
Special Article: Plant Cell
Ann Agric Crop Sci. 2024; 9(1): 1147.
Azulenes in Plant Cell: Clover as Their Useful Resource
Victoria V Roshchina*
Institute of Cell Biophysics, Federal Research Centre, Pushchino Scientific Centre for Biological Research, Russian Academy of Sciences, Institutskaya Str. 3, Pushchino, Moscow Region, 142290, Russia.
*Corresponding author: Victoria V Roshchina Institute of Cell Biophysics, Federal Research Centre, Pushchino Scientific Centre for Biological Research, Russian Academy of Sciences, Institutskaya Str. 3, Pushchino, Moscow Region, 142290, Russia. Email: roshchinavic@mail.ru
Received: December 05, 2023 Accepted: January 18, 2024 Published: January 24, 2024
Abstract
Location of azulenes in plant cell has been studied on three clover species Trifolium repens L., T. pratense L., and T. medium L. (fam. Fabaceae). By using of microspectrophotometer/ microspectrofluorimeter the absorbance spectra were recorded from the surface of intact leaves and intact isolated chloroplasts. Here the maxima in the range 580-620 nm, peculiar to blue pigments azulenes, were observed in all cases. The pigments’ picks 590-610 nm also have been found in the absorbance spectra of ethanolic extracts from the leaf surface and from the chloroplast suspension. The protector function of the azulenes for the plant cell has been discussed. Clovers studied are recommended for agriculture and pharmacy as useful resource of azulenes.
Keywords: The absorbance spectra; Cell surface; Chloroplasts; Microspectrophotometry
Introduction
The blue pigments azulenes are found in many types of plants, fungi, and invertebrate organisms [1,2]. Due to its physicochemical properties, azulene and its derivatives with many double bonds and their conjugated biologically active compounds have found application in technology, especially in optoelectronic devices [1-6]. Azulenes are also included in polymers, oligomers and conjugates with furanes and phenols in order to create new chromophore materials [2,3,5].
History of azulene-containing plants is beginning in ancient times for medical and cosmetic purposes. Natural azulenes and synthesized, such as azulenic retinoids, may have antitumor activity, immunomodulatory, and antifungal activity (guaiazulene derivatives isolated from the marine gorgonian) [4] or have an antioxidant effect [5] or affect dopamine receptors [6]. Moreover, in some cases they can affect other living organisms, including negative effects [7]. The technological direction is associated with the study of the physicochemical properties of azulenes and their application in the optical and chemical-analytical industries [2,3].
Unlike a burst in technological use of azulenes, there is small information about biological role of azulenes in plants themselves, especially of medicinal ones. For many years, chemists discovered azulenes by distilling essential oils from the medicinal herbs of wormwood bitter Artemisia absinthium L. or common yarrow Achillea millefolium L, which acquired a blue color [8]. But such pigments were also found in extracts of organic solvents in mosses of the hepatic Calypogeia azurea and others [9,10], pollen of different flowering species and pollen collected by bees [11, 12], cells of horsetail microspores Equisetum arvense L. [13], needles of the blue spruce Picea excelsa [14], isolated chloroplasts of pea Pisum sativum L. and clover Trifolium repens L. [14,]. The role of azulenes for the plants themselves has not yet been considered in the literature, although there is only one interpretation of their properties as growth regulators belonging to water soluble azulene [15] and protocols of the studies of the artificial pigment on the germination of pollen and vegetative microspores of horsetail [16,17]. In experiments with exogenous azulene on vegetative microspores of horsetail, the properties of this sesquiterpene lactone as antioxidant and as a histamine antagonist are shown [18]. Moreover, it may be the electron donor in model experiments on isolated chloroplasts [19-21].
In 2019-2023 there were works that search azulenes in plants, which have been connected with tolerance to tropospheric ozone and ultra-violet irradiation [22]. It was shown that short time-extracts by organic solvents from leaves having blue color in some woody plants contained azulenes [22]. The fact gives us the idea that their tolerant ability may be connected with the defensive role of azulenes. Short time of the azulene appearance in 10 min-extracts showed a possibility of the compounds’on the leaf surface as a barrier or filter for active ultra-violet radiation and ozone as the origins of dangerous oxidants [20-23]. The surface in transmitted light was analyzed by sensitive Invitro Evos M5000 microscope showed the presence of blue wax plates on the leaf surface of Eucalyptus cinerea, in both covered secretory structure (glands) and parts lack of the glands [20,22]. Also it has been carry out spectral analysis with use microspectrophotometer/microspectrofluorimeter for the receiving of spectra of autofluorescence and absorbance in order to test the composition of compounds outside the cell wall [21,23,26].
Although today this pigment is known and applied for medicine, cosmetics and technics, the attention to the functions and location of the natural metabolite for the plant itself was far from the study many years. It may be useful for practical use of our knowledge for agriculture of medicinal and nutritional plant species. In this connection we had a goal to analyse the location of azulenes in plant cell using three species of clover. For this task, it should need to observe the leaf surface and penetrate into the cells of model objects for the isolated organelles such as chloroplasts.
Materials and Methods
Objects
Leaves and isolated chloroplasts from three clover species were chosen: white clover Trifolium repens L., red clover Trifolium pratense L., and zigzag clover Trifolium medium L. collected on natural reservation “Lugovoi” near Oka river.
Isolation of Chloroplasts
Isolation of intact chloroplasts from leaves was carried out according to Robinson with co-workers [27] in the phosphate buffer medium containing 0.3 M mannitol,0.08 M KCl, 0.066 M KH2PO4, 0.066 M Na2HPO4 pH 7.25 .
Spectral Measurements
Absorption (absorbance) of the intact leaves was measured directly on slides using the microspectrophotometer/microspectrofluorimeter MSF-15 (LOMO, St. Petersburg, Russia) [20,21]. The position of the maxima in the absorption spectra of intact cell surfaces was determined according to the Zolotarev method by the option of the reflection spectra differentiation [28]. The absorption and fluorescence spectra of extracts with 100% acetone or 95% ethanol from cells (1:10 w/v for 10 min to 1 hour or more) in 1-0.5 cm cuvettes or on paper chromatograms were recorded using the Unicam Helios-epsilon spectrophotometer (USA), spectrophotometer Specord M-40 (Germany) and Perkin Elmer 350 MPF-44 B spectrofluorometer (Great Britain) [20-23].
Extractions of Azulenes
Azulenes in sample extracts with ethanol or acetone for 10-30 minutes of exposure were determined spectrophotometrically at 580 nm, as described earlier [22]. The average error of the experiment of three to four repetitions was calculated for each variant and control, respectively.
Detection of Azulenes
Azulenes in sample extracts with 95% ethanol and acetone for 10-30 minutes of exposure were determined spectrophotometrically at 580 nm, as described earlier [20-22]. To detect azulenes, the extracts were chromatographed on Whatman Paper No. 1 without or after the impregnation with Vaseline oil or on thin-layer plates of Silufol silica gel, as previously described for pollen of a number of seed plants and horsetail microspores [11-13]. Their absorbance spectra were then recorded to compare them with data obtained on intact cells. The concentration of azulenes (A) was estimated in solutions of ethanol or acetone extracts, according to the formula: A=D580 /e×l, where D580 is the optical density at 580 nm, e is the coefficient of molar extinction of 328 M–1 cm–1, l is the thickness of the liquid layer in the cuvette, cm. The average error of the experiment of three to four repetitions was calculated for each variant and control, respectively.
Results and Discussion
Since from 50-60 years of 20 century scientific interest to azulenes based on the pharmacological knowledge and experiments with the blue pigments as by -products of distillated plant oil [3]. Here we applied spectral methods to testing of presence of azulenes using microspectrophotometer/microspectrofluorimeter MSF-15 [20-22 below we represented new data received not only leaf surface, but also intact chloroplasts of three clover species.
Spectral Analysis of Plant Cells Surface and Isolated Chloroplasts
Spectral studies of leaf surfaces and isolated chloroplasts were carried out by microspectrophotometry, which makes it possible to record the absorbance spectra in individual cells. On Figure 1-3 visible leaf absorbance spectra of three clover species and isolated from intact chloroplasts with marked areas of absorption of azulenes and chlorophyll with significant maxima, indicated by circles and polygons, respectively.
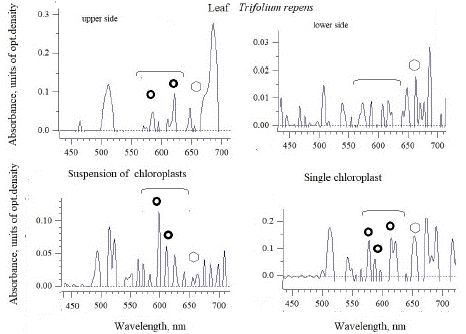
Figure 1: The absorbance spectra of white clover (T. repens) leaf and intact chloroplasts isolated from the leaves of this species. The absorption regions with maxima related to azulene and chlorophyll, were marked circles and polygons, respectively.
In white clover, the maxima of 580-620 nm on the upper side of the leaf and the maximum of 660 nm related to chlorophyll are already visible in the intact leaf. However, on the upside of the leaf (it looks whitish, and only small maxima are visible in the absorption region of both azulenes and chlorophyll). These data for blue pigments of azulenes refer mainly to the surface of the leaves [20-22], and the presence of these compounds inside the cell should be assessed. When comparing the nature of the absorption spectra of the leaf surface and the isolated chloroplasts (Figure 1), it is clearly seen that about five to ten times more significant maxima 600 and 615 nm appear in the spectrum of the intact chloroplasts’ suspension. Moreover, the absorbance spectrum of single chloroplasts, apparently, contains even more significant and noticeable maxima of 580,590 and 615 nm, characteristic of azulene.
In the contrast to white clover, red or meadow clover (Figure 2) showed lower maxima of azulenes and chlorophyll in the absorbance spectra not on the lower, but on the upper side of the leaf, (these maxima are 580 and 615 nm), where there is a significant part of whitish spots. The absorbance spectrum of the suspension has already shown a higher maximum of 615 nm, and in single chloroplasts - three significant maxima of 580, 600 and 620 nm, characteristic of azulene. The differences may be in the fact that a maximum of 615 nm is visible in the mass of chloroplasts with an intact outer membrane, while the spectrum from a single organelle can also show the contribution of azulenes of internal membranes-thylakoids
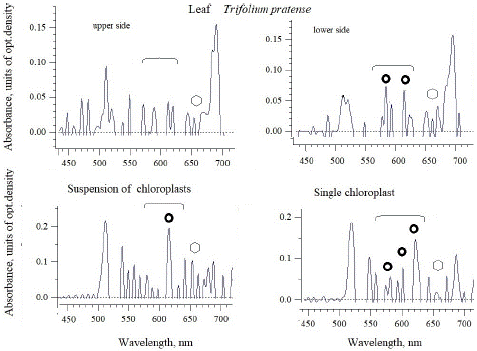
Figure 2: The absorbance spectra of red clover (T.pratense) leaf and intact chloroplasts isolated from the leaves of this species. The absorption regions of azulenes and chlorophyll with maxima were marked as circles and polygons, respectively.
For zigzag clover T. medium, there are the absorption maxima of azulenes from both leaf surfaces (upper and lower ones), but they are small for quantitative assessment (Figure 3), but quite comparable to the magnitude of the chlorophyll peak. A significant and noticeable increase in the maxima in the absorption region of azulenes (as well as chlorophyll at 666 nm) was noted in the suspension of chloroplasts – 580, 595 and 620 nm. However, single chloroplasts have the highest maximum of 610 nm inherent in azulene, and it is almost twice as high as the actual chlorophyll maximum of 666 nm.
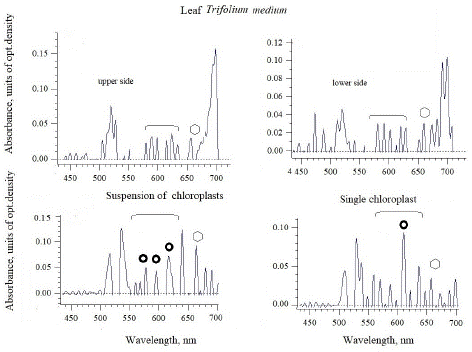
Figure 3: The absorbance spectra of zigzag clover leaf and intact chloroplasts isolated from the leaves of this species. The absorption regions of azulenes and chlorophyll with maxima, were marked as circles and polygons, respectively.
Plant surface and chloroplasts may be possible accumulators of blue pigments. Earlier we studied the plant responses to ozone, modelling its negative effects on the individual cells, and showed how the atmospheric gas acts on the leaves washings from woody plants [22]. Moreover, under O3 –action, the color and fluorescence of the surface secretory and non-secretory systems of were changed that was suitable for the express-testing of cell damage [22]. Among the woody species studied some tolerant to ozone have silver or blue color of leaves and contain azulenes washed by organic solvents from their surface. We concluded that the compounds may be optic and antioxidant filters for action of unfavorable factors such as tropospheric ozone of urbanistic regions or/and ultra-violet irradiation.
Extraction of Blue Pigments
To confirm the testing of azulenes in intact leaves and isolated chloroplasts, pigments should be extracted with ethanol or acetone [22]. As a whole there was no chlorophyll in 10 min-extracts from the intact leaves of woody plants with silver or blue color (chlorophyll was determined by the absorbance and fluorescence spectra with maxima of 660 and 680 nm, respectively). The appearance of chlorophyll in this extract already indicates the penetration of the solvent inside cell to chloroplasts [22]. In our experiments the absorbance spectra of 10 min - ethanolic extracts from the surface of intact leaves or isolated chloroplasts of three clover species have maxima in the range 580-620 nm, and we use the value of optic density at 580 nm for the determination of the pigment concentration (Table 1).
Species
Leaves
?hloroplasts
Trifolium repens
2,8±0.03
38±2.0
2,4±0.03
170±5.3
0.24±0.04
200± 9.2
Table 1: The content of azulenes in 10-minute ethanol extracts (mg/g of fresh leaf weight) from the surface of leaves or chloroplasts.
preliminary estimation of the content of azulenes on the surface of leaves and chloroplasts based on fresh weight of leaves is shown in table 1. In 10-minute ethanol extracts (washes) from the surface of all three species studied, it can be seen that white clover Trifolium repens and red clover Trifolium pratense have about 10 times more blue pigments compared to Trifolium medium clove. In ethanol extracts from isolated intact chloroplasts, on the contrary, the plastids of the first species have the least azulene, while in the other two species it exceeds this value for blue pigments by 5-6 times. Moreover, it was noted that in all the studied samples, based on fresh weight, the concentration of azulenes in chloroplasts significantly (up to 20 or more times) exceeds that washed out from the surface of the leaves.
Thus, it can be concluded that azulenes are present in cells both in the surface layer of leaf cells (in the cell wall, and mainly in the cuticle) and inside the cells – in chloroplasts. In Table 1 we see the calculation based on fresh weight of leaves and on the surface of cells azulenes are more 10 times lesser then in chloroplasts.
If we refer to the data of Figures 1-3 for testing azulenes by the absorbance spectra of leaf surfaces, we can see that there is a correlation between the height of the maxima and the content of these pigments. Moreover, the height of the maxima can be judged on the ratio of pigments between those on the surface of the leaf and in the suspension of chloroplasts. In chloroplasts, a higher height of azulene peaks is noted than on the surface. This implies the reliability of testing the presence of azulenes by spectral characteristics. The color of leaves and chloroplasts is also determined by the ratio of azulene: chlorophyll. It depends on the thickness of the cuticle and cell wall [22]. Chlorophyll of chloroplasts shines through the cell wall covered with azulenes or waxes with azulenes, as in the ashy eucalyptus Eucalyptus cinerea [19-21]. Earlier it was shown, if the extraction from intact leaves with an organic solvent lasts 10-15 minutes, blue color and, accordingly, the maxima are 580-630 nm in the absorbance spectra were seen in extracts from silver or blue colored leaves of woody species [20-22]. Sometimes there was no chlorophyll in similar extracts (determined by the absorbance and fluorescence spectra with maxima of 660 and 680 nm, respectively), then we supposed that this part of the azulenes is located on the surface of the leaf cells. Often, for example, in eucalyptus ash, blue color is present in wax plates [20-22]. In 10 - minute ethanol extracts, many plants with a silver or blue color on the surface of leaves and needles do not yet have a chlorophyll maximum of 666 nm.
The absorption maxima of azulenes and chlorophyll can overlap in the region of 620-660 nm. After chromatography of the extract on Whatman 1 paper, azulenes and chlorophyll are usually visible as one common band. In view of this, their separation may be achieved by adding concentrated sulfuric acid to the extract up to 50% concentration, and the resulting brown solution is passed through a glass filter, where the precipitated azulene crystals remain on the filter, and the brown pheophytin formed in reaction with the acid is removed with the liquid.
The precipitate was again dissolved by passing an organic solvent through a filter - 95% ethanol or 100% acetone. The resulting blue solution contained pure azulenes; the probe is without chlorophyll. Chromatography of this blue solution (the example of zigzag clover Trifolium medium) on Whatman 1 paper led to separation into three main bands - with Rf 0.66, 0.44 and 0.12 (Figure 4). After chromatography one see that all three band have characteristic maxima 600-605 nm peculiar to azulenes, most intensive optical density was for first of them.
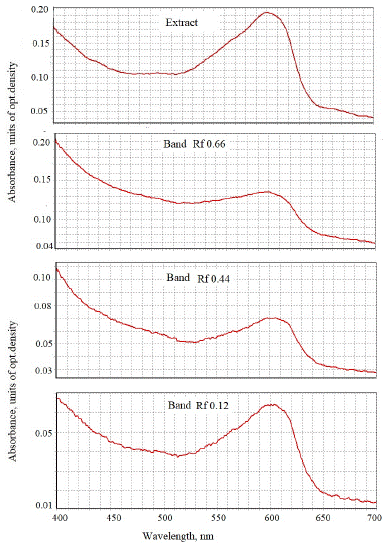
Figure 4: The absorbance spectra of blue bands after chromatographic purification of ethanol extract of intact chloroplasts of zigzag clover, pre-treated with 50% sulfuric acid on Whatman paper 1.
Discussion
The data received should be discussed in several directions in order to mark possible functions of azulenes in azulene-bearing plants such as clovers looking for perspectives for pharmacy.
Azulenes on the Plant Cell Surface
There is information from earlier publications [22,23] that some subtropical woody species are tolerant to intensive ultraviolet irradiation and tropospheric ozone, which may damage the leaves, contain azulenes on the leaf surface, for example, Eucalyptus cinerea, Picea excelsa and so on. When to compare the azulene content after short-time and long time-exposures in organic solvents, one could mark different time of the solvents ‘ way into the cell interior, controlled by the appearance of chlorophyll maxima in the extracts – from 10 min (average for Eucalyptus cinerea, Picea excelsa) to severel hours (for Cedrus atlantica).
Azulenes may be contained in cuticle and cell wall of plant cell. In some species studied they are included in wax covered all leaf surface like in Eucalyptus cinerea [26,24]. The pigments also may contacted and react with secretory products of the same cell releasing out and with exogenous metabolites (allellochemicals, biogenic amines, phenols, etc) such found on the leaf surface due to interactions with other inhabitants of biocenosis – plants, animals and microorganisms. They can protect from any damage because act as antioxidants [23-26].
Reactions to ozone of surface cells of leaves with blue or silver color in plant species: meadow rue Thalictrum minus L., oleaster Elaeagnus L., white willow Salix alba L., buckthorn Hippophae rhamnoides L., creeping clover or white clower Trifolium repens L, red clover Trifolium pratense L., ryegrass pasture Lolium perenne Engels were studied [23] . It has shown that the surface layers of the cuticle and the cell wall of these plants contained azulenes. In the leaf absorption spectra, the maxima characteristic of these blue pigments of 580-585 nm and 608-610 nm, respectively, were noted, and in the fluorescence spectra – 410 or 430 nm. It has assumed that these pigments may be the primary targets for ozone in species with blue or silvery leaf color, and their antioxidant properties determine low sensitivity to ozone. In this case, the blue pigments serve as a protective optical filter against ultraviolet radiation and ozone formed with its participation
The spectral characteristics of the surface cells of plants appeared on various evolutionary levels - from unicellular (diatoms, horsetail and fern spores) to multicellular (woody and herbaceous species) organisms- have been studied [20,24,25]. It was shown that the surface layers of the cuticle and cell wall of some analyzed plants included blue pigments azulenes as antioxidants.
Using histochemical methods, it has been discovered that here neurotransmitter compounds (biogenic amines) are present in the excretions by the entire surface or from specialized secretory structures of leaves [26]. Under conditions of high salt concentration, dopamine and histamine are secreted, which is blocked by the addition of exogenous azulene and proazulene grosshemine [18,20]. It is assumed that the azulene -containing surface protects it from the formed reactive oxygen species and toxic biogenic amines in high concentrations.
Azulenes in Chloroplasts
Azulenes can be found not only in the cuticle and cell wall, but also in chloroplasts and are somehow bound to chlorophyll as we saw in the experiments with ethanolic extracts from chloroplasts. Often it is difficult to separate both pigments by paper or thin laeyer chromatography without preliminary addition of acid that led to formation of pheophytin, which may be separated. Particular importance should be given to the relationship of chlorophyll and azulene in chloroplasts [26]. Figure 5 also shows their absorbance spectra of experiments earlier reported on the conference [26], the absorbance and fluorescence spectra were obtained in a mixture of individual azulene and a + b purified chlorophyll fraction. The absorbance spectra of artificial individual azulene include a total region of 650-680 nm, which can overlap a maximum of 665 nm of chlorophyll in the mixture (Figure 5). But the fluorescence spectra of solutions are very different, where azulene has a maximum in the region of 400-430 nm, and chlorophyll - 460-470 and 680 nm [20]. In the absorbance spectra of intact leaves in the range 630-650 nm the picks of azulene and chlorophyll may be overlapped as we saw on Fig.5 in experiments with individual compounds that reported on the conference materials [26]. We hypothesized [26] that in comparing of the molecules in both compounds there are common parts that may be originated from the common precursor in their metabolism. The 5-chains rings are present in them. Azulene moiety may serves as electron reservoir in positively charged systems [29].
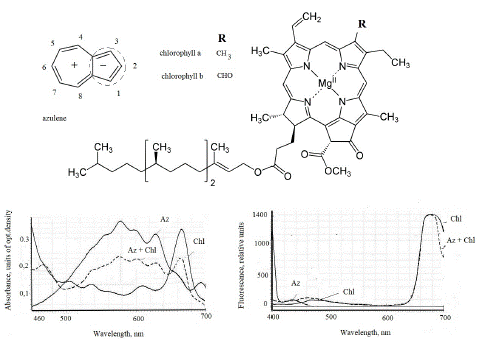
Figure 5: The absorbance and fluorescence spectra (excitation 360 nm) of solutions in azulene ethanol (2 mg) and chlorophyll a + b (2 mg) from common nettle leaves in a mixture (1: 1). Modified figure from conference material [26]. AZ and Chl – azulene and chlorophyll, relatively.
In chloroplasts azulenes and chlorophyll may form aggregates. According to experiments of Matenová et al [30] with aggregates of bacteriochlorophyll with azulene and azulene-derivatives, only azulenes with sufficient hydrophobicity are able to induce self-aggregation of bacteriochlorophyll C. Azulene derivatives possessing a conjugated phenyl ring were capable of efficient (50%) excitation energy transfer to bacteriochlorophyll molecules. These aggregates represent an artificial light-harvesting complex with enhanced absorption between 220 and 350 nm compared to aggregates of pure bacteriochlorophyll C. The results provide insight into the principles of self-assembly of similar aggregates and suggest an important role of the p-p interactions in efficient energy transfer. This fact explains difficulties in the separation of azulenes and chlorophyll by chromatography marked in our experiments because they may form self- assembled aggregations and aggregates between azulenes and chlorophyll. Since the azulene-containing fraction was isolated from isolated chloroplasts of clovers studied, it is likely that this pigment plays a role in thylakoids. It is possible to be a protective compound, since it has the properties of an antioxidant.
Besides antioxidant and aggregative capacities of azulenes, it should pay attention to redox characteristics of some azulenes in acetonitrile studied by Plemenkov with co-authors [31], who have shown their voltammograms. The large polarization of the p-electron system over the seven and five rings gives to azulene electrophile property a pronounced tendency to donate electrons to an acceptor, substituted at azulene 1 position (Figure 5). In some cases, azulene can transfer electrons to a suitable acceptor [29]. This supposition has been confirmed in several experimental publications demonstrated donor electron features in model system of isolated chloroplasts of Kalanchoe pinnata which lack of azulenes [19-21]. In experiments with additions of individual azulene to the chloroplasts and some individual components of photosynthetic electron transport chain (electron carriers cytochrome C 553 (f), plastocyanin and ferredoxin) and electron acceptors NADP+, ferricyanide and dichlorphenolindophenol the donation of electrons by azulene to electron transport chain has been shown. Block of the chain by inhibitors of non-cyclic and cyclic electron transport was overcome with azulene as electron donor. Therefore, the azulene presence in clover chloroplasts may defense function at the damage of electron transport chain.
Possible Practice of Azulenes
The azulene effects on the mammalians were rarely studied yet. In vitro and in vivo biological activities of azulene derivatives may be applied in medicine [1]. In medicine, the ingredients of these plants have been widely used for hundreds of years in antiallergic, antibacterial, and anti-inflammatory therapies [1]. Herein, the applications of azulene, its derivatives and their conjugates with biologically active compounds are presented. The potential use of these compounds concerns includes anti-inflammatory effects with peptic ulcers, antineoplastic with leukemia, antidiabetes, antiretroviral with HIV-1, antimicrobial, including antimicrobial photodynamic therapy, and antifungal [1]. Moreover, the antihistamine properties of azulene-containing medicinal plants like Artemisia absinthium, Achillea millefolium and Matricaria chamomilla in nutrition, cosmetics and dermatology may be explained by the presence of azulenes found here. Recently antihistaminic influence of azulene and natural proazulenes was demonstrated on the living model of single cell microspores of horse-tail Equisetum arvense [18].
In the paper, we consider perspectives for clovers cultivation, keeping in mind the dual roles of the azulene-containing plants – nutritional (the resource enriched in valuable proteins for the cattle fodder) and medicinal both for human and cattle-breeding. The plants are used in mixtures as herbal components only for folk medicine described in some reviews [32,33], it also may be used in official medicine too. In folk medicine dried clover leaves were used to relieve asthma. Tea made from the flowers, leaves and seeds was used as a remedy for the common cold, bronchitis and heartburn. Mainly, the known red clover - Trifolium pratense is used for the production of herbal medicines, an alternative to the conventional hormonal replacement therapy. The biological activity and potential therapeutic effects of other Trifolium species have gained a considerable scientific interest. Extracts obtained from various clovers have been shown to possess antioxidative and anti-inflammatory activities, inhibiting angiogenesis and displaying anti-cancer properties. Trifolium pratense has also gained popularity due to research into its use for the treatment for menopausal symptoms [34,35]. Dryedherb of Trifolium pratense is also used by Russian pharmacy [37] because it has expectorant, emollient, diuretic, diaphoretic, anti-inflammatory and antiseptic properties. In folk medicine, it is used as a general tonic for chronic lung diseases: tuberculosis, pneumonia, bronchitis, bronchial asthma.
The presence of azulenes in significant amounts both on the leaf surface and in chloroplasts in three studied Trifolium species should made the plants a perspective resource for the Future as a pharmaceutic raw. Extracts obtained from the aerial part of clovers are inexpensive and available. The economic importance lies in the availability and low cost of raw materials for the production of extracts [1].
Conclusion
The presence of azulenes in significant amounts both on the leaf surface and in chloroplasts in the clover species should made the plants a perspective resource for the Future as pharmaceutic raw. Described methods of their analysis with use of microspectrophotometry are recommended for the primary testing of the pigments in plants.
Author Statements
Acknowledgments
Many thanks to my constant helper - leading constructor Alexander R. Kun’ev for the support in tuning of microspectrophotometer. Author is also thankful to the Optical Microscopy and Spectrophotometry core facilities, ICB RAS, Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences” for possibility to work in optic department and especially to the department ingineer Nadezhda K. Prizova and technical assistant Lubov’ M. Khaibulaeva for their help.
Grants
Author has no grants for the experiments and writing of the paper.
Ethical Approval
The author declare that the study was carried out following scientific ethics and conduct. This study did not involve any use of animals, hence no ethical approval has been obtained from the concerned committee.
References
- Bakun P, Czarczynska-Goslinska B, Goslinski T, Lijewski S. In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med Chem Res. 2021; 30: 834-46.
- Murfin LC, Lewis SE. Azulene – a bright core for sensing and imaging. Molecules. 2021; 26: 353-62.
- Shoji T, Okujima T, Ito S. Development of heterocycle-substituted and fused azulenes in the last decade (2010-2020). Int J Mol Sci. 2020; 21: 7087-92.
- Asato AE, Peng A, Hossain MZ, Mirzadegan T, Bertram JS. Azulenic retinoids: novel nonbenzenoid aromatic retinoids with anticancer activity. J Med Chem. 1993; 36: 3137-47.
- Rekka E, Chrysselis M, Siskou I, Kourounakis A. Synthesis of new azulene derivatives and study of their effect on lipid peroxidation and lipoxygenase activity. Chem Pharm Bull (Tokyo). 2002; 50: 904-7.
- Löber S, Tschammer N, Hübner H, Melis MR, Argiolas A, Gmeiner P. The azulene framework as a novel are nebioisostere: design of potent dopamine d4 receptor ligands inducing penile erection. ChemMedChem. 2009; 4: 325-8.
- Sweet LI, Meier PG. Lethal and sublethal effects of azulene and longifolene to Microtox®, Ceriodaphnia dubia, Daphnia magna, and Pimephales promelas. Bull Environ Contam Toxicol. 1997; 58: 268-74.
- Konovalov DA. Natural azulenes. Rastitelnie resursi. Russia: Plant Resources. 1995; 31: 101-30.
- Nakagawa S, Katoh K, Kusumi T, Komura J, Nomoto K, Konno H, et al. Two azulenes produced by liverwort, Calypogeia azurea, during in vitro culture. Phytochemistry. 1992; 31: 1667-70.
- Siegel U, Mues R, Dönig R, Eicher Th, Blechschmidt M, Becker H. Ten azulenes from Plagiochila longispina and Calypogeia azurea. Phytochemistry. 1992; 31: 1671-8.
- Roshchina VV, Melnikova EV, Spiridonov NA, Kovaleva LV. Azulenes, the blue pigments of pollen. Dok Biol Sci. 1995; 340: 93-6.
- Roshchina VV, Melnikova EV, Kovaleva LV. The changes in the fluorescence during the development of male gametophyte. Russ Plant Physiol. 1997; 47: 45-53.
- Roshchina VV, Melnikova EV, Yashin VA, Karnaukhov VN. Autofluorescence of intact spores of horsetail Equisetum arvense L. during their development. Biophysics (Russia). 2002; 47: 318-24.
- Roshchina VV. Mechanisms of cell-cell communication. In: Narwal SS, editor. Allelopathy update. Enfield, NH: Science Publishers. 1999; 2: 3-25.
- Muir RM, Hansch C. Azulene derivatives as plant growth regulators. Nature. 1961; 190: 741-2.
- Roshchina VV. Cellular models to study the allelopathic mechanisms. Allelopath J. 2004; 13: 3-16.
- Roshchina VV, Yashin VA, Vikhlyantsev IM. Fluorescence of plant microspores as biosensors. Biochem (Mosc) Suppl Ser A Membr Cell Biol. 2012; 6: 105-12.
- Roshchina VV, Konovalov DA. Single Cell Plant Model of Equisetum arvense for the Study Antihistamine Effects of azulene and Sesquiterpene Lactones. Future Pharm. 2022; 2: 126-34.
- Roshchina VV. Possible role of azulenes in plant life: experiments with models. SMP Environ Sci Technol. 2022; 1: 1-10.
- Roshchina VV. Plant leaf surface as a sensory system in allelopathic relations: 1. Role of azulenes. Allelopath J. 2023; 59: 109-22.
- Roshchina VV, Yashin VA, Kunyev AR. Study of the Spectral characteristics of the plant cell surface: occurrence of azulenes and biogenic amines. Biochem Moscow Suppl Ser A. 2023; 17: 276-85.
- Roshchina VV, Kuchin AV, Kunyev AR, Soltani GA, Khaibulaeva LM, Prizova NK. The presence of azulene on the surface of plant cells as a test for ozone sensitivity. Biochem Moscow Suppl Ser A. 2022; 16: 167-74.
- Roshchina VV, Prizova NK, Khaibulaeva LM. Azulenes of the leaf surface as a protective optical filter. Russ J Biol Phys Chem. 2022; 7: 36-9.
- Roshchina VV, Soltani GA, Fateryga VV, Prizova NK, Khaibulaeva LM. Spectral studies of the cell surface to detect azulenes in plants. Bulletin of the Nikitsky Botanical Garden of Russian Academy of Sciences. 2023; 147: 90-5.
- Roshchina VV, Kunyev AR, Fateryga VV, Shovkun MM. Application of microspectrofluorimeter/ microspectrophotometer for the study of the surface of plant cells. Russ J Biol Phys Chem. 2023; 8: 137-42.
- Roshchina VV, Yashin VA, Kunyev AR, Fateryga VV, Soltani GA, Prizova NK, et al. Sensitivity of the surface of a plant cell – a sensor of neurotransmitters (biotransmitters). In: Berezhnov AV, Zinchenko VP, editors. Receptors and intracellular signaling. proceedings international conference. Vol. 2. Pushchino, Russia: Fifth Continent., Serpukhov. 2023; 657-62.
- Robinson SP, Edvards GE, Walkere DA. Established methods for the isolation of intact chloroplasts. In: Reid E, editor. Plants organelles. Chichester: Ellis Horwood. 1979; 13-24.
- Zolotarev VM. Application of differentiation in reflection spectroscopy. Opt Spectrosc. 2012; 112: 150-4.
- Razus AC. Azulene moiety as electron reservoir in positively charged systems; A short survey. Symmetry. 2021; 13: 526.
- Matenová M, Lorelei Horhoiu V, Dang FX, Pospíšil P, Alster J, Burda JV, et al. Energy transfer in aggregates of bacteriochlorophyll C self-assembled with azulene derivatives. Phys Chem Chem Phys. 2014; 16: 16755-64.
- Plemenkov VV, Yanilkin VV, Morozov VI, Palei RV, Maksimyuk NI, Bakopki BN. Reactions of single-electron oxidation and reduction of sulfides of the azulene series. Russ J Gen Chem. 2001; 71: 457-63.
- Sabudak T, Guler N. Trifolium L. a review on its phytochemical and pharmacological profile. Phytother Res. March 2009; 23: 439-46.
- Kolodziejczyk-Czepas J. Trifolium species-derived substances and extracts--biological activity and prospects for medicinal applications. J Ethnopharmacol. 2012; 143: 14-23.
- Lethaby A, Marjoribanks J, Kronenberg F, Roberts H, Eden J, Brown J. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013; 2013: CD001395.
- Newton KM. Isoflavones hold limited promise for the treatment of menopausal vasomotor symptoms. Evid Based Med. 2014; 19: 178.
- Kolodziejczyk-Czepas J, Sieradzka M, Wachowicz B, Nowak P, Oleszek W, Stochmal A. The anti-adhesive and anti-aggregatory effects of phenolics from Trifolium species in vitro. Mol Cell Biochem. 2016; 412: 155-64.
- https://zdorov.ru › catalog › klever-trava-72015.