Co-inoculation effects of phosphate solubilizing microorganisms and Glomus fasciculatum on greengram Bradyrhizobium symbiosis. Turk J Agric For. 2006; 30: 223-30.