
Special Article: Mixed Farming
Ann Agric Crop Sci 2024; 9(2): 1153.
Assessing the Efficacy of Mass Releases of Podisus maculiventris Treatments for Biocontrol of Mexican Bean Beetle Populations Infesting Snap Beans
Pasco B Avery1,2*; Erin Barbeau2; Lizbeth Ayala2; Paris L Lambdin1
¹Entomology and Plant Pathology, University of Tennessee, Knoxville, TN, USA
²Entomology and Nematology, University of Florida, Institute for Agricultural Sciences, Indian River Research and Education Center, Ft. Pierce, FL, USA
*Corresponding author: Pasco B Avery Entomology and Nematology, University of Florida, Institute for Agricultural Sciences, Indian River Research and Education Center, Ft. Pierce, FL, USA. Tel: 772-577-7335 Email: pbavery@ufl.edu; pascoavery@yahoo.com
Received: February 16, 2024 Accepted: March 16, 2024 Published: March 22, 2024
Abstract
all EMR treatments ranged from 60-94% compared with the DWGF treatments (0-25%) for both years. SSB nymphs become predacious as 3rd instars and the ratio of SSB 3rd instars available for EMR: DWGF middle and highest treatments at 7 days post-egg deposition (PED) was 39:1 for Year 1 and 20:0 for Year 2. A decrease in the number of SSB nymphs observed for 7 days PED was negatively correlated to heavy rainfall and other abiotic factors during both year evaluations. Overall, seasonal abundance of the MBB stages was lower on plants with the middle and highest treatment of 125 and 250 total eggs per plot for both years; however, there was no significant differences (P > 0.05) noted among means for the defoliation index. Mean pod yield was significantly greater for treatments containing 125 and 250 total eggs per plot, compared to all the different rates of dewinged gravid female releases and no release. SSB clearly demonstrated potential for use as a biological control agent against MBB populations infesting snap beans. SSB egg mass release treatments did appear to be a promising method for augmentative biological control of MBB for areas where snap beans are economically important. However, practical application of augmentative releases of SBB for control of MBB on snap beans will require further research. This will include the timing of releases, the numbers required, methods of mass production, distribution, and evaluation of field efficacy.
Keywords: Epilachna varivestis; Mexican bean beetle; Podisus maculiventris; spined soldier bug; Phaseolus vulgaris; snap beans; dewinged gravid females; egg mass releases; cold storage; dispersal; biological control; defoliation index
Abbreviations: MBB: Mexican bean beetle; SSB: spined soldier bug; DWGF: dewinged gravid females; EMR: egg mass releases; PED: post-egg deposition; BCA: biological control agent; DI: defoliation index
Introduction
The Mexican bean beetle, Epilachna varivestis Mulsant (Coleoptera: Coccinellidae) which first appeared in Virginia in 1922 and became established in the eastern part of the state in 1928 [1], is an annual invasive pest found on snap beans, Phaseolus vulgaris L. produced in the southeastern United States. In addition, in 1922, this beetle pest had invaded Georgia, North Carolina, South Carolina, Virginia, Tennessee, and Kentucky [2]. Between 1925-1929, the beetle had spread to Ohio, Pennsylvania, New Jersey, and Connecticut. At present, the beetle is established all over the continental United States. In Canada, this invasive beetle is a common pest found in the eastern provinces, from Ontario to New Brunswick to British Columbia [2]. Adults and larvae feed on plant tissue with chewing mouthparts; however, Howard [3] described the mechanism as more like the rasping and sucking technique used by thrips. Beetles use their mandibles to scrape the leaf surface, piling plant tissue together, compress the dislodged tissue, and extract the plant juices. The plant juices are ingested, while solid matter is discarded.
Most of the feeding injury of the Mexican bean beetles on bean plants occurs with third and fourth instar larvae [4]. Beetles generally feed on the lower leaf surface while avoiding veins, creating a lacy, skeletonized appearance of the remaining leaf [3]. Foliar feeding injury results in decreased photosynthetic activity and desiccation of the plant [5]. Though beetles feed primarily on the foliage, they also feed on pods and flowers once present [6,7]. Damage to pods is very critical during the time the beans are filling and maturing [8-10]. Even minor pod feeding can render the fruit unmarketable, as well as increasing the opportunity for plant pathogen entry [11]. As of 2020 [2], fresh market total snap beans planted in the United States was 95,344 ha at a value of $441 million. According to Capinera [2], the key pest of bean crops in many areas of the United States is the Mexican bean beetle. In Tennessee, infestations by these pests left uncontrolled may result in major defoliation of bean crops and even crop failure. For example, in 1978, only 5,917 ha were harvested from over 7,000 ha of snap beans planted in Tennessee resulting in a loss of over $1,014,000 [12]. Based on figures from the late 1980’s, the estimated loss of snap beans in the United States due to insect damage was $11.8 million [2]; however, these losses do not account for the cost incurred by the grower to prevent insects from causing even greater damage to their crop.
Control of the Mexican bean beetle has been primarily in the form of insecticide applications, and increasing resistance by the pest has diminished effective management with the expansion of agricultural crops into extensive monocultures. Therefore, there is an urgent need for more effective and sustainable control techniques [13]. Many arthropods are known to feed upon Mexican bean beetles; however, few native predators have proven effective at reducing populations of this pest [14]. The most common native predators of Mexican bean beetle include predatory stink bugs such as the soldier bug, Stiretrus anchorago (Fabricus), the spined soldier bug, Podisus maculiventris (Say) (Hemiptera: Pentatomidae) [15], and ladybeetles (Coleoptera: Coccinellidae). Other predators in the families Anthocoridae (Hemiptera), Nabidae (Hemiptera), and Chrysopidae (Neuroptera) have been observed to feed on the Mexican bean beetle life stages [14]. However, the indigenous predator of North America, the spined soldier bug, Podisus maculiventris, has been recorded as the most common predator in Mexican bean beetle infested areas east of the Mississippi River [14]. Prior to 1936, this predator was noted as the only effective predator of the Mexican bean beetle in Florida [16], South Carolina [17] and Virginia [1].
In addition to natural predators of the Mexican bean beetle, an exotic eulophid wasp Pediobius foveolatus (Crawford) (Hymenoptera: Eulophidae) from India was accessed in the United States for its potential as a classical biological control agent of this coccinellid pest and to verify that it would not parasitize native coccinellids and other beneficial insects [18-20]. Initial screenings demonstrated this wasp was unable to successfully complete its life cycle by parasitizing native beneficial coccinellids, but that it was successful only in the target pests, the Mexican bean beetles, and squash beetles, Epilachna borealis (F.) [20-21]. After release in the United States, this wasp appeared to be the most successful classical biological control agent for management of Mexican bean beetle populations [18-19]; however, the wasp was unable to establish populations due to the cold weather and its inability to overwinter in a larval host. In addition, P. foveolatus cannot survive cold winter months because all North American Epilachna hosts overwinter as adults, not larvae [21]. Therefore, these wasps had to be released annually in the United States to provide control of Mexican bean beetle populations which can be an exorbitant cost to the grower [18], whereas the spined soldier bugs can overwinter as adults in the duff or surrounding vegetation in the field. Also, in contrast to the wasp that parasitizes only the larval stages, the spined soldier bugs can feed on all life stages of the Mexican bean beetles, but prefer the larval, pupal, and adult stages [22].
Populations of P. maculiventris have been reared successfully in the laboratory [23-27] and were utilized as augmentative control agents for Diprion similis (Hartig), Hyphantria cunea (Drury), Pieris brassicae (L.) and Leptinotarsa decemlineata (Say) [28-32] and E. varivestis. The utility of predators in augmentative biological control programs is often not effective where they are released under field conditions, because they tend to disperse after 24 h in many directions, and not necessarily where the target pest is located. However, greater effectiveness with the P. maculiventris adults has been achieved by dewinging the females [33]. Waddill and Shepard [15] studied the dispersal of the nymphs in soybeans and found they dispersed along the rows rather than between them. In cotton fields, after 96 h post release of the dewinged P. nigrispinus, oviposition rates on the release site were about three times greater for dewinged compared to winged females [15]. Ignoffo et al. [33] recovered 85% of dewinged P. maculiventris predators released compared to 12% of the normal winged adults after 72 h and found greater numbers of eggs deposited by the dewinged predators. Their finding indicated that limiting flight induces females to stay and lay their eggs on the foliage; thus, allowing for local establishment of a new generation of predators. Another technique utilized in biological control programs to augment the natural population of P. maculiventris populations is to mass produce the eggs under laboratory conditions, place them in cold storage for a short duration and then disseminate them in the field prior to hatching [25]. However, in our study the eggs were not placed in cold storage.
Therefore, the objective of this study was to evaluate the efficacy of augmentative mass releases of P. maculiventris treatments at different rates (dewinged gravid female adults vs. egg mass releases) for biological control of E. varivestis in small plots of snap beans in East Tennessee. Foliar damage to the snap bean leaves by E. varivestis life stages using a damage index and subsequent bean pod yield was assessed amongst the different treatments compared to no releases.
Materials and Methods
Rearing of Mexican Bean Beetles
Rearing conditions for the Mexican bean beetles (MBB) were as follows: Snap beans, Phaseolus vulgaris cv. ‘Bush Blue Lake’ seeds were planted in three rows with seven beans per row in styrofoam containers (6.25 cm x 11.0 cm x 20.0 cm) in autoclaved sterile Holston silt loam soil. Styrofoam containers were moistened daily with ~40 ml of water and fertilized as needed with MiracleGro (Scotts Miracle-Gro Co., Marysville, OH). Plants 20-30 days after planting were transferred to insect cages (20.5 cm x 30.0 cm x 40 cm). MBB adults (2-3 mating pairs) collected from the Plateau AgResearch and Education Center fields in Crossville, TN were transferred to the new bean plants. Styrofoam containers with mature plants were replaced ~once every 3 d or when totally defoliated.
Rearing of Spined Soldier Bugs
Rearing conditions for the spined soldier bugs were modified from that described by Mukeiji and LeRoux [23], Warren and Wallis [24], and Evans [35]. Native populations of spined soldier bug (SSB) P. maculiventris previously captured in the field at the East Tennessee AgResearch and Education Center-Plant Sciences Unit in Knox County, TN, were brought back to the University of Tennessee, Knoxville to be reared in a laboratory room held at 30.2 ± 0.3°C, 40-50% RH under a 16 h light: 8 h dark photoperiod using florescent lighting. In the laboratory room there were several racks which held cafeteria trays (35 cm x 45 cm) making a shelf. Under each shelf there was a 1.2 m fluorescent light fixture fitted with two Sylvania Supersaver™ 34-watt tubes located 5 cm above each rearing area. In addition, a Paragon automatic timer set for the desired photoperiod was plugged into the light fixture. On each shelf there were inverted rearing Petri dish chambers (hereafter referred to as rearing chambers) containing a SSB life stage ranging from eggs to adults. Each inverted rearing chamber was lined with a filter paper circle ( 9.0 cm diameter with a coarse surface) and replaced once every 4 days or less or as needed. In each rearing chamber on top of the paper circle, a glass shell vial (12 x 35 mm) previously filled with water and plugged with cotton was placed to provide moisture and water. A piece of clay (~ 7 x 7 x 1 mm) was placed on the side of each shell vial away from the opening to hold it stationary and allow maximum absorbance of water by the cotton plug. Colonies of the greater wax moth, Galleria mellonella (L.), reared in the laboratory on Bio-Serv™ wax moth diet were provided as a constant source of live food for all nymphal stages beginning with the 2nd instar and ending with the 5th instar. The 1st instars are not predaceous, but feed only on the egg yolk and water [24]. Nymphs (2nd instars) were maintained together until the third instar with minimal loss to cannibalism with a sufficient food supply. After molting to the third instar, the nymphs were placed in individual rearing chambers as described above. The nymphs were maintained separately in individual dish chambers through the remaining instars, and as adults. Wax moth larvae were offered immediately upon completion of each molt and ad libitum thereafter. Once the 5th instars molted to the adult stage, newly eclosed and sclerotized adults were placed in a separate clean rearing chamber with MBB as food. Adults were sexed, placed in separate rearing chambers which were labelled, dated, and placed on a separate tray shelf with the other newly sclerotized adults. After 48 h post-ecdysis, a single adult SSB male was transferred to a different chamber and paired with a female SSB adult. This rearing chamber contained MBB instars and a clean glass shell vial with water for moisture. Once SSB adults were observed in copula, they were left undisturbed and allowed to copulate for ~24 h or until they separated naturally. Once separated, male SSB adults were removed from the female’s chamber and placed back into their original or a new clean rearing chamber with food and water provided as before. Gravid SSB females were provided E. varivestis as their food source prior to oviposition in the rearing chambers for both types of mass release treatments. Gravid female SSB adults 2-6 d old were used in the field release experiments. To maintain the reared colony, female SSB were paired approximately once a month to maintain fertile egg production [24,25].
Field Releases of Spined Soldier Bug Treatments
Dewinged gravid female treatments: Gravid normal winged females (80 total) were anesthetized with CO2 and one hemelytron was clipped with iridectomy scissors at the axillary region to inhibit flight and maximize predation potential. Dewinged gravid females each were color-coded by marking the scutellum with Testors® model enamel paint (Testor Corp., Rockford, IL) to differentiate them from the indigenous population (Figure 1a).
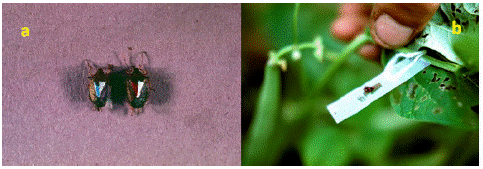
Figure 1: Photos of Podisus maculiventris release treatments. a) dewinged gravid female release treatments b) egg mass release treatments.
One color represented the block where the female was released and the other color the treatment in the block (Figure 1a). Females once dewinged and color coded were placed back into the individual rearing chambers to air dry, allowed to feed on E. varivestsis and monitored for survival for 7 d for premature mortality. Surviving females after this monitoring duration were then randomly chosen as dewinged gravid female (DWGF) treatments to be used for the field releases.
Egg Mass Release Treatments
To produce the egg mass release (EMR) treatments, a green acetate strip (1 x 27 x 0.1 cm) was placed lining the side of the Petri dish bottom of the rearing chamber described above. Green acetate strip was determined to be the preferred color for the highest egg deposition (unpublished data – Avery). This strip provided a substrate for egg deposition because gravid adult SSB females were observed to lay eggs in clusters on the side of the dish [25,36]. A single gravid female per chamber was allowed 24-48 h to lay eggs on the strip. After egg deposition, gravid females were removed from that chamber and placed into another clean rearing chamber with a new green acetate strip as described above. Each acetate strip containing 25 eggs per cluster, or more were removed from the original chamber, placed into an empty rearing chamber containing only a filter paper circle, dish was labelled according to the date of deposition and placed on a cafeteria tray to incubate until time for releasing in the field, i.e. eggs were reddish to orangish bronze color. EMR treatments were either 25, 125 or 250 eggs or a density ratio of eggs: plants (1:10, 5:10, 10:10), respectively; per row plot. The number of eggs per acetate strip added up to the total number expected to be released per treatment, e.g. 25, 125 or 250 eggs per row plot. On the day prior to releasing in the field, acetate strips containing one or more egg masses incubating on the tray were randomly chosen and removed from the chambers. The acetate strips were cut carefully with scissors between egg clusters, a hole was punched gently in one end and threaded with a string for tying to the plant stem (Figure 1b). Egg masses attached to the acetate strips were 4 d old before being released in the field.
Experimental Field Study
Snap beans, Phaseolus vulgaris cv. ‘Bush Blue Lake’ seeds were planted into Holston silt loam soil at the East Tennessee AgResearch and Education Center-Plant Sciences Unit in Knox County, TN on 2 May (Year 1) and 25 April (Year 2) for the field release of SSB treatments. Plots consisted of single row treatments which were 3.1 m long and 1.0 m wide with seeds spaced 5-7 cm apart. Plants were thinned from 30 to 10 plants per row plot per treatment for a total of 40 plants per treatment. The mean number of MMB insect stages per plant was determined by visual counts taken pre-release and then 4, 7, 14 and 21 days from 7 randomly selected plants per plot. Treatments were arranged in a randomized complete block design with four replications. No herbicides for weed control were applied for either year. MBB adults obtained from field populations at the Plateau AgResearch and Education Center in Crossville, TN were released at a rate of 5 adults per row plot in Year 1 to increase the population size prior to release and obtain an economic threshold or economic injury level of 1 - 1.5 larva/ plant [37]. In Year 2, due to the presence of at least ~4.9 - 6.9 larva / plant per row plot counted in the field, no additional releases were needed.
DWGF treatments were released on a selected plant per row at a rate of 1, 5, and 10 per row plot on 2 July (Year 1) and 5 June (Year 2). For the single DWGF treatments, the insect was placed on a selected plant per row plot. Multiple releases of DWGF treatments were evenly distributed along the row and all females were allowed 4 days to oviposit prior to the placement of EMR treatments. Marked females when found were counted, recorded as to the new location, and returned to the initial release row plot twice weekly.
EMR treatments (green acetate strips containing the SSB eggs) were tied as described above (Figure 1b) with string on stems of snap bean plants within the designated row at rates of 25, 125, and 250 eggs total per plot. Visual counts of strips tied on all plants per plot were taken 4-, 7-, 14-, and 21-days post-egg deposition (PED). Egg masses found deposited on foliage from DWGF treatments were counted, recorded, signified by flag tape as to location within the row plot and observed throughout the study to determine percent hatch. Egg masses released in EMR treatments on acetate substrates tied to a specific plant per row plot per treatment followed the same protocol as with the DWGF treatments above to determine percent hatch. An open operculum was used as the criterion of a hatched egg. Egg masses either deposited in the DWGF plots by SSB adults on foliage or EMR treatments were also observed and assessed for parasitization by endemic parasitoids naturally present in the field. An exit hole in the SSB egg chewed by the parasitoid was used as criterion that it was parasitized.
Defoliation Index
Foliar damage by the MBB feeding stages (larvae and adults) was recorded using a rating index from 0-5 with percentage defoliation rating of the plant as follows: 0 = none, 1 = 1-20%, 2 = 21-40%, 3 = 41%-60%, 4 = 61-80%, 5= 81-100%. Percent defoliation was determined by dividing the number of leaves skeletonized by the total number of leaves per plant × 100.
Yields of Snap Beans
Mature snap beans were harvested on 7 August (Year 1) and 7 July (Year 2). Yield data based on pod mass (kg/ha) were recorded after non-marketable pods were extracted.
Statistical Analysis
Data were submitted to a one-way ANOVA after square root arcsine transformed and if significant, ad hoc mean values were compared among treatments using Fisher’s Least Significant Difference HSD test or Duncan’s Multiple Range test (a = 0.05). A regression analysis was conducted to determine if there was any correlation between the decreasing number of SSB predators available on the foliage and the amount of precipitation over time. Statistical analyses were primarily conducted using SAS Software 9.4 programs (2002-2012 by SAS Institute Inc., Cary, NC, USA). Weather records were obtained from data collected at the East Tennessee AgResearch and Education Center – Plant Science Unit (NOAA).
Results
Year 1 (July to August)
Environmental conditions: Environmental conditions during the field releases of SSB treatments for management of the MBB from 2 July to 7 August were as follows: Average temperature ranged from 23.2 to 24.2°C (minimum: 18.4 - 18.7°C; maximum: 27.8 – 29.9°C) (Figure 2).
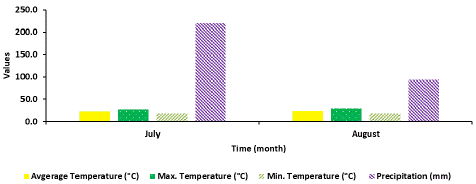
Figure 2: Environmental conditions in East Tennessee AgResearch and Education Center in Knox County.
Precipitation (rainfall) during the field release study was 220.2 mm in the month of July, and then decreased to 94.0 mm in August. During that time, a decrease in the number of nymphs on the foliage was observed between 4 and 7 days after release of all the EMR treatments. A significant negative correlation (r = -0.833; df = 3; P = 0.001) was found between the amount of rainfall and the decreasing number of nymphs observed on the foliage over time.
Mass Release Treatments: One week after release of all EMR treatments, a higher mean number of SSB eggs (23) were found deposited by 10 DWGF adults on the foliage in field row plots, compared with either 1 (0), 5 DWGF (5) adults released or control (Table 1).
Treatmenta
No released / 3.1 m row plot
No. eggs observed
% hatchedb
% eggs parasitizedc
DWGF
1 adult
0
0.0
0.0
DWGF
5 adults
5
6.0
83.0
DWGF
10 adults
23
2.0
0.0
EMR
25 eggs
19
94.0
0.0
EMR
125 eggs
73
80.0
0.0
EMR
250 eggs
40
66.0
0.0
Control (no releases)
---
0
0.0
0.0
aDWGF adult treatments were released 4 days prior to the placement of the EMR treatments.
bAn open operculum was used as the criterion of a hatched egg.
cParastization was based on observing exit hole of eggs by parasitoid.
Table 1: Mean number of P. maculiventris eggs deposited by dewinged gravid female (DWGF) adult treatments or observed per egg mass release (EMR) treatment per row plot. The percentage number of eggs hatched and parasitized were calculated over the 21 days evaluation period.
The mean number of eggs observed on the acetate strips 7 days PED tied to plants was highest for those with 125, followed by the those with 250 and 25 eggs per row plot. The percentage mean number of eggs that hatched for all EMR treatments (66-94%) was much higher compared with the DWGF treatments (0-6%). The percentage mean number of eggs that hatched was 94, 80, and 66% for the EMR treatments with 25, 125 and 250 eggs, respectively. Of the eggs deposited on leaves with 5 DWGF adults per row plot, 1 mass of 15 eggs was found 100% parasitized; mean percentage of eggs parasitized for this treatment was 83%. None of the egg masses released in the EMR treatments per row plot were parasitized.
Only 1 SBB predator as a 3rd instar was available to consume the MBB population in the highest DWGF treatments 7 days post release of the EMR treatments, whereas, the EMR treatments had a total of 21 and 41 SSB predators as 2nd and 3rd instars available, respectively (Figure 3).
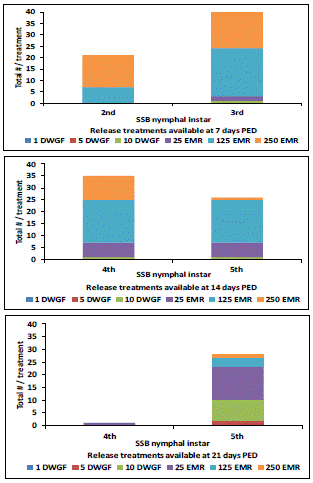
Figure 3: Total number and stage of P. maculiventris (SSB) nymphal instars available on the plants per DWGF and EMR treatments at 7, 14, and 21 days post-egg deposition (PED).
DWGF adult treatments were released 4 days prior to the placement of the EMR treatments.
Release treatments: DWGF: dewinged gravid female; EMR: egg mass release.
After 14 days PED, 4th and 5th SSB instar predators were observed on plants in DWGF and EMR treatment plot(s) (Figure 3). For the DWGF release treatments, only a single 4th and 5th instar SSB predator was observed in the highest release. There was a total of 6, 18, 10, and 6, 18, 1 for 4th and 5th instar SSB predators available from the lowest, medium and highest EMR treatments, respectively, available for management of the MBB population feeding on the snap bean foliage per row plot. At the last count, 21 days PED, only SSB predators at the 5th instar stage were available for the DWGF release treatments; however, both 4th and 5th SSB instar predators were available in the EMR treatment plots (Figure 3). A total of 2 and 8 of 4th and 5th instar SSB predators were observed in the row plots for the DWGF treatments; respectively. There were no SSB predators found in the no-release plots throughout the observation period.
Suppression of MBB Populations: Only the DWGF treatments with 5 adults per row plot had a significantly (P < 0.05) higher mean number of larvae prior to release of the EMR treatments compared to the other treatments and control; however, the number of larvae was balanced with the other treatments and control 4 days later (Table 2).
Treatmentb
No. released / 3.1 m row plot
Mean no. of E. varivestis life stagesa
Pre-release
Days post-egg deposition
4
7
14
21
Larvae
DWGF
1 adult
0.00a
93.75 a
173.50a
124.50c
32.25a
DWGF
5 adults
0.00a
108.75a
104.25a
69.75abc
41.25a
DWGF
10 adults
25.3b
124.50a
188.75a
148.00c
34.25a
EMR
25 eggs
0.00a
104.50a
194.00a
115.75c
37.75a
EMR
125 eggs
4.75a
121.75a
79.00 a
41.75ab
75.50a
EMR
250 eggs
6.75a
69.25 a
79.50 a
26.00 a
39.50a
Control (no releases)
---
3.75a
96.00 a
122.50a
94.75bc
30.25a
Pupae
DWGF
1 adult
0.00a
0.00a
0.00a
27.00a
42.75bc
DWGF
5 adults
1.25a
0.00a
0.00a
61.5a
44.25bc
DWGF
10 adults
0.75a
10.50a
0.00a
47.75a
77.50c
EMR
25 eggs
0.00a
0.25a
0.00a
31.50a
31.75b
EMR
125 eggs
0.00a
3.50a
0.00a
9.50a
5.75 a
EMR
250 eggs
0.00a
4.75a
0.00a
10.25a
4.00 a
Control (no releases)
---
0.00a
2.50a
0.00a
44.75a
56.75bc
Adults
DWGF
1 adult
5.00ac
0.50a
0.25a
0.50a
24.50a
DWGF
5 adults
5.00a
0.00a
1.50a
3.25a
55.50a
DWGF
10 adults
5.00a
3.25b
0.75a
1.00a
37.50a
EMR
25 eggs
5.00a
0.25a
0.75a
3.00a
23.00a
EMR
125 eggs
5.00a
0.50a
3.25a
0.50a
10.75a
EMR
250 eggs
5.00a
0.50a
2.75a
1.50a
17.25a
Control (no releases)
---
5.00a
0.25a
3.75a
4.00a
43.00a
aMeans were transformed prior to analysis; untransformed mean values displayed in a column not followed by the same letter per life stage section are significantly different (Least Significant Difference HSD test, P=0.05).
bTreatment: DWGF: Dewinged Gravid Female; EMR: Egg Mass Release
cMBB adults were released 18 June (10 days before the release of DWGF treatments)
Table 2: Effect of P. maculiventris mass release treatments for biocontrol of E. varivestis (MBB) life stages counted on snap beans in Knox County, TN.
Due to the low number of MBB adults counted 10 days prior to the release of the DWGF treatments, 5 MBB adult previously collected as described above were released in each row plot per treatment and control. The number of MBB larvae did not differ significantly (P > 0.05) between treatments compared to the control throughout the 21-day evaluation period, except for EMR treatments with 125 and 250 eggs at 14 days PED which was significantly (P < 0.05) lower. The mean number of pupae found on plants with EMR treatments with 125 and 250 eggs per row plots were significantly (P < 0.05) lower than the control and all DGF treatments at 21 days PED. The mean number of MBB adults did not differ significantly between all release treatments compared to the control but increased throughout the 21-day evaluation period.
Field Releases of the Spined Soldier Bug treatments (Year 2)
Environmental conditions: Environmental conditions during the field releases of SSB treatments for management of the MBB from 5 June to 7 July were as follows: Average temperature ranged from 22.9 to 27.1°C (minimum: 16.2 - 20.1; maximum: 29.6 - 30.0°C), (Figure 4).
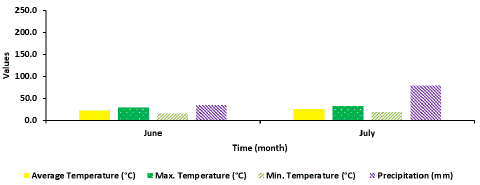
Figure 4: Environmental parameters in East Tennessee AgResearch and Education Center in Knox County.
Precipitation (rainfall) during the field release study was 36.3 mm in the month of June, and then increased to 79.8 mm in July. A decrease in the number of nymphs on the foliage was observed between 4 and 7 days after release of all the EMR treatments. A significant negative correlation (r = -0.639; df = 3; P < 0.001) was found between amount of rainfall and the decreasing number of SSB nymphs observed on the foliage over time.
Mass release treatments: One week after release of all EMR treatments, the mean number of SSB eggs observed deposited on the foliage was observed highest in the 10 DWGF adult release treatments per row plot, compared with the other adult release plots or control (Table 3).
Treatmenta
No released / 3.1 m row plot
No. eggs observed
% hatchedb
% eggs parasitizedc
DWGF
1 adult
0
0.0
0.0
DWGF
5 adults
1
25.0
0.0
DWGF
10 adults
6
5.0
0.0
EMR
25 eggs
24
76.0
0.0
EMR
125 eggs
28
60.0
0.0
EMR
250 eggs
42
67.0
0.0
Control (no releases)
---
0
0.0
0.0
aDWGF adult treatments were released 4 days prior to the placement of the EMR treatments.
bAn open operculum was used as the criterion of a hatched egg.
cParastization was based on observing exit hole of eggs by parasitoid.
Table 3: Mean number of P. maculiventris eggs deposited by dewinged gravid female (DWGF) adult treatments or observed per egg mass release (EMR) treatment per row plot. The percentage number of eggs hatched and parasitized was calculated over the 21 days evaluation period.
The mean number of eggs observed on the acetate strips 7 days after the EMR treatments were tied to plants was highest to lowest for those with 250, followed by the those with 125 and 25 eggs per row plot. The percentage mean number of eggs that hatched for all DWGF treatments ranged from 0-25%, whereas the EMR treatments were much higher and ranged from 60-76%. The percentage number of eggs that hatched was 76, 60, and 67% for the EMR treatments with 25, 125 and 250 eggs, respectively. None of the egg masses deposited by the DWGF adults or released in the EMR treatments per row plot were parasitized.
For the middle and highest DWGF adult treatments, the only stage of SSB predator available to feed on the MBB life stages 7 days PED were 2nd instars, whereas, the EMR treatments had both 2nd and 3rd instars available (Figure 5).
For the middle and highest DWGF adult release treatments, the total number of SSB 2nd instar predators observed was only 2 and 1, respectively. There was a total of 1 and 6 SSB predator(s) observed as 2nd instars on plants in the middle and highest EMR treatment, whereas there were 3, 9, and 11 of the 3rd instar predators from the lowest to highest release per row plot, respectively. On 14 days PED, there were 4th and 5th instar SSB predators observed on the plants in both DWGF adult and EMR treatments (Figure 5). For the DWGF adult treatments, there was a single 4th instar SSB predator observed on plants in the lowest and highest release row plots, and of the 5th instars, a total of 2 were found on plants in the highest release plots. There was a total of 2, 1, 2, and 5, 6, 13, for 4th and 5th instar SSB predators observed on plants in the lowest, medium, and highest EMR treatment row plots, respectively. At the last count, 21 days evaluation period, no SSB predators at the 4th, 5th or 6th instar stage were observed in any of the treatments. There were no SSB predators found in the no-release row plots throughout the observation period.
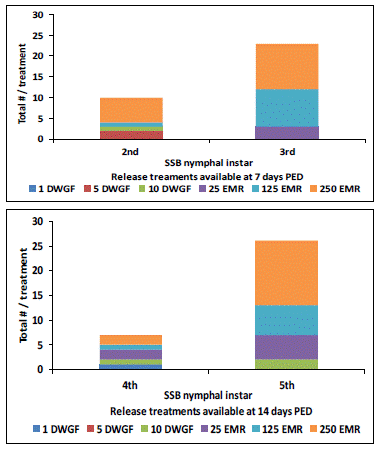
Figure 5: Total number and stage of P. maculiventris (SSB) nymphal instars available on the plants per DWGF and EMR treatments at 7 and 14 days post-egg deposition (PED).
DWGF adult treatments were released 4 days prior to the placement of the EMR treatments.
Release treatments: DWGF: dewinged gravid female; EMR: egg mass release.
Suppression of MBB Populations: None of the DWGF or EMR treatments had a significantly (P < 0.05) higher mean number of MBB larvae prior to release of the EMR treatments compared to the control (Table 4).
Treatmentb
No. released / 3.1 m row plot
Mean no. of E. varivestis life stagesa
Pre-release
Days post-release of EMR treatments
4
7
14
21
Larvae
DWGF
1 adult
69.00a
77.50a
53.25a
20.25a
22.50a
DWGF
5 adults
84.00a
68.00a
56.50a
4.50 a
23.00a
DWGF
10 adults
50.75a
49.25a
57.75a
10.00a
25.75a
EMR
25 eggs
55.25a
62.00a
58.00a
15.75a
10.50a
EMR
125 eggs
65.00a
52.25a
54.00a
0.25 a
3.25 a
EMR
250 eggs
49.00a
52.75a
52.50a
4.75 a
12.50a
Control (no releases)
---
50.75a
56.25a
58.00a
0.75 a
3.25 a
Pupae
DWGF
1 adult
0.00a
0.00a
0.00a
61.25a
2.25a
DWGF
5 adults
0.00a
0.00a
0.00a
50.25a
3.25a
DWGF
10 adults
0.00a
0.00a
0.00a
38.25a
6.50a
EMR
25 eggs
0.00a
0.00a
0.00a
38.75a
0.50a
EMR
125 eggs
0.00a
0.00a
0.00a
35.25a
0.00a
EMR
250 eggs
0.00a
0.00a
0.00a
27.00a
0.50a
Control (no releases)
---
0.00a
0.00a
0.00a
50.00a
0.50a
Adults
DWGF
1 adult
0.75a
1.00a
0.25a
1.75a
17.75a
DWGF
5 adults
0.25a
0.25a
0.75a
2.25a
17.75a
DWGF
10 adults
0.00a
0.50a
0.25a
1.00a
20.75a
EMR
25 eggs
0.25a
0.25a
1.50a
0.25a
8.00 a
EMR
125 eggs
0.75a
0.25a
0.25a
0.25a
8.25 a
EMR
250 eggs
0.75a
0.25a
0.25a
2.00a
13.50a
Control (no releases)
---
0.50a
0.50a
0.00a
0.75a
15.25a
aMeans were transformed prior to analysis; untransformed mean values in a column not followed by the same letter per life stage section are significantly different (Least Significant Difference HSD test, P=0.05).
bTreatment: DWGF: Dewinged Gravid Female; EMR: Egg Mass Release
Table 4: Effect of P. maculiventris mass release treatments for biocontrol of E. varivestis (MBB) life stages counted on snap beans in Knox County, TN.
There were no significant (P > 0.05) differences in the number of larvae observed on plants in any of the release treatments compared to the control row plots throughout the evaluation period. The number of pupae found on plants in all treatments and control were not significantly different (P > 0.05) throughout the entire 21 day evaluation period. MBB adult numbers were similar (P > 0.05) to the control throughout the duration of the study but were higher at 21 days post-release compared to the previous evaluation days.
Defoliation Index: The mean defoliation rating index values continued to increase throughout the evaluation period for both Years 1 and 2 (Table 5).
Treatment
No. released / 3.1 m row plot
Mean defoliation rating indexa days
post-egg deposition4
7
14
21
Year 1
DWGF
1 adult
1.75 ab
2.01 a
3.76 a
4.34 a
DWGF
5 adults
1.98 a
2.42 a
3.71 a
4.16 a
DWGF
10 adults
1.81 a
2.42 a
3.89 a
4.55 a
EMR
25 eggs
1.81 a
2.13 a
3.46 a
3.96 a
EMR
125 eggs
1.77 a
2.16 a
3.39 a
4.08 a
EMR
250 eggs
1.76 a
1.91 a
3.20 a
3.68 a
Control (no releases)
---
2.03 a
2.52 a
4.07 a
4.30 a
Year 2
DWGF
1 adult
2.61 a
3.19 a
3.63 a
4.18 a
DWGF
5 adults
2.49 a
2.81 a
3.25 a
3.90 a
DWGF
10 adults
2.47 a
2.98 a
3.13 a
3.63 a
EMR
25 eggs
2.41 a
3.03 a
3.28 a
4.33 a
EMR
125 eggs
2.53 a
2.90 a
3.00 a
3.83 a
EMR
250 eggs
2.36 a
2.88 a
3.00 a
3.63 a
Control (no releases)
---
2.30 a
2.97 a
3.23 a
4.28 a
aDefoliation rating index values: 0 = no damage; 1 = 1-20%; 2 = 21-40%; 3 = 41- 60%; 4 = 61-80%;
5 = 81-100%.
bMeans in a column not followed by the same letter are significantly different (Least Significant Difference HSD test P=0.05).
Table 5: Effect of mass release of SSB treatments on mean defoliation of snap bean foliage by MBB populations, Knox Co. TN for Years 1 and 2.
In Year 1, there were no significant (P > 0.05) differences in the mean defoliation rating indices for plants observed in the release treatments (1-20% defoliated) compared with the control throughout the 21 days evaluation period; however, only the plants in the control were 21-40% defoliated at 4 days PED. Prior to harvest on 21 days PED, defoliation rating indices for plants in all treatments were very high (~61-80%) and similar to the control with no releases. None of the plants in the release treatments or control were completely defoliated at the 21 days PED. In Year 2, none of the release treatments defoliation rating indices differed significantly (P > 0.05) from the control and all plants were defoliated by 21-40% only at 4 days PED. Defoliation ratings for all treatments and control were similar; all plants were 61-80% defoliated on day-21 post-release of EMR treatments. None of the plants in the release treatments or control were completely defoliated at 21 days PED.
Yields of snap bean pods: In year 1, the total mean yield of snap bean pods harvested was significantly (P < 0.05) higher for EMR treatments with 125 and 250 eggs released per row plot compared with no releases (Table 6).
Treatment
No. released / 3.1 m row plot
Year 1
Year 2
DWGF
1 adult
752.0 ab
21.58 a
DWGF
5 adults
750.4 ab
44.24 ab
DWGF
10 adults
756.8 ab
40.08 ab
EMR
25 eggs
913.4 abc
42.70 ab
EMR
125 eggs
1042.5 bc
50.10 bc
EMR
250 eggs
1247.5 c
67.83 c
Control (no releases)
---
626.1 a
21.58 a
aMeans in a column not followed by the same letter are significantly different (P < 0.05) (Duncan's multiple range test). Treatment: DWGF: Dewinged Gravid Female(s); EMR: Egg Mass Release(s). Snap bean pods were harvested on both 24 July for Year 1; 7 July for Year 2.
Table 6: Total mean yield (kg/ha) of snap bean pods harvested per treatment per row plot in Knox Co., TN for Years 1 and 2.
Also, the total mean yield of snap bean pods harvested for all DWGF treatments and the lowest EMR treatment per row plot were similar to the control with no releases.
In Year 2, the total mean yields of snap bean pods were significantly (P < 0.05) higher for EMR treatments with 125 and 250 eggs released per row plot compared to the control with no releases. The total mean yield of snap bean pods harvested for all DWGF and the lowest EMR treatment per row plot were similar to the control with no releases.
Discussion
Environmental Conditions
In this study, SSB life stages from egg to adult were reared at 30.2 ± 0.3°C under laboratory conditions for both years. The temperatures in the field for year 1 (2 July - 7 August) ranged from 23.2 - 24.2°C and for year 2 (5 June - 7 July) ranged from 22.9 - 27.1°C. Baek et al. [38], in determining the thermal developmental conditions for SSB eggs to adult, found completion of egg development was optimal at 13.2–32.7°C, whereas nymphs successfully developed into adults from 18.4–32.7°C. However, when developmental rates at the eight temperatures were fitted with a nonlinear Briere model, Baek et al. [38] found that the estimated optimal temperatures for development were 31.2, 30.6, and 30.6°C for egg, nymph, and egg to adult, respectively. Therefore, the thermal conditions recorded in the field for both years were within the optimum temperature range and conducive for the development of eggs to nymphs and from nymphs to adults.
The amount of precipitation (rainfall) for both years was negatively correlated with the number of SSB nymphs available to feed on the MBB life stages present in the row plots; this scenario was especially true of the EMR treatments. SSB gravid female adults lay their eggs upright with the posterior pole attached to the leaf or acetate surface by a gluey spumescent mass [36]. When the acetate strips with egg masses were being prepared for field release, it was observed that some of the eggs could easily become dislodged from the acetate substrate. Also, because the acetate strip was tied only at one end to the plant stem under the canopy, movement of the strip due to wind could dislodge the eggs before hatching. For both years, heavy rainfall had a significant negative effect and washed some of the attached SSB eggs off the acetate strips; thus, the developing egg mass or masses were unaccounted for and lost.
Mass Release Treatments
The loss in potential viable eggs led to a decrease in the number of potential predators available per EMR treatment to feed on the MBB life stages. In contrast, of the eggs deposited on the foliage in the DWGF treatments in year 1, some were observed to be parasitized based on the parasitoid’s exit holes. Parasitism of SSB eggs by Telenomus podisi (Ashmead) had been noted in the field after four days exposure [39-40]. The exit holes were not verified to be from T. podisi; however, in another study, Tillman [41] observed in a cornfield over six consecutive years with naturally deposited SSB eggs, that a mean of 97.9 ± 1.3% of eggs per egg mass was parasitized by T. podisi. Overall, the percentage egg hatch during both years was significantly higher for all EMR treatments compared with the DWGF treatments. This could be related to the low number of potential eggs deposited on the leaf surface to hatch observed in the DWGF adult treatments, compared to EMR treatments. Also, eggs attached to the acetate strips in the EMR treatments had only about 3 days before they hatched, so there was a higher number of potential viable eggs available to hatch simultaneously compared to the DWGF treatments. The egg deposition by the gravid SSB adult females released in the field was coordinated so that the gravid adult females in the laboratory were allowed to deposit their eggs on the acetate strips inside of the Petri dish chambers at the same time. Therefore, the effect of both release methods can be assessed as post-egg deposition (PED). In addition, eggs deposited in the field were unprotected until hatching from parasitization; whereas the eggs deposited on the acetate strip were protected and required only ~3 more days to hatch.
Of the low number of eggs being deposited in the field by the DWGF adults, the percentage of hatching was very low and ranged from 0-25%. This percent hatching was much lower than what was experienced in the EMR treatments which was 60-94%. Possible reasons for the low number of adults remaining in the field over time and/or low viability of the SSB eggs deposited by DWGF adults in the field row plots could be: 1) the inability of the SSB adults reared under optimum laboratory conditions to adjust and survive after being released into suboptimal natural field conditions [42]; the scarcity of MBB prey being immediately available upon release into the field, resulting in the gravid female laying fewer eggs than normal [43]; 3) less prey being available everyday will result in lower body weight and egg hatch rates [44] 4) longer developmental time in the field required for eggs deposited by females to hatch can allow for potential parasitization [45], high exposure of light colored eggs laid on the on the top leaf surface to solar UV radiation can reduce survival of nymphal instars by negative carryover effects [46] and 5) impairment of a single attached hemelytron post release can result in premature death of individual SSB adults [27].
Generalist predators in augmentative biological control programs are often not effective where they are released under field conditions, because they tend to disperse after 24 h in many directions, and not necessarily where the target pest is located. However, greater effectiveness with the SSB adults has been achieved by dewinging the females [27,33]. Although dewinging the female SSB was reported by Ignoffo et al. [33] and Lambdin and Baker [27] to increase predatory effectiveness, our data does not support such findings. However, in their studies, the females had both hemelytron’s cut off at the axillary region with an iridectomy scissors, not just one as in our study. The potential effect of premature death by having only one hemelytron attached was considered above. Also, in Ignoffo et al. [33] and Lambdin and Baker [27] the evaluation period was only 72 h or 51 days, respectively, whereas our study was over 21 days for each year. Nevertheless, results from our study indicated that there was a continual decrease in the number of DWGF adults alive and egg masses laid/hatched observed over the 21-days PED in the field for both years. Possible reasoning why the adults decreased over time in our study could be as follows: 1) Due to the lack of third instar MMBs feeding on the snap bean foliage at the time of release, the DWGF adults may have dispersed in search of other food. López et al. [47] reported that SSB females are more efficient predators of third instar MBB compared with first or second instars. 2) Some of the DWGF adults were eaten by avian predators; similar avian predation was also noted by a researcher studying a pentatomid species Grabarczyk et al. [48]. The occurrence of avian predation could be due to the bright colors painted on the scutellum which would make the DWGF adults more conspicuous and less able to camouflage in the snap bean canopy, especially as the canopy is being defoliated over time. 3) Due to the increase in defoliation of the snap bean leaves over time, the available space for the DWGF adult to lay eggs was severely limited.
Suppression of MBB Populations
When evaluating the efficacy of a generalist predator as a biological control agent (BCA) after being released into an open field agroecosystem, it is virtually impossible to control both the abiotic and biotic factors inherent within that system. The abiotic factors inherent within this snap bean crop agroecosystem and how each component may affect the multi-trophic interaction of the plant-predator-parasitoid/prey relationship has been addressed above; however, the biotic factors that may influence the efficacy of the BCA in this snap bean-SSB-MBB interaction investigation within open field conditions has not. Therefore, firstly the suppression of the MBB population numbers may or may not have been affected by other BCAs that were present in the snap bean agroecosystem during both years. These other BCAs include the convergent ladybird beetle (Hippodamia convergens Guérin-Méneville) adults and larvae, twelve-spotted ladybird beetle (Coleomegilla maculata de Geer, 1775) adults and larvae, and nabid (Nabid americoferus Carayon, 1961) adults which have all been observed by various researchers to feed on the eggs and small larvae of the MBB [7,14,22,49-50]. Besides the MBBs, occasional pests observed in this agroecosystem feeding on the snap bean foliage and/or the developing pods were unidentified aphid species, southern green stinkbug ((Nezara viridula (L.)) adults, and cabbage looper ((Trichoplusia ni (Hübner)) larvae. We can only assume that some of the occasional pests could have provided alternative supplemental food for the developing SSB nymphs and/or BCAs as well; however, this predation was not assessed. Also, the focus of this two-year study was to evaluate the efficacy of SSB mass release treatment methods for managing the MBB population on the snap beans compared to no release.
SSB nymphs do not require animal food in the first or second instar. It is approximately 7 days after hatch that the nymphs become predacious as 3rd instars [23]. Suppression of the MBB larvae was significantly evident at 14-days PED during Year 1; however, a similar trend was also evident in Year 2, although not significantly compared to no release. The mean number of pupae was reduced significantly in the two highest EMR treatments only during Year 1; however, as with the larvae in Year 2 discussed above, the numbers were lower, but not significantly compared to no release. MMB adults can fly; therefore, it is difficult to access the “true” suppression of their population at each day PED due to their constant fluctuations over time. Therefore, in this study, the counting of 7 randomly chosen plants out of 10 possible per row plot (times 4 per treatment), represented our best assessment when comparing different release treatment methods at days PED for management of the MBB population, but could change due to fluctuations over time with those MBB adults that have winged mobility. Judge et al. [51] indicated that fluctuations in the MBB adult beetle numbers may be attributed to probable migration from plot to plot. In general, both the middle and highest SSB EMR treatments suppressed the pest population of MBB as there were more 3rd instars SSB nymphs available as predators 7-days PED compared to the number of the DWGF treatments which was reflected in the final yield of the snap bean pods for both years. The ratio of SSB 3rd instars available in the middle and highest treatments for EMR: DWGF was 39:1 for Year 1 and 20:0 for Year 2.
Defoliation Index and Yield of Snap Bean Pods
The defoliation indices increased over time for both years; however, the values were lower starting at 4-days PED, compared to Year 2, because the overwintering returning population of MBB was much higher after the snap beans were planted in Year 2 of the study. Although no significant differences (P > 0.05) were noted among means for the defoliation index per treatment methods, higher yields were obtained from row plots with the two highest release rates of SSB EMR treatments. Higher yields are reflective of less pod damage by the MBB during the critical time when the beans are filling and maturing [10]. In addition, the data indicates that as the number of SSB eggs released in the EMR treatments increased per row plot, there were a lower number of MBB larvae available to damage the pods which subsequently resulted in higher yields. In another study, Ridgeway and Jones [52] found that an increase in the number of Chrysopa carnea eggs released to control Heliothis on cotton, resulted in higher yields compared to no releases. Also, in corroboration, Montemayor and Cave [53] observed that the increase in the number of SSB 3rd instars released against the yellowmargined leaf beetle in bok choy plots, resulted in higher yields compared to no releases. The total mean yield of snap bean pods was much lower in Year 2 for all treatments compared to Year 1, because a much higher endemic population of MBB was already present in the field prior to the experiment. Only in Year 1 was it necessary to augment the endemic populations of MBB with other field collected adult beetles to reach an economic threshold/injury level of 1-1.5 larva/plant [37] for the study; in Year 2, the endemic overwintering MBB population reached the threshold level at the pre-count date. The yield for Year 1 in kg/ha for the two highest DWGF adult treatment releases was 2× higher compared to Lambdin and Baker [27] who conducted a similar experiment using DWGF adult treatments, except they clipped both hemelytra and the mean number of larvae in their study was ~ half that counted in our study.
Conclusion
In the release of SSB treatments for biological control of the MBB, percent egg hatch for both years was higher for the EMR treatments compared to the eggs deposited from DWGF treatments. The two highest EMR treatments were effective in suppressing the pest population after 14 days for all tests conducted. A decrease in the number of SSB nymphs observed 7 days after release in the field for the EMR treatments was negatively affected by heavy rainfall and other abiotic factors during both year evaluations. Snap bean pod yields in both years were significantly higher for plants with the highest EMR treatment.
In conclusion, the effective control of MBB obtained in the field experiments using the two highest EMR treatments demonstrated the potential of the generalist predator, SSB as a BCA in areas where this pest is economically important. With further studies evaluating the compatibility of certain insecticides or biopesticides with this predator, SSB could be utilized in an integrated pest management program. Mass releases of this predator as EMR treatments could minimize the continued usage of insecticidal sprays and enhance the endemic predator-prey-parasite complex found in snap bean agroecosystems. Practical application of augmentative releases of SSB for control of MBB on snap beans will require further research. This will include the timing of releases, the numbers required, methods of mass production, distribution, and evaluation of field efficacy.
Limitations
This study was conducted both years in a small plot area, which could have affected the dispersal of the DWGF treatments. Given a larger area for the SSB DWGF adults to disperse may have a more positive effect on the number of predators available for each release treatment and allow for a more robust mean comparison of the data statistically amongst the various treatments. If conducted again, a specific number of plants per treatment row would be selected from 6 or more row plots and data would be collected per plant from those randomly selected plants, giving a higher number of data values per row treatment for comparison. In addition, the interval of time between assessments would be shorter, being daily instead of weekly; however, this change in experimental design would require more personnel.
Author Statements
Conflicts of Interest
Authors have no conflicts of interest.
Acknowledgments
Thanks to Ilene Maddox for her technical assistance in rearing the Podisus maculiventris, and the farm crew for sterilizing the soil for the snap bean plants used for rearing the Mexican bean beetles.
Funding
Funding was through a research stipend from the University of Tennessee, Department of Entomology and Plant Pathology.
Disclosure
Authors declare no conflicts of interest.
References
- Chapman PH, GE Gould. The Mexican bean beetle in eastern Virginia. Bull VA Truck Exp. 1928; 65: 677-697.
- Capinera JL. Handbook of Vegetable Pests. Academic press. 2020.
- Howard NF. Feeding of the Mexican bean beetle larva. Ann Entomol Soc Am. 1941; 34: 766-769.
- McAvoy TJ, Smith JC. Feeding and developmental rates of the Mexican bean beetle on soybeans. J Econ Entomol. 1979; 72: 835-836.
- Peterson RK, Higley LG, Haile FJ, Barrigossi JA. Mexican bean beetle (Coleoptera: Coccinellidae) injury affects photosynthesis of Glycine max and Phaseolus vulgaris. Environ Entomol. 1998; 27: 373-381.
- Howard NF, English LL. Studies of the Mexican bean beetle in the Southeast. USDA. 1924.
- Capinera JL. Handbook of Vegetable Pests. Academic Press. 2001.
- McAlister DF, Krober OA. Response of soybeans to leaf and pod removal. Agron J. 1958; 50: 674-677.
- Kincade RT, Laster ML, Hartwig EE. Simulated pod injury to soybeans. J Econ Entomol. 1971; 64: 984-985.
- Smith RH, Bass MH. Relationship of artificial pod removal to soybean yields. J Econ Entomol. 1972; 65: 606-608.
- Krupke CH, Obermeyer JL, Bledsoe LW. Soybean insect control recommendations. Purdue Extension Publication No. E-77-W West Lafayette, IN. 2015.
- Hobson R. Tennessee Agricultural Statistics, Ann Bull. 1979; 27.
- van den Bosch R. The cost of poisons. Environment: Sci Policy Sustainable Develop. 1972; 14: 18-31.
- Howard NF, Landis BJ. Parasites and predators of the Mexican bean beetle in the United States. USDA. 1936.
- Waddill V, Shepard M. Dispersal of Podisus maculiventris nymphs in soybeans. Environ Entomol. 1975; 4: 233-234.
- Howard NF. The Mexican bean beetle in its bearing on Florida citrus growing. Fla St Plt Bd Qtly Bull. 1921; 6: 15-24.
- Eddy CO, McAlister Jr LC. The Mexican bean beetle. SC Agr Exp Stn Bull. 1927; 236: 38.
- Stevens LM, Steinhauer AL, Coulson JR. Suppression of Mexican bean beetle on soybeans with annual inoculative releases of Pediobius foveolatus. Environ Entomol. 1975; 4: 947-952.
- Fess TL. Organic management of Mexican bean beetle (Epilachna varivestis Mulsant) in snap bean (Phaseolus vulgaris L.). West Virginia University. 2008.
- Angalet GW, Coles LW, Stewart JA. Two potential parasites of the Mexican bean beetle from India. J Econ Entomol. 1968; 61: 1073-1075.
- Schaefer PW, Dysart RJ, Flanders RV, Burger TL, Ikebp K. Mexican bean beetle (Coleoptera: Coccinellidae) larval parasite Pediobius foveolatus (Hymenoptera: Eulophidae) from Japan: field release in the United States. Environ Entomol. 1983; 12: 852-854.
- Nottingham LB, Dively GP, Schultz PB, Herbert DA, Kuhar TP. Natural history, ecology, and management of the Mexican bean beetle (Coleoptera: Coccinellidae) in the United States. J Integr Pest Manag. 2016; 7: 2.
- Mukerji MK, LeRoux EJ. Laboratory rearing of a Quebec strain of the pentatomid predator Podisus maculiventris (Say) (Hemiptera: Pentatomidae). Phytoprotect. 1969; 46: 40-60.
- Warren LO, Wallis G. Biology of the spined soldier bug, Podisus maculiventris (Hemiptera: Pentatomidae). J Ga Entomol Soc. 1971; 6: 109-116.
- Maddox II. Cold storage of Podisus maculiventris (Say): effect on survival, longevity, fecundity and egg viability. Master’s Thesis, University of Tennessee. 1981.
- Baker AM, Lambdin PL. Fecundity, fertility, and longevity of mated and unmated spined soldier bug females. J Agr Entomol. 1985; 2: 378-82.
- Lambdin PL, Baker AM. Evaluation of dewinged spined soldier bugs, Podisus maculiventris (Say), for longevity and suppression of the Mexican bean beetle, Epilachna varivestis Mulsant, on snap beans. J Entomol Sci. 1986; 21: 263-266.
- Coppel HC, Jones PA. Bionomics of Podisus spp. associated with the introduced pine sawfly Diprion similis (Htg), in Wisconsin. Trans WI Acad Sci. 1962; 51: 31-56.
- Kim CW, Kim JI, Kim SH. Biological control of fall webworm, Hyphantria cunea Drury in Korea. II. Studies on the natural enemies imported. Entomol Res Bull. 1968; 4: 37-56.
- Tadic M. Process of adaptation of indigenous parasites and predators on the fall webworm (Hyphantria cunea Dr.) in Yugoslavia in 1963-1972. Zastita Bilja. 1975; 26: 247-267.
- Hokyo N, Kawauchi S. The effect of prey size and prey density on the functional response, survival, growth and development of a predatory pentatomid bug, Podisus maculiventris (Say). Popul Ecol. 1975; 16: 207-218.
- Sazonova RA, Shagov EM, Stradimova LA. Podisus—a predator of the American white butterfly and the Colorado beetle. Zashchita Rastenii. 1976; 8: 52.
- Ignoffo CM, Garcia C, Dickerson WA, Schmidt GT, Biever KD. Imprisonment of entomophages to increase effectiveness: evaluation of a concept. J Econ Entomol. 1977; 70: 292-294.
- Neves RC, Torres JB, Vivan LM. Reproduction and dispersal of wing-clipped predatory stinkbugs, Podisus nigrispinus in cotton fields. BioControl. 2009; 54: 9-17.
- Evans EW. Consequences of body size for fecundity in the predatory stinkbug, Podisus maculiventris (Hemiptera: Pentatomidae). Ann Entomol Soc Amer. 1982; 75: 418-420.
- Lambdin PL, Lu GQ. External morphology of eggs of spined soldier bug, Podisus maculiventris (Say) (Hemiptera: Pentatomidae). Proc Entomol Soc Wash. 1984; 86: 374-377.
- Michels Jr. GJ, Burkhardt CC. Economic threshold levels of the Mexican bean beetle on pinto beans in Wyoming. J Econ Entomol. 1981; 74: 5-6.
- Baek S, Son Y, Park Y-L. Temperature-dependent development and survival of Podisus maculiventris (Hemiptera: Pentatomidae): implications for mass rearing and biological control. J Pest Sci. 2014; 87: 331-340.
- Yeargan KV. Parasitism and predation of stink bug eggs in soybean and alfalfa fields. Environ Entomol. 1979; 8: 715-719.
- Buschman LL, Whitcomb WH. Parasites of Nezara viridula (Hemiptera: Pentatomidae) and other hemiptera in Florida. Fla Entomol. 1980; 63: 154-162.
- Tillman, PG. Parasitism and predation of stink bug (Heteroptera: Pentatomidae) eggs in Georgia corn fields. Environ Entomol. 2010; 39: 1184-1194.
- Stoner A, Metcalfe AM, Weeks RE. Development of Podisus acutissimus in relation to constant temperature. Ann Entomol Soc Amer. 1974; 67: 718-719.
- Ramalho ES, Mezzomo J, Lemos WP, Bandeira CM, Malaquia JB, Silva JPS, et al. Reproductive strategy of Podisus nigrispinus females under different feeding intervals. Phytoparasitica 2008; 36: 30-37.
- Lambert AM. Effects of prey availability, facultative plant feeding, and plant defenses on a generalist insect predator. Arthropod-Plant-Interact. 2007; 1: 167-173.
- Mohaghegh J, de Clercq P, Tirry L. Effects of maternal age and egg weight on developmental time and body weight of offspring of Podisus maculiventris (Heteroptera: Pentatomidae). Ann Entomol Soc Amer. 1998; 91: 315-322.
- Gaudreau M, Guerra-Grenier E, Abram PK, Brodeur J. Photoprotective egg pigmentation reduces negative carryover effects of ultraviolet radiation on stink bug nymph survival. J Insect Physiol. 2021; 133: 104273.
- López Jr JD, Ridgway RL, Pinnell RE. Comparative efficacy of four insect predators of the bollworm and tobacco budworm. Environ Entomol. 1976; 5: 1160-1164.
- Grabarczyk EE, Cottrell TE, Schmidt JM, Tillman PG. Low incidence of avian predation on the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae), in southeastern orchard systems. Insects. 2023; 14: 595.
- Chittenden FH, Marsh HO. The bean ladybird. USDA Bull. 1920; 843: 1-21.
- DeLong DM. Parasites and predators of the Mexican bean beetle in the United States. USDA Circ. 1924; 418: 8.
- Judge FD, Mcewen FL, Rinick Jr HB. Field testing candidate insecticides on beans and alfalfa for control of Mexican bean beetle, potato leafhopper, and plant bugs in New York State. J Econ Entomol. 1970; 63: 58-62.
- Ridgway RL, Jones SL. Inundative releases of Chrysopa carnea for control of Heliothis on cotton. J Econ Entomol. 1969; 62: 177-180.
- Montemayor CO, Cave RD. Evaluation of the predation capacity of Podisus maculiventris (Hemiptera: Pentatomidae) on Microtheca ochroloma (Coleoptera: Chrysomelidae) in field cages. J Econ Entomol. 2012; 105: 1719–1725.