
Special Article: Horticulture
Ann Agric Crop Sci. 2024; 9(3): 1156.
Separation of Phycocyanin from Spirulina platensis Using a Non-Conventional System: Evaluation of Photostability and Antioxidant Activity
Yonca Duman*; Hilal Aktürk
Kocaeli University, Faculty of Arts and Sciences, Department of Chemistry, Section of Biochemistry, Umuttepe Campus, 41380 Izmit-Kocaeli/TURKEY.
*Corresponding author: Yonca Duman Kocaeli University, Faculty of Arts and Sciences, Department of Chemistry, Section of Biochemistry, Umuttepe Central Campus, 41380 Izmit-Kocaeli / Turkey. Tel: 90-262-3032019; Fax: 90-262-3032003 Email: yavci@kocaeli.edu.tr
Received: April 15, 2024 Accepted: May 13, 2024 Published: May 20, 2024
Abstract
In present study, the combinable approach of freezing and thawing and aqueous two-phase partitioning system was designed for simple, rapid and cost friendly extraction and purification of Spirulina plantesis phycocyanin. From cell extract, C-PC was purified 1.38-fold 51.35% recovery by ammonium sulphate fractionation then PEG3000/KH2PO4/K2HPO4 system at pH 7. Contrary to expectations, increasing neutral salt concentrations resulted in decreased C-PC recovery, deviating from the typical enhancement of biomolecular partitioning performance in aqueous two-phase systems. The C-PC showed remarkable in vitro antioxidant and radical-scavenging activity. 200 µg/mL C-PC, performed a maximum absorbance by phosphomolybdenum and ferric ion reducing assay, 0.55 and 0.45, respectively. H2O2 scavenging activity was defined as 93.67%. Notably, modified C-PC (m-C-PC) exhibited improved photostability under yellow light exposure compared to unmodified C-PC, showcasing a 1.064-fold enhancement after 540 minutes. However, m-C-PC showed increased susceptibility to white light, UV-A and UV-B irradiation, suggesting a failure to enhance stability under these conditions.
Introduction
Phycocyanin Spirulina platensis, which is the microalgae, includes proteins, vitamins-minerals, and contains many essential amino acids and fatty acids, can be used as a useful component to produce quite nutritious foods. Gamma-Linolenic Acid (GLA), which is a metabolite of Linolenic Acid (LA) and one of the intermediates in the conversion of LA to Arachidonic Acid (AA), is an imperative polyunsaturated fatty acid with economic concern, and S. platensis is a crucial reservoir of GLA. The microbial production of polyunsaturated fatty acids is considered an economical alternative to producing them with high-cost methods [21,14] Spirulina is a low-priced source of blue pigment. Due to its rich metabolites, the culture of Spirulina is engaging in several commercial aims, as well as a nutritional additive for humans, and provides active metabolites in the pharmaceutical, food, and cosmetic industry [1,28,47]. Phycobiliproteins are associated with photosynthetic pigments localized in the cell as phycobilisomes, which are affiliated to the thylakoid membrane of chloroplast [4]. Cyanobacterial phycobiliproteins, that are widely used in medicines, foods, cosmetics can be divided into three critical classes; Phycoerythrin (PE –bright pink, red), Phycocyanin (PC –dark blue), and allophycocyanin (AP –brighter blue) [15]. Phycocyanin is a phycobiliprotein and is the main pigment produced by the Spirulina platensis [48]. A blue water-soluble pigment, phycocyanin, which has an important antioxidant and free radical properties; it is also carried out as a natural coloring in food such as ice cream, jellies, chewing gum, and also dye in cosmetics and medicine due to they are not toxic and not carcinogenic [28,41]. In medicinal applications, particularly fluorescent analysis, pigment purity is of the most importance, [43]. C-PC purity is defined as the commercial ability, which is defined by the ratio between its peak absorbance at 620 nm and the absorbance of proteins at 280 nm. When this ratio is = 0.7, it is reflected in food grade purity [11]. Pure and stable C-PC is essential to commercially apply ability. Although all the benefits, there are still restrictions for C-PC implementation especially because of the extraction methods, that consequence in low purity extracts, and low stability of the pigment under storage and during food processing. Different cell disruption techniques were reported for Spirulina as freezing and thawing cycles, pulsed electric field, bead milling, and mixing, ultrasound (bath) and homogenization [16,18,33]. Freezing and thawing method is commonly carried out at laboratory scale to cells for C-PC extraction from Spirulina. The main profits of freezing and thawing method are the comparatively high purity of the extracts and its effortlessly [45].
Due to the industrial and commercial important of the C-PC, researchers have improved a number of procedures for the purification previously. But these procedures have been represented by expensive, lots of steps and low recovery. Moreover, the scaling-up of these processes was troublesome and high cost. Using of Aqueous Two-Phase System (ATPS) to separate the C-PC has been an engaging option to reduce the disfavor conditions [23,35].
Despite the widespread usage of C-PC, its poor durability constrains its utility. According to reports, solid phycocyanin from Spirulina platensis degrades easily when the substance experienced degradation upon exposure to light [10] More than 50% of the sample exhibits degradation after being exposed for a month. It can occur due to phycocyanin simply dissociates to monomers at low levels. The photostability properties of phycocyanin can be enhanced through the use of a crosslinking agent. This results in a more stable and durable phycocyanin pigment that can withstand exposure to light. Additionally, the use of this technique allows for the preservation of the pigment's native structure and biological activity. Overall, these advancements showcase the potential of phycocyanin and its practical applications in various industries.
Crosslinking is the process of forming three-dimensional structure networks by connecting polymer chains (Azeredo and Waldron, (2016). It aims to enhance the capacity and usability of the material. Several natural crosslinks, including disulphide bridges, can be utilized for proteins. Formaldehyde and glutaraldehyde are likely the most frequently used crosslinkers due to their low cost and accessibility.
In this research, formaldehyde (HCHO) was selected as the crosslinker due to its ease of use, varied reaction specificity and remarkable adaptability [27].
The aim of this study is also to investigate the possibility of improving the photostability properties of phycocyanin using the crosslinking technique. Phycocyanin loses its strong absorption [49] and high fluorescent quantum efficiency [50] at concentrations below 10-6M.
In the current investigation, the optimization of phycocyanin extraction from Spirulina was achieved through the implementation of the freezing and thawing method. Subsequently, the partitioning of the extracted phycocyanin was conducted using an aqueous two-phase system. The influences of inorganic salt concentration, pH, and temperature on the partitioning of the pigment were systematically analyzed. The primary objective of this study was to establish an efficient extraction and purification methodology for phycocyanin sourced from Spirulina. Additionally, the research aimed to assess the antioxidant activity and photostability of the purified C-PD, with a specific focus on its potential applications in industrial settings.
Material and Methods
Chemicals
All chemicals used were purchased from Sigma-Aldrich (Auckland, New Zealand), unless specifically stated otherwise. Dried S. platensis was obtained from local producer in Çanakkale/Turkey region.
Extraction Methods
Phycocyanin was acquired from the Spirulina by using the following procedures:
i)Homogenization in a mortar and pestle: Dried biomass was homogenized in a mortar. ii) Freezing and thawing: Cells were subjected to freezing and thawing for 1, 2, 3, and 24 hours. In the second case (2 hours), the freezing and thawing procedure was repeated twice, with 24 hours intervals. iii) Ultrasonic treatment: Spirulina cells were homogenized with the sonicator (QSonica, Q500) which was equipped with a standard needle titanium probe (1/2 inch), and was kept immersed about 5 mm into the samples. Sonication experiments were done at 20 kHz.15 mL volumes of samples were placed in a 25 mL erlenmeyer flask at room temperature. In all cases, the duty of the cycle was 20 s, with the generator acting for 10 s intervals with 10 s of rest. After extraction, the samples were centrifuged and the supernatant was used for further analysis.
Partitioning of Phycocyanin in Aqueous Two-Phase System (ATPS)
ATPS was carried out in 25-mL centrifuge tubes by adding the predetermined quantities of stock solutions of PEG and salt and the total weight was made up to 5 g with crude extract and buffer (KH2PO4/K2HPO4; (1:1.82; g:g), pH 7). To analyze the effect of different salts on phycocyanin partitioning, ammonium chloride, sodium chloride, and sodium sulfate were determined by the constant total level of PEG and salt in the system as the 15%. The system parameters were chosen according to previous reports [13,30]. Salt, PEG, buffer, and deionized water were first mixed for the averting of phycocyanin precipitation, then the addition of 1 mL crude extract to the phase system was carried out. The mixture was softly shaken for 60 min at room temperature and separated by centrifugation for 5 min at 2500 g. The upper phase was properly isolated from the lower phase by using a Pasteur pipette then dialyzed. The volumes of the separated phases were measured. Each of the phases was analyzed for analytical analysis. The partition experiments were conducted in duplicates.
Analytical Measurements
Analytical measurements of C-PC were done using UV–vis spectrophotometer (Bio-Rad SmartSpec 3000 UV/Vis Spectrophotometer. Wavelength range: UV and Visible ranges 200–800 nm. Wavelength accuracy: ±2 nm). The C-PC concentration in mg.mL-1 was calculated according to Equation 1 (Bennett and Bogorad, 1973), by using the optical densities at 652 and 620 nm. The ratio of A620 to A280 gives the purity of C-PC, wherein A620 is the maximum absorbance of C-PC and A280 is the absorbance of total proteins.
Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Molecular weight of purified invertase was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Laemmli on a Mini Protean II gel electrophoresis unit (Bio-Rad Laboratories, Richmond, CA). Electrophoresis was studied at a constant current of 100 V, 400 mA, for about 2 hr.
Antioxidant Activity of Pigment
The antioxidant activity of phycocyanin was studied as below methods: phosphomolybdenum assay, ferric ions reducing assay, DPPH scavenging assay and H2O2 scavenging assay.
Phosphomolybdenum Assay
Phosphomolybdenum method is one of the total antioxidant activity assays of the phycocyanin [38]. Different concentrations (200 µg/mL, 100 µg/mL, 50 µg/mL, 25 µg/mL and 5 µg/mL) of 1 mL C-PC of were mixed with 1 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The reaction mixture was incubated at 95°C for 90 min. After cooling to room temperature, the absorbance was measured at 695 nm against blank.
Ferric Ions Reducing Assay
Reduction of ferric ion by phycocyanin was carried out according to the report of Oyaizu (1986) with minor modifications. Various concentrations C-PC (200 µg/mL, 100 µg/mL, 50 µg/mL, 25 µg/mL and 5 µg/mL) were combined with 0.75 mL 0.1 M (KH2PO4/K2HPO4, pH 7) buffer and 0.75 mL of 1% potassium ferrocyanide [K4Fe(CN)6]. The solution was incubated at 50 °C for 20 min. 0.75 mL of trichloroacetic acid (10%) was added to the reaction mixture, and was centrifuged at 3000 rpm for 10 min. The top layer of the mixture was combined with 2.5 mL of distilled water and 0.1 mL of 0.1% FeCl3. Then were incubated at room temperature for 20 min and the absorbance was measured at 700 nm.
Hydrogen Peroxide free Radical Scavenging Assay
Hydrogen peroxide radical scavenging assay was carried out to measure the scavenging activity of free radicals by C-PC (Roche et al., 1989). 1 mL of different concentrations of 1 mL C-PC (200 µg/mL, 100 µg/mL, 50 µg/mL, 25 µg/mL and 5 µg/mL) was quickly combined with 2 mL of hydrogen peroxide solution (50mM hydrogen peroxide in KH2PO4/K2HPO4 buffer, pH 7). After 10 min of incubation at 37ºC; absorbance was measured at 230 nm. Non included H2O2 solution was used as blank. Scavenging performance of pigment as percentage was calculated using the formula 2. A0 and A1 refers to absorbance of control and absorbance of test sample, respectively.
Lipid Peroxidation Products
The level of lipid peroxidation of C-PC was carried out in the way with Malondialdehyde (MDA) ingredient according to the method of Turan and Tripathy (2013) with some modifications. MDA was detected using the Thiobarbituric Acid (TBA) reaction. 0.5 mL C-PC was added to 0.5 mL of TBA reagent (containing 15% w/v, trichloroacetic acid; 0.375% w/v, TBA in 0.25 M HCl). The mixture was heated to 95°C for 15 min then cooled fastly in ice bath and centrifuged at 2000 g for 20 min. Absorbances were read (Bio rad Smartspec 3000) at 532 and 600 nm. The amount of MDA current was calculated from the extinction coefficient of 156 mM-1cm-1 and predicted using the following equation: [(A532-A600) / 156] x 103x dilution ratio.
Modification of C-PC Using Formaldehyde and Evaluation of Photostability of Modified C-PC (m-C-PC)
The modification of phycocyanin was conducted by employing formaldehyde, as outlined in literature with certain modifications [27,34]. The addition of 10 mL of 37% formaldehyde supplement to 50 mL purified C-PC was performed using a magnetic stirrer for 24 hours at +4°C. Following this, the solution underwent dialysis overnight. The resulting modified phycocyanin solution was then transferred to a dark bottle and stored at +4°C for later process.
To assess the resistance of C-PC to light, a photostability measurement was conducted using various light sources. C-PC was exposed to cool white-light fluorescent illumination from a set of Philips white LED lights (18 W), yellow light from a set of yellow LED lights S Lighting (14 W), UV A (8 W) lamps, and UV B Neon lighting tools. The irradiation conditions were adapted from a previous study with certain modifications [27,36]. The photostability analysis included continuous exposure of C-PC to light for 540 minutes (9 hours). Measurements were taken at, 30, 60, 90, 120, 180, 240, 300, 360, 420, 480, 540 minutes of exposure using a UV–vis spectrophotometer, with absorbance readings recorded at 620 nm. The experimental design aimed to explore alterations in the photostability of C-PC under diverse light conditions during the 9 hours exposure period. Specific time points for measurement were selected to provide a thorough understanding of how the samples reacted to extended light exposure. The UV–vis spectrophotometer was utilized to quantify changes in absorbance at designated wavelengths, yielding valuable insights into the photostability of C-PC under the experimental conditions.
Results
Extraction of C-PC from Spirulina plantesis and Selection of ATPS system
We evaluated three different methods for extracting C-phycocyanin from dried cultures: mechanical cell disintegration using a mortar and pestle, sonication, and freezing–thawing. The results, as presented in Table 1, indicate that freezing–thawing was the most effective method for C-PC extraction when compared to the other methods.
Purity and phycocyanin concentration (mg/mL) values of C-phycocyanin from S. platensis cells using various treatments. 1) homogenization by mortar and pestle; 2) Sonication; 3) Freezing and thawing for 1h; 4) Freezing and thawing for 2 h; 5) Freezing and thawing for 3 h; 6) Freezing and thawing for 24 h.
After the extraction of C-PC through freezing and thawing, the culture medium underwent centrifugation (10,000 g, 10 min, 4°C) to eliminate S. platensis cells. The dissolved proteins in the resulting supernatant were precipitated using solid ammonium sulfate with two steps: first, at 25% saturation, and then at 65% saturation. Following the 25% saturation step, the medium was subjected to centrifugation at 10,000 g for 10 min at 4°C, and the supernatant was collected. The precipitate was collected after centrifugation at 12,000 g for 10 min at 4°C during the second precipitation step and subsequently dissolved in a 50 mM KH2PO4/K2HPO4 buffer at pH 7.
The separation of biomolecules can be an expensive and time-consuming process. As an alternative method to traditional bioseparation techniques, Aqueous Two-Phase Systems (ATPS) have gained popularity due to their cost-effectiveness, simplicity, and efficiency at a larger scale.
PEG and salts were examined to determine the optimal partitioning of phycocyanin into one of the two phases. To investigate the influence of PEG molecular weight on C-PC partitioning, various ATPS with PEG1000, PEG2000, PEG3000, PEG4000, and PEG6000 were designed. All experiments were conducted at pH 7.0 using KH2PO4/K2HPO4 as the phase-forming salt. The results are presented in Table 2. In all experiments, two phases were observed, with C-PC predominantly present in the top phase. Therefore, only data from the top phase were considered in this study to analyze the partitioning behavior of Spirulina platensis C-PC in aqueous two-phase systems.
C-PC partitioning at different concentrations of KH2PO4/K2HPO4 from 10 to 20% (w/w) in ATPS planned by using 15%(w/w) PEG3000, PEG4000 and PEG6000 at pH 7.0 was carried out. The effect of salt concentration on enzyme partition is presented in Figure 1, Figure 2 & Figure 3.
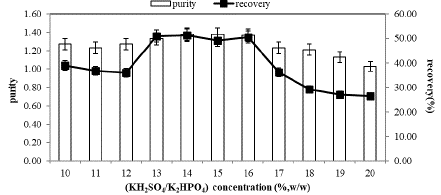
Figure 1: Effect of KH2PO4/K2HPO4 (w/w) concentration on partitioning of Spirulina plantesis C-PC in PEG3000 at pH 7.0 in the top phase.
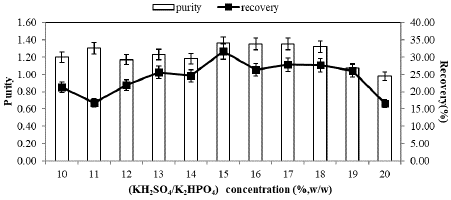
Figure 2: Effect of KH2PO4/K2HPO4 (w/w) concentration on partitioning of Spirulina plantesis C-PC in PEG4000 at pH 7.0 in the top phase.
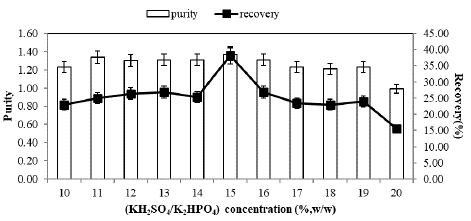
Figure 3: Effect of KH2PO4/K2HPO4 (w/w) concentration on partitioning of Spirulina plantesis C-PC in PEG6000 at pH 7.0 in the top phase.
Although significant changes in purity and recovery were not observed within the concentration range of 13%, 14%, 15%, and 16%, as indicated in Figure 1, the highest C-PC recovery and purity were consistently achieved at a constant 15% PEG3000 concentration and 14% salt concentration, measuring 51.35% and 1.38, respectively. This suggests that C-PC partitioning in the PEG4000/KH2PO4/K2HPO4 system is fundamentally influenced by polymer molecular weight and salt concentration, as depicted in Figure 2. In the PEG4000 system, purity and recovery were found to increase up to a 15% salt concentration (purity and recovery of C-PC at 1.36 and 31.60%, respectively), beyond which these values decreased.
To explore the impact of phase-forming salt concentration on C-PC partitioning, experiments were conducted in different phase systems with salt concentrations ranging from 10% to 20% (w/w), while maintaining a constant 15% (w/w) PEG6000 concentration. The results, as shown in Figure 3, reveal that C-PC recovery and purity were highest at a 15% (w/w) KH2PO4/K2HPO4 concentration in the top phase, reaching 40.12% and 1.37, respectively.
As in the salt concentration polymer concentration also affects the phase separation and partition of biomolecules in ATPS (Albertson, 1986). Accordingly, C-PC partition at various PEG3000 concentrations (10–20%, w/ w) using 10% (w/w) KH2PO4/K2HPO4 salt at pH 7.0 was carried out. The results showed that the partitioning of protein was clearly affected by the PEG concentration (Figure 4). With the use of 15% (w/w) PEG3000, the highest purify (1.38) and recovery (51.30%) values were received. Above this concentration, both decreased nearly to 40% and 54%, respectively.
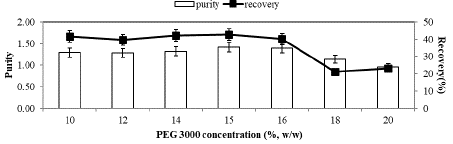
Figure 4: Effect of PEG3000 concentration on the partitioning of Spirulina plantesis C-PC in PEG/ KH2PO4/K2HPO4 system at the constant potassium salt concentration (15%) and at pH 7.0 in the top phase.
Effect of pH and Temperature on ATPS
In the partitioning of proteins within aqueous two-phase systems, pH emerges as another significant parameter. Consequently, the partitioning behavior of C-PC was investigated across a range of pH values spanning from 5.0 to 12.0 in a mixture containing 14% (w/w) PEG3000 and 15% (w/w) KH2PO4/K2HPO4. As depicted in Figure 5 (A), the highest partitioning efficiency was observed at pH 7.0. However, as the pH was increased from 7.0 to 12.0, both the purity and recovery values decreased. Conversely, below pH 7.0, the partitioning performance of C-PC also exhibited a decline.
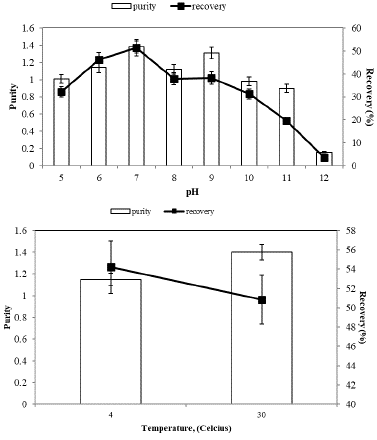
Figure 5: pH (A) and temperature (B) effect on partitioning of Spirulina plantesis C-PC.
Furthermore, we examined the partitioning of Spirulina platensis C-PC within an aqueous two-phase system at two distinct temperatures: 4 and 30 °C (Figure 5(B)). In this study, we closely monitored the temperature's impact on the purity and recovery of C-PC partitioning.
Effect of Neutral Salts on ATPS
Figure 6 illustrates the impact of increasing concentrations of sodium sulfate (A), ammonium chloride (B), and sodium chloride (C) as neutral salts on Spirulina platensis C-PC. In our study, it was observed that the purity of C-PC in the top phase remained nearly constant at 1.37, while the recovery decreased by approximately 45%, 30%, and 42%, respectively, with an increase in salt concentration.
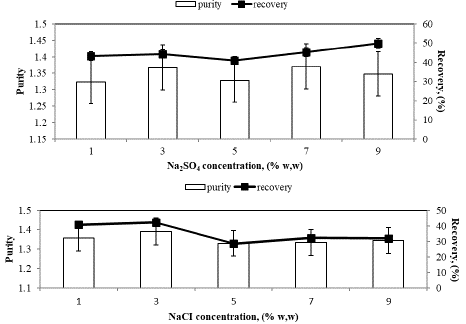
Figure 6: A: Increasing Sodium sulfate concentrations effect on purity and recovery of Spirulina plantesis C-PC. B: Increasing ammonium chloride concentrations effect on purity and recovery of Spirulina plantesis C-PC. C: Increasing Sodium chloride concentrations effect on purity and recovery of Spirulina plantesis C-PC.
The molecular weight of C-phycocyanin was reported to be approximately 17 kDa for the Β subunit and 16 kDa for the a subunit [20,44]. In this study, polyacrylamide gel electrophoresis (SDS-PAGE) was conducted to separate and visualize the protein bands. The resulting bands were stained and visualized using a silver stain method, which allowed for the identification of two distinct bands, corresponding to the a and Β subunits.
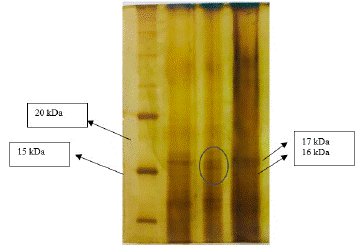
Figure 7: SDS-PAGE analysis of C-PC.
Antioxidant Activity of C-PC
The antioxidant activities exhibited a concentration-dependent increase, with a maximum absorbance of 0.55 observed at a concentration of 200 µg/mL and a minimum absorbance of 0.36 at 5 µg/mL (Figure 8). Figure 8 also illustrates the optical density of the reaction medium at 700 nm, which is directly related to the reducing power of C-PC. The increase in OD at 700 nm, indicating the formation of a Fe+2 complex, suggests that C-PC possesses significant reducing capabilities.
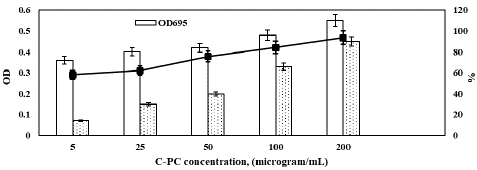
Figure 8: Antioxidant properties of Spirulina plantesis C-PC.
Hydrogen peroxide is produced in vivo by the superoxide dismutase enzyme and exhibits strong oxidizing potential towards cell membranes and biomolecules. In our study, we evaluated the ability of partitioned C-PC to scavenge hydrogen peroxide radicals, as depicted in Figure 8. The maximum scavenging activity of 93.67% for C-PC was observed at a concentration of 200 µg/mL, while a minimum scavenging activity of 58.13% was recorded at the lowest C-PC concentration.
Photostability of C-PC
The evaluation of photostability under various light exposures, including yellow light, white light, UV-A light, and UV-B light, was conducted on both C-PC and m-C-PC, aiming to understand their resilience in different spectral regions representative of sunlight. Figure 9 illustrates the impact of yellow light on the photostability of C-PC and m-C-PC. Notably, C-PC exhibits a time-dependent reduction in absorbance, while m-C-PC maintains relatively constant absorbance. The residual absorbance percentages for m-C-PC over the 30-540 minutes yellow light exposure range from 94.83% to 91.6%, showcasing its enhanced stability compared to C-PC. This suggests that the modification with formaldehyde contributes to the improved resistance of m-C-PC against yellow light. The residual absorbance values for m-C-PC, ranging from 85% to 65% during 30-540 minutes of white light exposure, demonstrate its susceptibility to this type of irradiation. In comparison, C-PC exhibits a residual absorbance of 75% after 540 minutes. This implies that m-C-PC, despite its enhanced stability against yellow light, shows a notable decrease in photostability under white light, unlike the native C-PC.
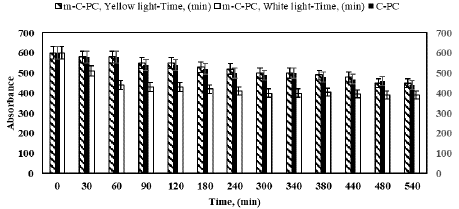
Figure 9: Photostability analysis of C-PC and m-C-PC from Spirulina platensis with formaldehyde under increasing exposure time to yellow and white light.
Figure 10 provides insights into the effects of UV-A and UV-B light on both C-PC and m-C-PC. Both variants exhibit a decrease in absorbance under UV-A light, with m-C-PC showing a comparable reduction to C-PC. However, under UV-B light, m-C-PC experiences a more significant decrease in absorbance (from 87.90% to 39.54%) compared to C-PC (from 96.7% to 80%). This suggests that m-C-PC is more susceptible to UV-B light, indicating a potential limitation in its stability under this specific irradiation.
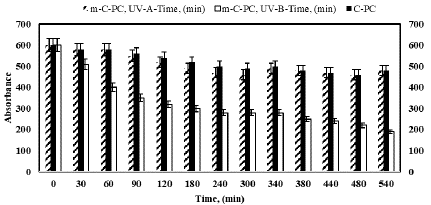
Figure 10: Photostability analysis of C-PC and m-C-PC from Spirulina platensis with formaldehyde under increasing exposure time to UV-A and UV-B.
Discussion
Due to the selection of a suitable extraction method is very important for the maximum recovery of C-PC, the most significant necessity to get phycobiliproteins from crude Spirulina plantesis extract is optimizing the extraction and purification steps. According to Table 1, purity and C-PC concentration vary between 0.33-0.44 and 0.85-1.98 mg mL-1, respectively. The highest purity and concentration of C-PC was reached when using freezing (- 20°C) and thawing (25 °C) for 2 h. Freezing and thawing cycles are considered the most used at the lab scale for disrupting cyanobacteria cells and showing higher C-phycocyanin content than the other methods and also allow superiority in the same way as reproducible, quick, simple, and free from biomass quantity was reported [16,45].
In aqueous two-phase extraction, which is an alternative purification method, the separation resulting from almost all of the proteins are predominantly differential partitioning of the target protein to one phase and the contaminant proteins to the other phase. The principle of the partitioning of biomolecules relates in general to the size, charge, and hydrophobicity of the protein [12]. The molecular weight of PEG is significant for ATPS to increase the protein partition into one phase. With higher molecular weights of PEG, the free volume area for protein becomes limited. Thus, proteins shift to the salt-rich phase. But then, low molecular weights of PEG supply more volumetric area for proteins which causes the partition of proteins to the top phase and increase partitioning due to low interfacial tension. [2,3]. There are some reports in the literature that C-PC mostly participates in the top phase of the ATPS system [9,23,30,40]. In our study, although the purity value was the highest (1.34) in the PEG4000 system,1.33 was calculated in PEG6000 system at the top phase. Moreover, most C-PC recovery was observed in the PEG3000 system. Thus, these three systems were selected for further experiments. The type and concentration of salt is as crucial in protein partitioning as the type of PEG. According to Patil et al. [31], the purity of the C-phycocyanin is interpreted from the ratio of the absorbances OD620/OD280, where a value of 0.7 or above is regarded to be of nutritive value, and this value was reached in the above three assays, in the system of ATPS prepared of PEG3000, PEG4000, PEG6000, and potassium phosphate. It has been previously studied that increasing salt concentrations cause a decrease in the protein value but an increase in the enzyme activity value at the bottom phase meaning that the contaminant protein load in the top phase results in an improved purification factor [6,25]. In PEG/salt systems, the partitioning of the proteins relies on the volume exclusion effect of the polymer in the polymer-rich (top) phase and salting out effect in the salt-rich (bottom) phase. The researchers described that [12] with an increased salt concentration in the salt-rich (bottom) phase, the solubility of biomolecules decreases, which results in increased partitioning of biomolecules to the top phase and is inferred as a “salting out effect.” In this study, the solubility limit was detected at 15% potassium phosphate; thus, maximum C-PC recovery (51.35%) with a purify (1.38) was observed at 15% (w%w) potassium phosphate and PEG3000 system.
Another critical parameter for partitioning of biomolecules in ATPS is the pH of the system due to it can change the net charge of protein or any of other charged molecules in the system [12]. The pH affects the target protein through electrostatic interactions and the charges of contaminant proteins, which may alter the electrical potential governing the partitioning process [12,19]. The results suggested that the partitioning of C-PC into the top phase was more efficient when the pH was adjusted to 7.0.
Temperature effect on the phase content is confused because of the fact that the electrostatic interactions and the hydrophobic inter- actions are all affected by the temperature. Increasing or decreasing effects of temperature on partition coefficient, and, no temperature effect on partition of biomolecules in ATPS systems were reported [42]. This might be due to there was change to phase composition in the PEG3000/ KH2PO4/K2HPO4 (14–15% (w/w)) at pH 7 system when temperature was changed. Therefore, 30 °C was selected for further studies (Figure 5B).
While neutral salts can enhance biomolecular partitioning performance in ATPS [19], our study observed a decrease in recovery with increasing neutral salts concentrations, which could be attributed to the reduced partitioning of C-PC into the top phase due to higher concentrations of neutral salts [26].
Antioxidant Activity and Photostability of C-PC
The antioxidant activity of C-PC is related about its amino acid residues like Glu, Asp, Ala, Leu, Arg, Ile, Ser and Gly. Antioxidant activity of C-PC is conducted by both through scavenging the already produced ROS via redox reaction and by reducing the oxidized metal ions, which can promote ROS production [38].
Results of different in vitro antioxidant analysis are reported in literature [8,37,41]. The total antioxidant activity of the C-PC was predicted as spectrometrically at OD695 by phosphomolybdenum assay method. The assay is established on the reduction of molybdenum (VI) to molybdenum (V) by the antioxidant which causes to change of green phosphate molybdenum (V) complex at acidic pH [38]. Moreover, the antioxidant activity of C-PC was also checked by measuring its ability to reduce the ferric ion (to ferrous ion) [44]. Antioxidant activities of phycocyanin were assessed using various assays, including the phosphomolybdenum method, ferric reducing activity, and H2O2 scavenging activity. Our findings indicate that the phycocyanin pigment isolated from Spirulina platensis could serve as a promising antioxidant agent and a potential biomolecule to mitigate oxidative stress-related issues.
Notably, m-C-PC exhibited an improved photostability up to 1.064-fold after 540 minutes of yellow light exposure compared to C-PC. Further investigations are required to comprehend the enhanced stability of m-C-PC at the molecular level under yellow light. In contrast to yellow light irradiation, the absorption spectra of m-C-PC decreased more significantly than those of unmodified C-PC under white light exposure. This outcome suggests that m-C-PC failed to enhance the stability of PC under white light exposure, indicating greater susceptibility to white light irradiation. The results highlight the nuanced photostability of C-PC and m-C-PC under different light conditions. While m-C-PC demonstrates improved stability against yellow light, its susceptibility to white light and UV-B light raises questions about the generalizability of its enhanced stability. Further investigations are warranted to understand the molecular mechanisms behind these observations and to explore potential applications and limitations of m-C-PC in diverse environmental conditions. Additionally, the study emphasizes the importance of considering specific light conditions when assessing the photostability of modified pigments.
Conclusion
In this study, the focus was on the purification and recovery of C-PC from Spirulina plantesis using an Aqueous Two-Phase System (ATPS). The impact of various experimental parameters on C-PC extraction was systematically analyzed.
The application of this simple and cost-effective technique resulted in a successful protein purification, achieving a 51.30% recovery and a 1.38-fold purification from S. plantesis. The partitioning behavior of C-PC in the PEG3000/KH2PO4/K2HPO4 (14–15% (w/w)) ATPS system at pH 7 revealed that the protein could be efficiently extracted into the top phase. Key factors such as PEG molecular weight, salt type, and concentration were identified as significant influencers on the partitioning of C-PC. Notably, ATPS emerged as a low-cost, straightforward, safe, and highly efficient method for protein purification when compared to conventional techniques. Additionally, the study aimed to characterize the C-PC partitioned by the PEG3000/KH2PO4/K2HPO4 ATPS. The optimized process holds promise for encouraging the utilization of C-PC in both pure science and applied research, facilitating a better understanding of partitioning processes in algae.
Antioxidant activities of the phycocyanin were assessed using various assays, including the phosphomolybdenum method, ferric reducing activity, and H2O2 scavenging activity. The total antioxidant capacity, determined by the phosphomolybdenum method, exhibited a maximum absorbance of 0.55 at 200 µg/mL. Furthermore, the hydrogen peroxide scavenging activity demonstrated a maximum scavenging activity of 93.67% at a phycocyanin concentration of 200 µg/mL. In conclusion, the study not only showcased the efficiency of ATPS in purifying C-PC but also shed light on the promising antioxidant potential of the isolated phycocyanin from Spirulina plantesis. The investigation into the photostability of modified phycocyanin (m-C-PC) highlighted improvements under yellow light exposure but revealed susceptibility to white light. Future research endeavors should focus on elucidating the molecular mechanisms behind these observations, thereby paving the way for broader applications and addressing potential limitations in various environmental conditions. The tailored consideration of specific light conditions in assessing photostability remains crucial for the practical utility of modified pigments in both industrial and environmental contexts.
Author Statements
Competing Interests
(The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.)
Author’s Contributions
(Yonca Duman: Supervision, Conceptualization, Methodology, Writing- Original draft preparation.
Hilal Aktürk: Software, Data curation, Visualization)
Availability of Data and Materials
(The data used to support to findings of this study are included within the article)
References
- Adjali A, Clarot I, Chen Z, Marchioni E, Boudier A. Physicochemical degradation of phycocyanin and means to improve its stability: a short review. Journal of Pharmaceutical Analysis. 2021.
- Albertson PA. Partition of Cell Particles and Macromolecules.3rd ed; Wiley: New York, USA. 1986.
- Albertson PA, Cajarville A, Brooks DE, Tjerneld F. Partition of Proteins in Aqueous Polymer Two-Phase Systems and the Effect of Molecular Weight of the Polymer. Biochim. Biophys. Acta. 1987; 926: 87–93.
- Arad SM, Yaron A. Natural pigments from red microalgae for use in foods and cosmetics. Trends in Food Science & Technology. 1992; 3: 92-97.
- Azeredo HM, Waldron KW. Crosslinking in polysaccharide and protein films and coatings for food contact–A review. Trends in Food Science & Technology. 2016; 52: 109-122.
- Babu BR, Rastogi NK, Raghavarao KSMS. Liquid–liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chemical Engineering and Processing: Process Intensification. 2008; 47: 83-89.
- Bennett A, Bogorad L. Complimentary Chromatic Adaptation in a Filamentous BlueGreen Alga. The Journal of Cell Biology. 1973; 58: 419-35.
- Bertolin TE, Farias D, Guarienti C, Petry FTS, Colla LM, Costa JAV. Antioxidant Effect of Phycocyaninon Oxidative Stress Induced with Monosodium Glutamate in Rats. Brazilian Journal of Medical and Biological Research. 2011; 54: 733–738.
- Chethana S, Nayak CA, Madhusudhan MC, Raghavarao KSMS. Single step aqueous two-phase extraction for downstream processing of C-phycocyanin from Spirulina platensis. Journal of food science and technology. 2015; 52: 2415-2421.
- Colla LM, Bertol CD, Ferreira DJ, Bavaresco J, Costa JAV, Bertolin TE. Thermal and photo-stability of the antioxidant potential of Spirulina platensis powder. Brazilian journal of biology. 2016; 77: 332-339.
- de Amarante MCA, Braga ARC, Sala L, Kalil SJ. Colour stability and antioxidant activity of C-phycocyanin-added ice creams after in vitro digestion. Food Research International. 2020; 137: 109602.
- Duman YA, Acemi A, Yuzugullu Y, Özen F. Separation of catalase from Amsonia orientalis with single step by aqueous two-phase partitioning system (ATPS). Separation Science and Technology. 2017; 52: 691-699.
- Gu Z, Glatz CE. Aqueous Two-Phase Extraction for Protein Recovery from Corn Extract. J. Chromatogr. 2007; 845: 38–50.
- Gustone FD. c-linolenic acid. Occurrence and physical and chemical properties. Progress in Lipid Research. 1992; 31: 145–161.
- Ilter I, Akyil S, Demirel Z, Koç M, Conk-Dalay M, Kaymak-Ertekin F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. Journal of Food Composition and Analysis. 2018; 70: 78-88.
- Jaeschke DP, Teixeira IR, Marczak LDF, Mercali GD. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Research International. 2021; 143: 110314.
- Jaeschke DP, Teixeira IR, Marczak LDF, Mercali GD. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Research International. 2021; 143: 110314.
- Kamble SP, Gaikar RB, Padalia RB, Shinde KD. Extraction and purification of C-phycocyanin from dry Spirulina powder and evaluating its antioxidant, anticoagulation and prevention of DNA damage activity. Journal of Applied Pharmaceutical Science. 2013; 3: 149-153.
- Karkas T, Önal S. Characteristics of Invertase Partitioned in Poly(ethylene Glycol)/Magnesium Sulfate Aqueous Two-Phase System. Biochem Eng J. 2012; 60: 142–150.
- Kathiravan A, Udayan E, Kumar RR. Bioprospecting of Spirulina biomass using novel extraction method for the production of C-Phycocyanin as effective food colourant. Vegetos. 2021; 1-9.
- Kennedy MJ, Reader SL, Davies RJ. Fatty acid production characteristics of fungi with particular emphasis on gamma linolenic acid production. Biotechnology and Bioengineering. 1993; 42: 625–634.
- Laemmli UK. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature. 1970; 227: 680–685.
- Liu Y, Feng Y, Lun J. Aqueous two-phase countercurrent distribution for the separation of c-phycocyanin and allophycocyanin from Spirulina platensis. Food and Bioproducts Processing. 2012; 90: 111-117.
- Liu Y, Feng Y, Lun J. Aqueous two-phase countercurrent distribution for the separation of c-phycocyanin and allophycocyanin from Spirulina platensis. Food and Bioproducts Processing. 2012; 90: 111-117.
- Madhusudhan MC, Raghavarao KSMS. Aqueous two-phase extraction of invertase from Baker’s yeast: Effect of process parameters on partitioning. Process Biochemistry. 2011; 46: 2014.
- Mittal R, Sharma R, Raghavarao KSMS. Aqueous two-phase extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum. Bioresource technology. 2019; 280: 277-286.
- Munawaroh HSH, Gumilar GG, Alifia CR, Marthania M, Stellasary B, Yuliani G, et. al, Photostabilization of phycocyanin from Spirulina platensis modified by formaldehyde. Process Biochemistry. 2020; 94: 297-304.
- Oliveira EG, Duarte JH, Moraes K, Crexi VT, Pinto LA. Optimisation of Spirulina platensis convective drying: evaluation of phycocyanin loss and lipid oxidation. International journal of food science & technology. 2010; 45: 1572-1578.
- Oyaizu M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. The Japanese journal of nutrition and dietetics. 1986; 44: 307-315.
- Patil G, Raghavarao KSMS. Aqueous two-phase extraction for purification of C-phycocyanin. Biochemical Engineering Journal. 2007; 34: 156-164.
- Patil G, Chethana S, Sridevi AS, Raghavarao KSMS. Method to obtain C-phycocyanin of high purity. Journal of chromatography A. 2006; 1127: 76-81.
- Patil G, Raghavarao KSMS. Aqueous Two-Phase Extraction for Purification of C-Phycocyanin. Biochem Eng. J. 2007; 34: 156–164.
- Prabakaran G, Sampathkumar P, Kavisri M, Moovendhan M. Extraction purification of C-phycocyanin from dry Spirulina powder and evaluating its antioxidant, anticoagulation and prevention of DNA damage activity. Journal of Applied Pharmaceutical Science. 2020; 3: 149–153.
- Purohit A, Kumar V, Chownk M, Yadav SK. Processing-independent extracellular production of high purity C-Phycocyanin from Spirulina platensis. ACS Biomater Sci & Eng. 2019; 5: 3237-3245.
- Ranjitha K, Kaushik BD. Purification of phycobiliproteins from Nostoc muscorum. Sci Ind Res. 2005; 64: 372–375.
- Rastogi RP, Sonani RR, Madamwar D. Effects of PAR and UV radiation on the structural and functional integrity of phycocyanin, phycoerythrin and allophycocyanin isolated from the marine cyanobacterium Lyngbya sp. A09DM. Photochemistry and photobiology. 2015; 91: 837-844.
- Remirez D, Fernández V, Tapia G, González, R, Videla LA. Influence of C-Phycocyanin on Hepatocellular Parameters Related to Liver Oxidative Stress and Kupffer Cell Functioning. Inflammation Research. 2002; 51: 351–356.12.
- Renugadevi K, Nachiyar CV, Sowmiya P, Sunkar S. Antioxidant activity of phycocyanin pigment extracted from marine filamentous cyanobacteria Geitlerinema sp TRV57. Biocatalysis and agricultural biotechnology. 2018; 16: 237-24.
- Renugadevi K, Nachiyar V, Sowmiya P, Sunkar S. Antioxidant activity of phycocyanin pigment extracted from marine filamentous cyanobacteria Geitlerinema sp TRV57. Biocatalysis and agricultural biotechnology. 2018; 16: 237-242.
- Palomares MR, Nunez L, Amador D. Practical application of aqueous two-phase systems for the development of a prototype process for c-phycocyanin recovery from Spirulina maxima. Journal of Chemical Technology & Biotechnology. 2001; 76: 1273-1280.
- Romay Ch, González R, Ledán N, Remirez D, Rimbau V. C-Phycocyanin: A Biliprotein with Antioxidant, Anti-Inflammatory, and Neuroprotective Effects. Current Protein & Peptide Science. 2003; 4: 207–216.11.
- Sarubbo LA, Oliveira LA, Porto ALF, Takaki GMC, Tambourgi EB. Partition of proteins in aqueous two-phase systems based on cashew-nut tree gum and poly (ethylene glycol). Brazilian Archives of Biology and Technology. 2004; 47: 685.
- Sekar S, Chandramohan M. Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol. 2008; 20: 113–136.
- Sonani RR, Patel S, Bhastana B, Jakharia K, Chaubey MG, Singh NK, et. al. Purification and antioxidant activity of phycocyanin from Synechococcus sp. R42DM isolated from industrially polluted site. Bioresource technology. 2017; 245: 325-331.
- Tavanandi HA, Mittal R, Chandrasekhar J, Raghavarao KSMS. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Research. 2018; 31: 239–251.
- Turan S, Tripathy BC. Salt and genotype impact on antioxidative enzymes and lipid peroxidation in two rice cultivars during de-etiolation. Protoplasma. 2013; 250: 209-22.
- Utama-ang N, Kuatrakul I, Klangpetch W, Walter P, Kawee-ai A. Comparative evaluation of physicochemical, functional and texture properties and sensory acceptance of different instant rice varieties coated with Spirulina and edible polymers. International Journal of Food Science & Technology. 2022; 57: 4183-4193.
- Vonshak A. Spirulina platensis (Arthrospira) Physiology, Cell-Biology and Biotechnology. London: Taylor & Francis. 1997.
- Zhang Z, Li J, Fu J, Chen L. Fluorescent and magnetic dual-responsive coreshell imprinting microspheres strategy for recognition and detection of phycocyanin. RSC Advances. 2014; 4: 20677-20685.
- Zhang Z, Li J, Wang X, Shen D, Chen, L. Quantum dots based mesoporous structured imprinting microspheres for the sensitive fluorescent detection of phycocyanin. ACS Applied Materials & Interfaces. 2015; 7: 9118-9127.