
Research Article
Ann Agric Crop Sci. 2024; 9(1): 1144.
Pathogen Growth Inhibition in Ocimum Basilicum (L.), Malus Domestica (Borkh.), and Citrus Limon (L.) by Low-Dose UV-C LED Exposure
Paolo Di Lazzaro1*; Giulio Metelli2; Sarah Bollanti1; Antonia Lai3; Marco Montecchi4; Daniele Murra1; Guido Bernabei5; Loretta Bacchetta5
1ENEA, Department FSN-PLAS-PAX, Centro Ricerche Frascati via E. Fermi 45 00044 Frascati, Italy
2University of Tuscia, Dept. DAFNE via S. Camillo de Lellis 01100 Viterbo, Italy
3ENEA, Department FSN-TECFIS-DIM, Centro Ricerche Frascati via E. Fermi 45 00044 Frascati, Italy
4ENEA, Department TE RIN-STSN-ITES, Centro Ricerche Casaccia via Anguillarese, 301, 00123 Santa Maria di Galeria, Italy
5ENEA, Department SSPT-BIOAG-PROBIO, Centro Ricerche Casaccia via Anguillarese, 301, 00123 Santa Maria di Galeria, Italy
*Corresponding author: Paolo Di Lazzaro ENEA, Department FSN-PLAS-PAX, Centro Ricerche Frascati via E. Fermi 45 00044 Frascati, Italy. Tel: +390694005722 Email: paolo.dilazzaro@enea.it
Received: November 25, 2023 Accepted: January 02, 2024 Published: January 09, 2024
Abstract
In order to stimulate hormesis in basil, apple and lemon we used Ultraviolet C-band (UV-C) radiation emitted by an array of Light-Emitting Diodes (LEDs) at low irradiation doses followed by inoculation of the pathogens Botrytis cinerea and Penicillium digitatum. The LEDs are preferable to conventional mercury lamps when robustness and portability are required. Hormetic doses lower than those reported in the literature were used, in order to achieve short irradiation times and thus rapid treatment of crops. The results show that a dose of just 0.3 kJ m-2 released in a time interval of 3 to 14 seconds generates metabolites that inhibit, or dramatically slow, pathogen growth. After 75 days from irradiation immediately followed by inoculation, the fungal development on basil plants affected less than 30% of the epigeal part, compared to 90% in the unirradiated control. In addition, we obtained preliminary results of low-dose hormetic irradiation of lemons and apples. No fungal growth was observed in 75% of irradiated apples 15 days after irradiation and inoculation. Irradiated lemons showed complete inhibition of P. Digitatum growth. One can then infer that preventive irradiation by LED is beneficial to limit crop diseases in both pre-harvest and post-harvest without harming the plant or the environment, as a sustainable alternative to pesticides.
Keywords: Crop protection; Ecological agriculture; Hormesis; Post harvest; Sustainable agriculture; UV-C
Introduction
Overuse of pesticides in agriculture contributes to soil, water, and air pollution, putting the health of operators and consumers at risk. The proposed European target is to reduce the use of chemicals by 50% by 2030 [1]. This goal implies an urgent need to find alternatives to pesticides and copper compounds, the latter of which are used on organic farms and whose toxicity to farmers, birds, mammals, and soil organisms has been demonstrated in long-term assessments.
UV-C radiation (wavelength range of 200-280 nm) appears to be a promising alternative to protect plants and fruits from pathogens without harming the plant or the environment [2-9]. Depending on the dose, the UV-C irradiation generates a germicidal or hormetic response, the latter being an adaptive strategy through which performance is enhanced and mediated. Specifically, exposure to the right dose of UV-C causes stress in plant tissues, which stimulates the biosynthesis of defensive secondary metabolites with antimicrobial and antioxidant activity [3,6,7]. Several works have demonstrated the effectiveness of UV-C irradiation at the wavelength of 254 nm emitted by low-pressure mercury lamps to limit the spread of common pathogens [2,5,7,9]. However, since nearly 140 countries have joined a global agreement (Minamata Convention on Mercury) that is expected to reduce the production and trade of mercury-containing products [10], including mercury lamps, it would be interesting to test other mercury-free UV-C sources possibly with better portability and efficacy. Light-Emitting Diodes (LEDs) are another type of UV-C radiation source with less conversion of electrical power to radiation than lamps, but they are more compact, lightweight, easy to carry, shock resistant, have an immediate turn-on time, and require a smaller and lighter power supply. These characteristics make LEDs preferable to mercury lamps for applications requiring portability, such as field irradiation, without the disposal problems of mercury, which is highly toxic to the environment. Among crop fungal diseases, Botrytis cinerea (Pers. 1794) a necrotic fungus that causes grey mold disease, and Penicillium digitatum Sacc., the causative agent of green mold disease, are among the most dangerous pathogens that infect several species in pre-harvest and remain latent until the post-harvest period, during storage and transport,making their control throughout the supply chain critical [11]. They produce significant economic losses in crop production and storage [12,13]. In particular, B. cinerea, a highly polyphagous necrotrophic pathogen capable of infecting numerous agricultural species, causes significant economic damage to crops on more than 1,400 known hosts in 586 plant genera and 152 botanical families [14]. Similarly, P. digitatum is a major cause of postharvest economic losses, accounting for up to 90% of total damage to citrus, especially in dry areas and subtropical climates [15,16]. Today, the application of chemical synthetic fungicides is the main method of controlling postharvest diseases caused by B. cinerea or P. digitatum [17]. However, there are two main problems with the application of fungicides: 1) toxicological residues of chemical fungicides are harmful to the environment and humans, and 2) the frequency of resistant strains to different categories of fungicides [18] increases crop management costs as well as the difficulty of controlling these pathogens with conventional methods [15].
In this work, it is explored the efficacy of the 277 nm wavelength emitted by an LED array to stimulate hormesis in basil plants and fruits after low-dose irradiation and subsequent exposure to the pathogens B. cinerea or P. digitatum. As case studies it has been chosen: basil plants, one of the main Mediterranean aromatic crops widely used in cooking and medicine; Golden Delicious, the most popular and easily available apple; and typical Italian lemons, of the Femminello cultivar.
Materials and Methods
Plants and Pathogens
Basil plants (Ocimum basilicum (L.), Genovese type) sown in March 2021 in the growth chamber (temperature 25°C; relative humidity 70%) and transplanted (3 plants per pot) at the emission of two true leaves were used. Growing conditions were as follows: 16h photoperiod; the average of PFD 120±10μmol photons m-2 s-1, actinic light. The substrate used for the experiment was the nursery soil with the main characteristics: pH (in H2O)=8; electrical conductivity EC=0.8 dS/m; dry bulk density =220 kg/mc; total porosity =82%. One month after transplanting, the plants were inoculated and irradiated as described in the following.
As for fruits, apples (Malus domestica (Borkh.) Golden Delicious variety) and lemons (Citrus limon (L.) Femminello variety) from organic farming were used.
The fungi were isolated from naturally infected material and subsequently grown in vitro to obtain enough inoculum. B. cinerea (Pers. 1794) was isolated from infected basil plants, while P. digitatum (Pers.) Sacc. 1881 was isolated from infected lemons, see Figure 1. Both fungal pathogens are non-obligatory hosts because once isolated they can be propagated in vitro on artificial culture media and kept in growth chambers.

Figure 1: Isolation of B. cinerea from previously infected Ocimum basilicum (L.) plants, and of P. digitatum from infected lemons.
They were then maintained on sterile Potato Dextrose Agar culture medium and kept at 25 °C in a LABnet 211DS incubator, until use.
Inoculation and Data Evaluation
The basil plants were inoculated with a conidia suspension. To obtain a sufficient inoculum concentration, conidia of B. cinerea, grown in vitro for one month were collected in sterile deionized water with a spatula. The suspension was adjusted to 9×105 conidia/ml using a Burker chamber microscope. The inoculation was carried out by spraying the conidia suspension on both irradiated and unirradiated plants, the latter being used as a reference. The plants were isolated, covered with plastic wrapping, and placed in a growth chamber at 25°C and a humidity of 90% that promotes the development of pathogenesis. The growth of basil plants was assessed by determining phenological and morphological stages at maximum fungal incidence. During the 3-month storage period, fungal development was evaluated as the percentage of the damage (incidence of pathology). Metabolic changes induced by irradiation and their correlation with pathogen response were assessed by fluorometric analysis.
Organically grown lemons and apples (purchased from the Natura SI store in Fiumicino, Italy) were surface sterilized with 80% ethanol to prevent uncontrolled infection. After solvent evaporation under sterile conditions and laminar air flow, each fruit was placed in sterile irradiated plant tissue culture containers (10x10x10cm, Fisher Scientific) and then each container was closed under aseptic conditions. Subsequently, the apples were infected by applying 1-cm-diameter pieces of B. cinerea fungal mycelium maintained in vitro [19]. The inoculum was collected with a cylindrical instrument at the periphery of the fungal colonies where growth is most effective and was placed on the surface of UV-C-irradiated or unirradiated fruits. The lemon fruits were infected following the same procedure, but using P. digitatum. Fungal mycelium was placed on each fruit, both irradiated and not irradiated, and its growth over time was observed. All inoculations were performed immediately after the application of UV-C treatment. Infected fruits were each stored in closed sterile containers to avoid cross-contamination and were placed at room temperature (22± 2°C), 80% humidity, and in dark conditions. The fungal growth response was monitored for six weeks. Fungal development on the fruits was analyzed by assessing the percentage of necrosis or the contaminated areas. Each trial was replicated three times.
UV-C Irradiation Device and Delivered Doses
The single LED is a 6 V, 0.1-0.4 A-powered, millimeter-sized solid-state source emitting a few tens of mW radiation. LEDs can be assembled in multi-element arrays, resulting in medium-power UV-C devices. At the ENEA Frascati Centre, have assembled and tested several Luminus XBT-3535-UV LEDs [20]. Accessed on 6 June 2023) which emit radiation whose spectrum is peaked at 277 nm, with a full width at half maximum bandwidth of 11 nm. The device shown in Figure 2 is made up of an array of 38 LEDs on a 90×34 mm² rectangular base. The associated electronics manage the timing and duty cycle of the irradiation.
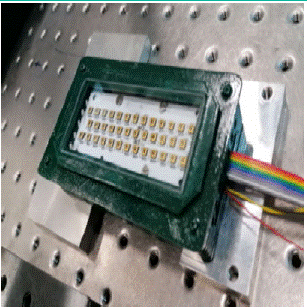
Figure 2: Array of 38 UV-C LEDs assembled and tested at ENEA Laboratories. In addition to the LEDs, the housing contains an appropriate cooling system, necessary to achieve optimal performance of UV-C emission.
A dedicated simulation software that calculates both the spatial distribution and the average value of the intensity of UV-C radiation vs. the distance from the LEDs was developed. The simulation results are in excellent agreement with the experimental data. As an example, Figure 3 shows the intensity distribution at different distances from the LEDs. Despite the aspect ratio ≈ 3 of the rectangular LEDs array, the spatial distribution of the radiation has a nearly circular shape at any distance larger than 50 mm.
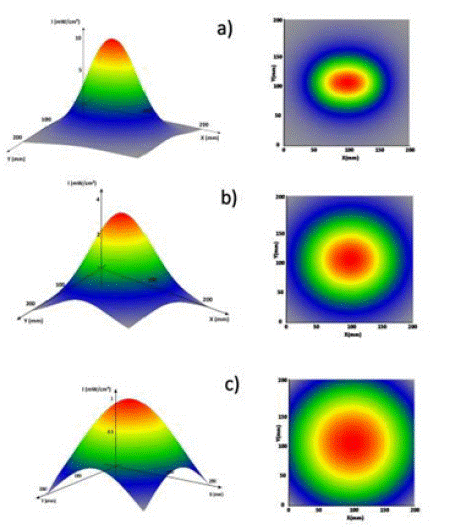
Figure 3: 3-D distribution of the UV-C intensity (left) and corresponding contour plot (right) at different distances from LEDs: a) at 50 mm, b) at 100 mm and c) at 200 mm.
The LEDs were positioned on top of each basil plant in the pot as shown in Figure 4.
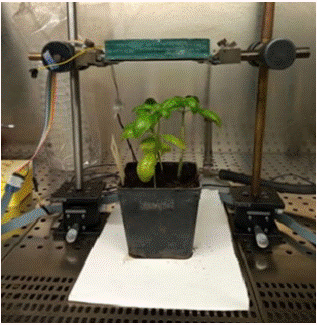
Figure 4: Apparatus of UV-C irradiation of a Ocimum basilicum (L.), Genovese type. The LEDs are in the green casing above the plant, and the UV-C radiation is directed downwards. Since UV-C radiation is not within the visible spectrum, a sheet of paper was placed under the pot that emits blue fluorescence when illuminated by UV-C, thus revealing that the LEDs are on.
UV-C intensity decreases with distance from the LEDs, so leaves placed at different heights receive slightly different doses of UV-C. The data refer to the average dose released to the plant, which is conventionally given by the radiation intensity (power per unit surface) on a plane located at 2/3 of the maximum height of the plant in the pot, multiplied by the exposure time (i.e., duration of irradiation). The dose was varied by changing the exposure time and keeping the position of the LEDs fixed. Depending on the height of the plant, exposure times ranged from 3 to 14 seconds.
Hormesis is a nonmonotonic/biphasic dose-response phenomenon [6], so accurate dose determination is crucial. The effective hormetic doses are dependent on the specific plant, the pathogen, and the irradiation wavelength. As a consequence, hormetic doses reported in the literature differ from each other. To make matters worse, not all doses reported in the literature have been measured by absolute detectors, increasing confusion between reliable dose values and others that are less so. Most hormetic doses are reported at the UV-C wavelength of 254 nm, ranging between 0.5 kJ m-2 and 9 kJ m-2 [2-5,8]. In these experiments, an extremely low hormetic dose was chosen, equal to 0.33±0.03 kJ m-2 as accurately measured by the absolute power-meter Hamamatsu C9536/H9535. Choosing a dose value much lower than those reported in the literature allows for shorter irradiation times and rapid treatment of crops.
Fluorometric Analyses
To assess irradiation-induced metabolic changes over time and correlate them with tolerance and response to pathogens, the Multiplex®3 UV-Visible portable fluorometer was used [21].
The measurements with Multiplex were carried out on basil plants both not irradiated and not inoculated controls versus irradiated and not inoculated. In the latter case, the leaves directly exposed to UV radiation were chosen to have homogeneous growth and age. The Multiplex analyzed the leaves of each plant for a total of 6 measurements per treatment.
Statistical Analysis
The data processing for the comparison between treatments was carried out with the ANOVA (ANalysis Of VAriance) parametric statistical analysis and the treatment-contrast pairs (t-Student test) by the software SPSS version 13.0 (Statistics BaseTM, Chicago, IL, USA) or GraphPad Prism 9 software for statistical analysis (Two-Way ANOVA). ANOVA verifies whether the data of the different groups are homogeneous and whether the observed differences between the groups are only due to normal statistical variability. More in detail, after the sampling campaign, a Shapiro-Wilk test for normality distribution on the values collected using GraphPad Prism was conducted, and the results pas sed validation. Next, it was performed an ANOVA test on the values obtained from the Multiplex flavonoid index collected at the end of sampling, and a Two-Way ANOVA comparing the values at the beginning of sampling, after UV-C irradiation, with those collected at the end. The goal was to understand whether elapsed time is the only factor on flavonoid accumulation or whether the stress performed also has an influence on flavonoids. Finally, for the two-way ANOVA the two independent variables are time (t0/t1) and exposure (S/N) and the dependent variable is the value itself, while for the one-way ANOVA the independent variable is only the exposure 163 (S/N).
Results
Irradiation Tests on Basil Plants
Although B. cinerea is not considered a basil-specific pathogen, under favorable conditions -usually in spring or fall- it becomes a common disease for basil crops [19,20]. Infected organs produce profuse off-white to gray mycelia covered with dark conidia on leaves and stems. Furthermore, stem cuttings are highly receptive to infection soon after harvest thus B. cinerea may also develop on the packed bunches during shipment to market, causing the entire package to rot. Farr and Rossman [23] listed records of B. cinerea on basil from Canada, Greece, Hungary, Italy, Poland, and Turkey, and recently de Oliveira Lopes et al. [24] reported B. cinerea damage on basil in Brazil for the first time. Therefore, the availability of new integrated control strategies is important to achieve the goal of reducing the use of synthetic fungicides. As shown in Table 1, basil plants irradiated at hormetic doses contrasted the fungal growth. After 75 days from irradiation followed by inoculation, the fungal development on plants affected less than 30% of the epigeal part, compared to 90% in the unirradiated control. The irradiation did not damage the basil plants. Indeed, the vegetative growth of both control and irradiated plants was similar to not irradiated and not inoculated plants. The control basil plants, inoculated and not irradiated, showed progressive deterioration of the epigeal part as a result of pathogen development leading to desiccation of the plant within 90 days.
Ocimum basilicum (L.) treatment
Height (cm)
Status of plants
Fungal growth (%)
CONTROL 1 Not irradiated and not inoculated
42±0.9
Post flowering, seed formation
0
Irradiated and not inoculated
40±0.5
Pre-flowering
0
Irradiated and inoculated
30±1.2
Vegetative stage
30 ± 3.21
CONTROL 2 Not irradiated and inoculated
25±1.4
Vegetative stage
90 ± 4.04
Table 1: Evaluation of necrosis extension (% mean value±Standard Deviation of the mean of three replicates) induced by B. cinerea on Ocimum basilicum (L.), Genovese type treated and not treated with UV-C after 75 days from irradiation with a hormetic dose of (0.33±0.03) kJ m-2.
The change in flavonols and chlorophyll was evaluated to determine whether low-dose UV-C irradiation induces metabolic change and promotes the formation of metabolites useful for plant defense. Table 2 shows the mean values of the Flav index, which is proportional to flavonoid content in plant leaves [25].
Days after irradiation
Sample treatment
FLAV
t-value
40
Irradiated
0.184±0.14
4.026**
Control
-0.015±0.03
50
Irradiated
0.150±0.16
1.906 ns
Control
0.001±0.09
60
Irradiated
0.304±0.14
2.049 ns
Control
0.135±0.14
Table 2: Flav index ±Standard Deviation of the mean of three replicates, as determined with Multiplex analysis on irradiated and control Ocimum basilicum (L.). Comparison of data collected at 40, 50, and 60 days after irradiation. NS=Not Significant; ** = 0.01≤ P≤0.05.
The data were collected by Multiplex at 40, 50, and 60 days after irradiation, showing a significant increase in flavonols content of irradiated plants compared to the unirradiated controls. This is indicative of the hormetic effect sought. Namely, small doses of UV-C directed against the organism elicited a protective response that is attributed to the accumulation of phytoalexins as a result of increased Phenylalanine Ammonia-Lyase activity, the key enzyme which leads to the synthesis of phenylpropanoid compounds [1,2,23].
Some authors [27,28] report the loss of the hormetic effect in fruits irradiated by UV-C and exposed to visible light. Namely, Stevens et al. [28] note that in peach fruits irradiated with hormetic doses of UV-C at 254 nm, the protective effect from Monilia frutticola disappears after exposure to visible light for 48 hours. In contrast, our results show that the hormetic effect of UV-C irradiation at 277 nm lasts over time and does not appear to be limited by plant exposure to visible light. During the two months following irradiation, the presence of flavonols in the leaves of irradiated plants remained consistently higher than that of the unirradiated control, as shown in Table 2.
The effects of time and irradiation on the Flav-index were statistically analyzed by 2-way ANOVA and the results are reported in Table 3. Analyses showed a positive effect of time on the concentration of flavonoids: elapsed time was responsible for 44% of the total variance. One month after irradiation, the Flav index was significantly different between control and irradiated plants, and 24% of the total variance was caused by irradiation itself, showing that irradiation produced a significant effect. The interaction between time and irradiation was not significant. Flavonoids are effective antifungal agents against a wide range of pathogens. According to [29], they can reduce or inhibit fungal growth in several ways, including alterations of plasma membrane metabolism, changes in the efflux-mediated pumping system, induction of mitochondrial dysfunction, and RNA and protein synthesis. Recently, Wang et al [30] reported natural flavonoid extracts that can inhibit the growth of B. cinerea by negatively affecting its cell membrane lipid metabolism.
Flav: variation source
% of total change
P-value
Time
43.92
<0.0001***
Irradiation
23.86
0.0008**
Time*Irradiation
1.32
0.3661 ns
Table 3: Variation of the Flav index due to time since irradiation, UV-C irradiation, and interaction between time and irradiation. ns = not significant; **=0.01 ≤P ≤0.05; ***= P≤0.0001.
The values of the SFR_R index 31], which is proportional to the chlorophyll content in plant leaves, are reported in Table 4, showing an appreciable decrease over time.
Day after irradiation
Sample treatment
SFR_R
t-value
40
Irradiated
1.219±0.08
2.455*
Control
1.061±0.15
50
Irradiated
0.939±0.05
1.073*
Control
0.803±0.20
60
Irradiated
0.659±0.15
1.169 ns
Control
0.761±0.14
Table 4: SFR_R index ±Standard Deviation of the mean of three repetitions, calculated from the results of Multiplex analysis on irradiated and control basil plants on different days after UV_C irradiation. ns=Not Significant; *=P≤0.05.
The decrease in chlorophyll is also observed in the unirradiated control, and it is attributable to natural leaf senescence. The dose of 0.33 kJ m-2 was not sufficient to stimulate new chlorophyll production. For example, in Chinese cabbage plants (Brassica oleracea var. Alboglabra) irradiated at much higher doses of 3.6 and 5.4 kJ m-2, Chairat et al. [32] observed a delayed yellowing of the leaves probably due to lower activity of chlorophyllase, an enzyme found in chloroplasts that degrades chlorophyll. Costa et al. [33] observed the positive effect of UV-C at 10 kJ m-2 on chlorophyll degradation and yellowing in broccoli, due to the inhibition of catalysis enzymes.
Preliminary Irradiation Test on Apples
As a preliminary investigation, four apples (Malus domestica Golden Delicious variety) from organic farming were irradiated with a low hormetic dose of 0.30±0.03 kJ m-2 (exposure time 11 s) and immediately inoculated with B. cinerea. At the same time, four unirradiated apples were inoculated and used as a control. Fifteen days after inoculation, all unirradiated controls had typical disease symptoms, while in 75% of the irradiated apples fungal development was almost absent and minimal in the remaining 25%, see Figure 5. After one month, the percentage of necrosis detected on the irradiated apples was 60% compared to 100% in the unirradiated control. As UV-C radiation is absorbed in a very thin layer of most biological materials, in this experiment UV-C rays are entirely absorbed by the exocarp [34], and the hormetic effect is limited to the surface of the apples. Figure 5 shows that on the one hand, the growth of the pathogen was inhibited or significantly slowed down by UV-C irradiation, on the other hand, in 25% of cases, the pathogen penetrated the thin exocarp barrier in a much longer time than in unirradiated apples, and reached the fruit. This can cause the apple to show symptoms far from where inoculated, such as the middle apple in the bottom row of Figure 5. An important factor is the physiological state of the apples at the time of irradiation. For example, de Capdeville et al. [35] observed that fresh apples were more responsive to UV-C treatment than apples stored for 3 months in a controlled atmosphere environment.
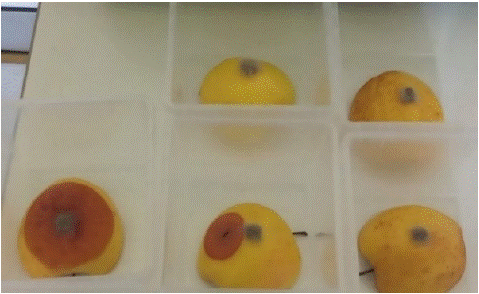
Figure 5: Malus domestica (Borkh.) fruits 15 days after inoculation with pieces of B. cinerea mycelium. One not irradiated control is on the left. On the right are the four apples subjected to 11 seconds of UV-C irradiation before inoculation.
Preliminary Irradiation Test on Lemons
Citrus spp. is one of the most popular fruits in more than 100 countries worldwide, also consumed for its health benefits. Due to fruit diseases and metabolic disorders, postharvest losses can reach 30-50% of total production [36]. Therefore, it would be important to extend the shelf life of high-quality fruit, because reducing losses has relevant implications for a sustainable production system.
As a preliminary investigation, four lemon fruits (Citrus limon (L.) Femminello variety) from organic farming were irradiated with a low hormetic dose of 0.30 ± 0.03 kJ m-2 (exposure time 11 s) and immediately inoculated with mycelium dowels of P. digitatum, the agent causing green mold. Unirradiated lemons were inoculated at the same time and used as a control. Forty days after treatment, the fungus has invaded the entire surface of the unirradiated lemons (Figure 6, left). In contrast, the irradiated lemons (Figure 6, right) show complete inhibition of pathogen growth.
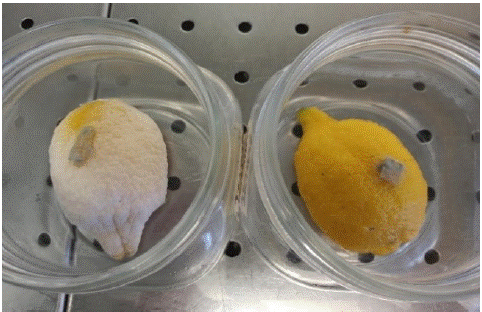
Figure 6: Citrus limon (L.) 40 days after inoculation with green mold mycelium. The lemon on the right was subjected to 11 seconds of UV-C irradiation before inoculation. The not irradiated control is on the left.
In the literature, there are examples of high-dose UV-C irradiation being able to limit the development of P. digitatum in Citrus spp [36]. The resistance to the pathogen has been attributed to the accumulation of phytoalexins such as scoparone and scopoletin [37,38]. Phonyiam et al [39] showed that application of a UV-C dose of 10 kJ m-2 on Satsuma mandarins was able to reduce green mold growth due to increased accumulation of jasmonic acid and bioactive compounds with high antioxidant activity. They also reported a reduction in lipid peroxidation, which helped maintain the integrity of the membrane structure. Similar results were previously reported by Barka et al. [40], who demonstrated the effect of UV-C irradiation (254 nm) on cell wall degradation enzymes, which led to a delay in fruit ripening and senescence process. The application of UV-C irradiation before storage, with a dose ranging from 3.4 to 10.5 kJ m-2, helped preserve the quality of lime fruits during storage [41]. In addition, UV-C technology has been extensively studied as an alternative pasteurization treatment and extension of beverage shelf life [42].
Discussion
An important question is whether there are any disadvantages or limitations to using low-dose UV-C radiation for plant growth and pathogen inhibition.
Regarding plant growth, in our experiments the value of the dose that generated hormesis is more than thirty times lower than the dose that can harm the plant. Therefore, the use of hormesis doses excludes unintentional damage to plant health.
In addition, one may wonder whether the cost of LEDs is competitive with that of mercury lamps, and whether there are potential health problems for operators when using LEDs in the field.
As for the cost of LEDs, it is no longer an issue because their massive use in various applications has generated a permanent trend toward cost reduction, so much so that today the cost of LEDs is a negligible part of the total cost of an automated machine to irradiate crops with UV-C light. Regarding operator health, the machines currently used to irradiate crops with UV-C lamps are automatic and driverless. The operator is away from the machine while it is operating. The lamps can be easily replaced by LEDs in these machines.
Conclusions
This paper summarizes the effects of 277-nm UV-C irradiations of basil, apples, and lemons followed by inoculation of the fungal pathogens B. cinerea and P. digitatum. The UV-C source is the compact 90×34 mm² LEDs matrix shown in Figure 2, and the UV-C dose delivered is much lower than those reported in literature, to test the possibility of reducing the irradiation time to achieve rapid treatments of crops.
Basil plants were irradiated with a dose of 0.33±0.03 kJ m-2, as accurately measured by the absolute power meter, and released in a time ranging from 3 to 14 seconds. Multiplex analysis revealed that the irradiated basil plants showed a significant increase in flavonols content compared with the unirradiated control. This means that a dose of 0.33 kJ m-2 of radiation at 277 nm generated metabolites with antimicrobial and antioxidant activities that contribute to the protection of basil plants. In contrast, the dose value was not sufficient to retard chlorophyll degradation.
Post-harvest lemons and apples were irradiated with a dose of 0.30±0.03 kJ m-2, released in 11 seconds. Forty days after P. digitatum inoculation, irradiated lemons show complete inhibition of pathogen growth, while green mold invaded the entire surface of the unirradiated lemons. Fifteen days after B. cinerea inoculation, fungal development was absent in 75% of irradiated apples and minimal in the remaining 25%, while unirradiated apples showed extensive necrosis over most of the surface of the fruits. The hormetic results in postharvest apples and lemons are very encouraging, and are in agreement with the literature. Further irradiations to confirm these results and investigate in detail the mechanisms of hormesis as a sustainable technique of fruit preservation are planned. The results presented in this paper show that preventive irradiation by LED UV-C is beneficial to limit crop diseases evaluated in this study in both pre-harvest and post-harvest, without harming the plant or the environment, as a sustainable alternative to pesticides. However, the work was carried out under laboratory conditions, and further research is needed to confirm the efficacy and safety of using low-dose UV-C radiation emitted by LEDs for plant growth and pathogen inhibition in real agricultural systems. In this context, the use of LEDs in field irradiations is facilitated by the availability of driverless, automated irradiation systems that currently use mercury lamps, as the replacement of mercury lamps with arrays of LEDs is straightforward.
Author’s Statements
Author Contributions
All authors contributed equally to the measurements and writing of the article
Funding
This research received no external funding
Conflicts of Interest
The authors report there are no competing interests to declare.
Data Availability
The data that support the findings of this study are available from the corresponding author, [PDL], upon reasonable request.
References
- See European Commission. Farm to Fork strategy.
- Stevens C, Khan VA, Lu JY, Wilson CL, Chalutz E, Droby S, et al. Induced resistance of sweet potato to Fusarium root rot by UV-C hormesis. Crop Prot. 1999; 18: 463-70.
- Calabrese EJ. Hormesis: changing view of the dose-response, a personal account of the history and current status. Mutat Res. 2002; 511: 181-9.
- Shama G, Alderson P. UV hormesis in fruits: a concept ripe for commercialisation. Trends Food Sci Technol. 2005; 16: 128-36.
- Turtoi M. Ultraviolet light treatment of fresh fruits and vegetables surfaces: a review. J Agroaliment Processes Technol 19. 2013;325-337.pdf:325-37.ro/admin/articole/22340L53_Vol_19_3.
- Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? npj Aging Mech Dis. 2017; 3: 13.
- Duarte-Sierra A, Charles MT, Arul J. UV-C hormesis: a means of controlling diseases and delaying senescence in fresh fruits and vegetables during storage. In: Palou L, Smilanick J, editors. Postharvest pathology of fresh horticultural produce. 1st ed ed. Boca Raton, FL: CRC Press. 2019; 539-94.
- Scott G, Dickinson M, Shama G. Preharvest high-intensity, pulsed polychromatic light and low-intensity UV-C treatments control Botrytis cinerea on lettuce (Lactuca sativa). Eur J Plant Pathol. 2021; 159: 449-54.
- Sidibé A, Charles MT, Lucier JF, Xu Y, Beaulieu C. Preharvest UV-C hormesis induces key genes associated with homeostasis, growth, and defense in lettuce inoculated with Xanthomonas campestris pv. vitians. Front Plant Sci. 2021; 12: 793989.
- United Nations (accessed on Jun 6]. Available from: https://www.mercuryconvention.org, Minamata Convention on Mercury. 2023; 2021.
- Williamson B, Tudzynski B, Tudzynski P, Van Kan JAL. Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol. 2007; 8: 561-80.
- Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, et al. The Top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012; 13: 414-30.
- Li H, Chen Y, Zhang Z, Li B, Qin G, Tian S. Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual Saf. 2018; 3: 111-9.
- Poveda J, Barquero M, González-Andrés F. Insight into the microbiological control strategies against Botrytis cinerea using systemic plant resistance activation. Agronomy. 2020; 10: 1822.
- Sánchez-Torres P. Molecular mechanisms underlying fungicide resistance in citrus postharvest green mold. J Fungi (Basel). 2021; 7: 783.
- Costa JH, Bazioli JM, de Moraes Pontes JG, Fill TP. Penicillium digitatum infection mechanisms in citrus: what do we know so far? Fungal Biol. 2019; 123: 584-93.
- Sholberg PL, Bedford K, Stokes S. Sensitivity of Penicillium spp. and Botrytis cinerea to pyrimethanil and its control of blue and gray mold of stored apples. Crop Prot. 2005; 24: 127-34.
- Harper LA, Paton S, Hall B, McKay S, Oliver RP, Lopez-Ruiz FJ. Fungicide resistance characterized across seven modes of action in Botrytis cinerea isolated from Australian vineyards. Pest Manag Sci. 2022; 78: 1326-40.
- Nybom H, Ahmadi-Afzadi M, Rumpunen K, Tahir I. Review of the impact of apple fruit ripening, texture and chemical contents on genetically determined susceptibility to storage rots. Plants (Basel). 2020; 9: 831.
- See Luminus product datasheet, available on line [cited Jun 6 2023]. Available from: https://download.luminus.com/datasheets/Luminus_XBT-3535-UV_Datasheet.pdf.
- See Multiplex UV-visible portable fluorometer [cited Jun 6 2023]. Available from: http://www.misure.net/sites/default/files/pdf/Brochure%20Multiplex.pdf.
- Garibaldi A, Gullino ML, Minuto G. Diseases of basil and their management. Plant Dis. 1997; 81: 124-32.
- Farr DF, Rossman AY. Fungal databases. Systematic Mycology and Microbiology Laboratory, Anaesthetics Research Society, United States Department of Agriculture; 2015. Available from: http://nt.ars-grin.gov/fungaldatabases/.
- de Oliveira LE, Salcedo-Sarmiento S, Weingart Barreto R. Botrytis cinerea causes grey mould on basil (Ocimum basilicum) in Brazil. Australas Plant Dis Notes. 2021; 16: 14-8.
- Cerovic ZG, Ounis A, Cartelat A, Latouche G, Goulas Y, Meyer S, et al. The use of chlorophyll fluorescence excitation spectra for the nondestructive in situ assessment of UV–absorbing compounds in leaves. Plant Cell & Environment. 2002; 25: 1663-76.
- Legrand M. Phenylpropanoid metabolism and its regulation in disease. In: Callow J.A. Ed., Biochemical Plant Pathology (Wiley, New York). 1983; 367-85.
- Wojciech J, Takeda F, Glenn DM, Camp MJ, Jurick II WM. Dark period following UV-C treatment enhances killing of Botrytis cinerea conidia and controls gray mold of strawberries. Phytopathology. 2016; 106: 386-94.
- Stevens C, Khan VA, Lu JY, Wilson CL, Pusey PL, Kabwe MK, et al. The germicidal and hormetic effects of UV-C light on reducing brown rot disease and yeast microflora of peaches. Crop Prot. 1998; 17) :75-84.
- Aboody MSA, Mickymaray S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics (Basel). 2020; 9: 45.
- Wang K, Zhang X, Shao X, Wei Y, Xu F, Wang H. Flavonoids from Sedum aizoon L. inhibit Botrytis cinerea by negatively affecting cell membrane lipid metabolism. Appl Microbiol Biotechnol. 2022; 106: 7139-51.
- Buschmann C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth Res. 2007; 92: 261-71.
- Chairat B, Nutthachai P, Varit S. Effect of UV-C treatment on chlorophyll degradation, antioxidant enzyme activities, and senescence in Chinese kale (Brassica oleracea var. alboglabra). Int Food Res J. 2013; 202: 623-8.
- Costa L, Vicente AR, Civello PM, Chaves AR, Martínez GA. UV-C treatment delays postharvest senescence in broccoli florets. Postharvest Biol Technol. 2006; 39: 204-10.
- Shama G. New role for UV? Shelflife extension of plant foods by UV-induced effects. IUVA News vol. Available from: https://uvsolutionsmag.com/stories/pdf/archives/110301Shama_Article.pdf. Vol. 11(3); 2009.
- de Capdeville G, Wilson CL, Beer SV, Aist JR. Alternative disease control agents induce resistance to blue mold in harvested’red delicious apple fruit. Phytopathology. 2002; 92: 900-8.
- Strano MC, Altieri G, Allegra M, Di Renzo GC, Paterna G, Matera A, et al. Postharvest technologies of fresh citrus fruit: advances and recent developments for the loss reduction during handling and storage. Horticulturae. 2022; 8: 612.
- Rodov V, Ben-Yehoshua S, Kim JJ, Shapiro B, Ittah Y. Ultraviolet illumination induces scoparone production in kumquat and orange fruit and improves decay resistance. J Am Soc Hortic Sci. 1992; 117: 788-92.
- D’hallewin G, Schirra M, Manueddu E, Piga A, Ben-Yehoshua S. Scoparone and scopoletin accumulation and 402 ultraviolet-C induced resistance to postharvest decay in oranges as influenced by harvest date. J Am Soc Hortic Sci. 1999; 124: 702-7.
- Phonyiam O, Ohara H, Kondo S, Naradisorn M, Setha S. Postharvest UV-C irradiation influenced cellular structure, jasmonic acid accumulation, and resistance against green mold decay in satsuma mandarin fruit (Citrus unshiu). Front Sustain Food Syst. 2021; 5: 684434.
- Barka EA, Kalantari S, Makhlouf J, Arul J. Impact of UV-C irradiation on the cell wall-degrading enzymes during ripening of tomato (Lycopersicon esculentum L.) fruit. J Agric Food Chem. 2000; 48: 667-71.
- Pristijono P, Bowyer MC, Papoutsis K, Scarlett CJ, Vuong QV, Stathopoulos CE, et al. Improving the storage quality of Tahitian limes (Citrus latifolia) by pre-storage UV-C irradiation. J Food Sci Technol. 2019; 56: 1438-44.
- Bazaraa WA, Eissa HA, Helmy SA, Ramadan MT, Aboelhaggag RM. Effect of ultra violet (UV-C) and cold storage on orange juice quality. Food Sci Technol Int. 2022; 29: 757-764.