
Review Article
Ann Agric Crop Sci. 2024; 9(4): 1159.
Changes in Approach towards Nitrogen Management: Nanofertilizers for Sustainable Agriculture
Pooja LR; Singh R*; Darjee S; Shrivastava M
Division of Environment Science, ICAR-Indian Agricultural Research Institute, New Delhi, 110012, India
*Corresponding author: Singh Division of Environment Science, ICAR-Indian Agricultural Research Institute, New Delhi, 110012, India. Email: renu_icar@yahoo.com
Received: May 02, 2024 Accepted: June 20, 2024 Published: June 27, 2024
Abstract
Nitrogen is a vital nutrient for plant growth and agricultural productivity. However, conventional nitrogen fertilization practices often lead to inefficiencies, environmental pollution, and health hazards. This review paper explores the evolving landscape of nitrogen management in agriculture, focusing on the advancements in nanofertilizers and their potential to revolutionize nutrient delivery, enhance efficiency, and promote sustainability. Nitrogen nanofertilizers have emerged as a promising tool in agricultural practices, offering targeted nutrient delivery mechanisms that optimize plant uptake while minimizing environmental impact. The role of these nanofertilizers in unlocking agricultural potential is underscored by their ability to overcome challenges such as nutrient leaching and volatilization, thus maximizing nitrogen use efficiency. However, the efficacy of nanofertilizers is influenced by various factors including particle size, surface charge, and composition, highlighting the importance of understanding the mechanisms governing their performance. Synthesis methods play a crucial role in tailoring nanofertilizer properties to meet specific agricultural needs, with controlled and slow-release formulations gaining traction for their ability to sustainably supply nutrients over extended periods. Moreover, nanofertilizers contribute to nitrogen dynamics within soil-plant systems, influencing nitrogen cycling and minimizing losses to the environment. Beyond agricultural benefits, these nanomaterials also hold promise in mitigating climate change by optimizing nitrogen utilization and reducing greenhouse gas emissions associated with conventional fertilization practices. However, the widespread adoption of nitrogen nanofertilizers necessitates a comprehensive evaluation of their potential impacts on human health, particularly within agricultural systems. Addressing concerns related to nanoparticle toxicity and accumulation in food chains is imperative to ensure the safe and sustainable integration of nanofertilizers into modern farming practices.
Keywords: Nanotechnology; Crops; Sustainable agriculture; Controlled release; Environmental impacts; Emissions
Introduction
The worldwide agricultural landscape reflects humanity's never-ending search for higher crop yields and food security. The extensive use of nitrogen fertilizers, which are critical for increasing agricultural output, is central to this effort. According to Anas et al. [8], the use of nitrogen fertilizers has become inextricably linked to current agricultural techniques, influencing the trajectory of world food production. However, this dependency has its own set of issues, notably in terms of managing nitrogen supplies sustainably in the face of rising demand. The International Fertilizers Association [35] predicts that global N supply will continue to expand, surpassing demand growth, which will be driven mostly by rising industrial demands. By 2050, the world population is expected to exceed 9.7 billion people, representing a 70% increase in food consumption above current levels [26,32,47]. This population explosion emphasizes the critical need to increase agricultural production to maintain food and nutrition security, especially given the restrictions of limited arable land. Fertilizer application appears to be a significant component in tackling this problem, and it is a vital tool for increasing crop yields. However, while chemical fertilizers have been shown to increase agricultural productivity, their widespread usage raises concerns about their effects on soil health and fertility. The indiscriminate application of synthetic fertilizers has been linked to disruptions in soil structure and microbial communities [81], endangering the long-term viability of agricultural systems. Furthermore, the ramifications go beyond soil health, as changes in food chains and heritable modifications in future consumer generations highlight the far-reaching effects of excessive fertilizer usage across a variety of environments.
Over the past three decades, nitrogen inhibitors, such as urease and nitrification inhibitors, have effectively reduced N losses in agricultural systems. However, their method of action, which largely involves the inhibition of enzymes critical to N dynamics, raises worries about their interference with natural nitrogen cycles. Furthermore, despite their potential benefits, these inhibitors have faced economic obstacles in terms of widespread adoption among farmers, with minimal evidence of considerable agricultural production increases [55,70]. As a result, the hunt for alternate techniques has escalated, leading to an increased interest in nanofertilizers. Within this framework, the incorporation of nanotechnology into agriculture emerges as a viable route, providing new opportunities for increasing crop yields while also boosting environmental sustainability. In light of the problems associated with traditional nitrogen fertilizers and the promising potential of nanofertilizers, it becomes imperative to explore innovative solutions that reconcile the imperatives of agricultural productivity with environmental stewardship. This review endeavors to delve into the emerging paradigm of nanotechnology in agriculture, offering insights into its transformative potential and implications for sustainable crop production in the 21st century.
Nitrogen Nanofertilizer
Finding the best N fertilizer product and linking it with crop production is the need of the hour. Nowadays the world is moving towards replacing urea which was the sole N fertilizer used for crop production but scientists are coming up with innovations in the fertilizer industry. The neem-coated urea which is normally used in India contains 46% N and when compared to all other N fertilizers it is cheaper and easily accessible to the farming community [55]. Nitrogen nanofertilizers are nanoscale (1-100 nm) particles or composites designed to deliver nitrogen to plants in a controlled manner, thereby improving crop yields and reducing N losses [33]. They are also developed by encapsulating the fertilizer molecules (urea) into nanomaterials like nanotubes, nanoporous materials coating urea with polymers, or preparing emulsions of nano-sized particles [47,54]. Due to the high surface area to volume ratio, it performs much better than all improved conventional N fertilizers. However, this approach often leads to significant losses through ammonia (NH3) volatilization, emissions of N oxides (NOx), surface runoff, leaching, and other pathways as explained in Figure 1 [15,20]. With the advent of nanotechnology, there's a growing opportunity to utilize nanoscale or nanostructured materials as carriers for fertilizers or controlled-release agents, leading to the development of "smart fertilizers" aimed at improving nutrient efficiency and reducing environmental costs (Adisa et al., 2019). Nano fertilizers can be classified into four main groups with different mechanisms of approach [45,87].
Nano-coated fertilizers: In this type, traditional fertilizers are coated with nanomaterials such as metal oxides, carbon-based materials, or polymers or incorporate the nutrients into nanomaterial or nanoparticles for example clay with high porosity. The coating serves several purposes, including controlled release of nutrients, protection against environmental losses (e.g., leaching, volatilization), and improved uptake efficiency by plants.
Nano-encapsulated fertilizers: These nanofertilizers involve encapsulating nutrient compounds within nanoscale capsules or matrices. This encapsulation protects the nutrients from degradation, enhances their solubility, and facilitates controlled release, ensuring a sustained and targeted supply of nutrients to plants.
Nano-scaled fertilizers: This category comprises nanomaterials engineered to deliver nutrients directly to plants. Examples include nanoparticles of essential elements like nitrogen, phosphorus, and potassium or nanoparticles functionalized with specific nutrients. Nano-scaled fertilizers aim to improve nutrient uptake efficiency by plants through enhanced solubility, increased surface area, and facilitated transport across cell membranes.
Nano additive fertilizers: formulation of nanofertilizer by combining suitable supplement nanomaterial along with conventional fertilizer.
Factors Affecting the nanofertilizer Efficiency
The efficiency of nanofertilizers depends on different factors, these factors affect the life cycle of the nanofertilizers like uptake, translocation, and accumulation in the different parts of the plant. It may be i) intrinsic factors of nanofertilizer or certain characteristics of nanofertilizer, ii) extrinsic factors or environmental factors, and most importantly iii) Mode of application [34]. The intrinsic factors involve the shape, size, surface charge, surface functionalization, and composition of the nanofertilizer. The extrinsic factors include mainly soil pH, soil texture, and soil organic matter content [59]. There are mainly two ways of application of these nanofertilizers one is through soil application where the required amount of nanofertilizer is directly applied to the soil, and another is through foliar application where a prescribed quantity of liquid nanofertilizer is mixed with water and other chemicals and sprayed on the foliage of the crops as shown in figure 1. The extraordinary properties of nanomaterial along with fertilizer increase the efficiency by increased absorption and précised delivery of nutrients (Zulfiqar et al. 2019). In an experiment comparison between the soil pH was observed and reported that initially, the soil pH of the conventional fertilizer and nanofertilizer treated soil was higher than the soil in the control. Then, on 4-5 consecutive days, the pH of nano fertilizer-treated soil was lower than that of the control and conventional fertilizer-treated soil [69].

Figure 1: Factors responsible for increased efficiency of nanofertilizers a) intrinsic factors, b) extrinsic factors, and c) mode of application of nanofertilizers.
Mechanism of Nitrogen Nanofertilizers in Increasing the Nitrogen use Efficiency
The world is now focusing on sustainability; nanotechnology gives an opportunity in every sector. N nanofertilizers are expected to increase NUE by improving the effectiveness of N delivery to plants reducing N losses to the environment and ensuring the overall development of mankind and biodiversity [54]. The high reactivity of nanomaterial enables increased usage efficacy and high and effective N absorption for plants, resulting in minimal losses when compared to conventional fertilizers like urea. In addition to features inherent to the nanoparticle, such as size and coating, the efficacy of the absorption, distribution, and accumulation of nanofertilizers depends on exposure to other conditions, including the pH of the soil, the amount of organic matter present, and the texture of the soil [31]. As an example, when compared to traditional fertilizers, nanofertilizers of macronutrients (N, P, and K) improve plant development by 19% and its growth is boosted by 29% [11].
The Working Mechanism of Nitrogen Nanofertilizers
The mechanism by which N nanofertilizers work can be broken down into two steps:
1. Protection of the N: The protective coating helps to keep the N in the soil, reducing its loss to the atmosphere through processes like volatilization, denitrification, and leaching. This allows the plants to access a steady supply of N over an extended period, rather than receiving a large amount all at once [7].
2. Controlled release of the N: The coating is designed to release the N gradually as the plants need it, allowing for more efficient use of the fertilizer. This slow release can be controlled by factors such as soil moisture and temperature, pH, and the presence of microorganisms [12]. As the plants absorb the N, the coating gradually degrades, releasing more N into the soil.
Popular Methods of Synthesizing Nitrogen Nanofertilizers for Enhanced Agricultural Sustainability
Synthesizing nitrogen nanofertilizers for agriculture involves various methods, each offering distinct advantages and producing nanofertilizers with specific properties as summarized in Table 1. Some common methods include:
Synthesis Method
Pros
Cons
References
Ball milling method
Scalable and relatively simple process
Requires high energy input
Gajraj Yadav et al., [27]
Allows for precise control over particle size and shape
Potential for contamination from milling media
Can be used for a wide range of materials
Limited to certain types of materials
High surface area and reactivity of resulting nanoparticles
Sol-Gel Method
Precise control over composition and structure
Requires careful control of reaction conditions
Kumar et al., [46]
Can produce nanoparticles with uniform size distribution
Time-consuming process
Versatile, suitable for a variety of materials
May involve the use of toxic precursors or solvents
Can incorporate various additives and dopants
May require post-treatment steps for desired properties
Hydrothermal Method
Low processing temperatures, suitable for heat-sensitive materials
Requires high-pressure equipment
Byrappa and Adschiri, [17]
Allows for control over particle size and morphology
Long reaction times
High purity of synthesized nanoparticles
Limited scalability for large-scale production
Enables synthesis of complex nanostructures
Energy-intensive process
Co-precipitation Method
Simple and cost-effective process
Limited control over particle size and shape
Batool et al., [14]
High yields and large-scale production capability
Agglomeration of nanoparticles may occur.
Suitable for producing a wide range of materials.
Post-treatment steps may be required for the desired properties.
Allows for doping and surface modification.
Green Synthesis
Environmentally friendly, using non-toxic precursors
Limited control over particle properties
Aslam et al., [10]
Low energy consumption
Synthesis conditions may be less reproducible
Biocompatible and suitable for biological applications
Longer synthesis times may be required
Potential for synthesis using renewable resources
Limited scalability for large-scale production
Table 1: Summary of pros and cons of various synthesis methods of nano fertilizers.
Name of N nano fertilizer
Method of preparation
Crop
Dosage
N Use Efficiency
Yield
Reference
Nanozeolite composite fertilize (NZCF)
Co-precipitation method
Lettuce (Lactuca sativa)
10g 42m-2
-
-
Khan et al. [44]
Nanofertilizer NPK (19:19:19)
Biological process [Developed by a private company (Pratishtha) in India in association with the Indian Council of Agricultural Research]
Potato (Solanum tuberosum)
350 Kg N ha-1
67.74
23.71 ton ha-1
Abd El-Azeim et al. [1]
N nanofertilizer
Modification of zeolite using hexadecyltrimethylammonium bromide (HDTMABr)
Kangkong (Ipomoea aquatic)
40 kg ha-1
92.8
76.00 g 100 plants-1 (Fresh Weight)
5.33 g 100 plants-1 (Dry Weight)Rajonee et al. [69]
Hybrid nanofertilizer (HNF)
Urea-modified hydroxyapatite + Cu2+, Fe2+, and Zn2+ NPs using ultrasound sonication (30 kHz for 1 h) technique
Ladies' finger (Abelmoschus esculentus)
50 mg week-1
-
-
Tarafder et al. [87]
Nitrate-doped CaP nanoparticles (nano-NPK)- nano U-NPK
Precipitation method
Durum Wheat (Triticum durum)
75 kg of N ha-1
-
-
Ramírez-Rodríguez et al. [72]
Chitosan based NPK nanofertilizer
Ionic gelation of tripolyphosphate and chitosan solution
Coffee seedlings (Coffea arabica)
50 mL for 20 coffee seedlings of each plot at 50 ppm of NPK nanofertilizer emulsion
-
-
Ha et al., [29]
ZnO Nanoparticles (ZnO NP)
-
Brinjal (Solanum melongena L.)
4500 mg ha-1
-
3106 g
Kale and Gawade [40]
Urea coated with ZnO NPs
-
Tomatoes
With 3% Zn w w-1 with urea
-
Increased biomass and yield
Pierre [61]
Nano hydroxyl apatite (nHA) application
-
Soybean
nHA as a source of phosphorous
-
20% higher seed yield as compared to conventional phosphorous application
Singh et al. [46]
Urea–HA nanohybrid
-
Tea (Camellia sinensis (L.) Kuntze)
28-30% conc. of N @ 675 kg ha-1 yr-1
-
14–16% increase in the yield
Raguraj et al. [66]
Urea-Hydroxyapatite-Polymer Nanohybrids
In-situ sol-gel method
Maize
-
-
Germination was accelerated, 124%, 147.6% increase in average biomass, root length respectively
Pabodha et al. [60]
Foliar application of chitosan-NPK fertilizer
Polymerization of meth acrylic acid
Wheat
25% nano NPK
-
Crop yield plant-1: 8.28 g
Abdel-Aziz et al. [2]
Nano-composite NPK
-
Red pepper
25% conc.
-
Promoted growth and yield
Abdel-Aziz et al. [3]
Table 2: Effect of different composition of nitrogen nanofertilizer on different crops and their yields.
1. Ball milling method: It is a type of mechanical attrition where high-energy mechanical devices produce nanoscale particles. It is facilitated by energetic ball mills like planetary, tumbler, or rod mills, which utilize containers filled with powder/flakes (<50 mm) and tungsten carbide or steel balls. The optimal ball-to-substance ratio is typically 2:1, and milling efficiency decreases if containers are overfilled. Collision temperatures can rise significantly, ranging from 100 to 1100°C [27]. The ball milling method was employed to refine clinoptilolite particles, reducing their diameter to 30 nm. Similarly, other naturally occurring nano-clays like halloysite, montmorillonite, and bentonite were subjected to ball milling, resulting in particles sized between 30-40 nm [84]. In a recent study conducted by Sebastian et al. [75], a planetary ball mill was employed for the physical synthesis of a novel potassium-infused nitrogenous nano-fertilizer using chitosan and potassium carbonate extracted from banana peel ash, featuring nitrogen content of 5.55 weight % and potassium content of 3.01 weight %.
2. Sol-Gel Method: This technique involves the formation of nanoparticles through the hydrolysis and condensation of precursor materials in a sol solution. It offers precise control over particle size and composition and allows the incorporation of nitrogen sources during synthesis [46]. This method is typically conducted at low temperatures and facilitates the synthesis of various materials such as aerogels, zeolites, and ordered porous solids through organic-inorganic hybridization. Utilizing this technique, nanotubes, nanoparticles, and nanorods are synthesized by forming a network through the creation of a liquid sol and subsequent connection of sol particles. Upon drying, this liquid transforms into powders, thin films, and even solid masses, offering versatility in material fabrication [58]. For instance, it has been employed for the biological synthesis of nano-sized hexagonal hydroxyapatite powder, as demonstrated in a study conducted by Priyam et [64]. Furthermore, this method has been instrumental in developing controlled-release urea formulations, as evidenced by Abhiram [4], and urea-silica nanohybrids by de Silva et al [22]. This approach has been explored for the nanotechnology-based controlled release of sustainable fertilizers, particularly metal oxide nanoparticles (Beig et al., 2022).
3. Hydrothermal method: The hydrothermal method stands as a versatile approach in the synthesis of monodispersed particles across a range of materials, encompassing metal oxides, sulfides, and carbon nanoforms. This method operates by orchestrating reactions involving metal salts and other requisite chemicals under controlled conditions of temperature and pH, often executing the process in a single step, thereby offering scalability for industrial applications [17]. Notably, recent studies have showcased the efficacy of hydroxyapatite nanoparticles functionalized with humic substances, achieved through a straightforward dipping process, revealing substantial enhancements in plant growth and nutrient utilization efficiency, thereby hinting at its potential for wide-scale agronomic use [97]. Furthermore, the application of supercritical hydrothermal synthesis presents an avenue for the rapid production of metal oxide nanocrystals, further augmented by the incorporation of organic materials to regulate nanoparticle dispersion across diverse media, thereby conferring advantages for the formulation of nanofertilizers [56]. In addition, Continuous Hydrothermal Flow Synthesis (CHFS) techniques emerge as cutting-edge methodologies for crafting inorganic nanoparticles endowed with size-dependent properties, an indispensable facet for numerous technological domains, including the development of nanofertilizers [21].
4. Co-precipitation Method: The co-precipitation method of synthesizing nanoparticles involves the simultaneous precipitation of multiple precursor ions from solution, typically yielding metal oxides or hydroxides. It entails the mixing of aqueous solutions containing precursor ions under controlled conditions, leading to a chemical reaction that forms insoluble nanoparticles directly within the solution. These nanoparticles nucleate and grow during the reaction, after which they are isolated, purified, and often stabilized to prevent agglomeration. Naseem et al [57] synthesized mesoporous ZnAl2Si10O24 nanofertilizers, which enable a high yield of Oryza sativa L.
5. Green Synthesis: This eco-friendly approach involves the use of natural sources or plant extracts as reducing and capping agents to synthesize nitrogen nanofertilizers. It offers sustainability and minimal environmental impact while producing nanofertilizers with desirable properties (Table 1).
Soil Application of Nanofertilizers
The working mechanism of nanofertilizer and conventional fertilizer is almost alike, in slow-releasing fertilizer the nutrients are released over 40-50 days over 4-5 days in conventional fertilizers [99]. The mobility and stability of these nanofertilizers in soil depend on their particle size and surface charge. In soil, on application, they interact with the soil microorganisms and other compounds and form aggregates which helps in the movement and absorption of these nanoparticles through root. The movement of these aggregates is governed by the Brownian motion towards macro and microspores present in the soil [68]. The aggregates' movement through soil pores is enhanced by absorption to certain soil-mobile colloids and also to be noted that their mobility can be inhered by the binding of these nanoparticles to non-mobile colloids [88]. The soil organic matter, humic acid, and soil water ionic strength also affect the mobility of these nanoparticles. Any nanofertilizer when applied to soil undergoes a slow decomposition process. For example, the urea molecule that is released from the nanofertilizer will undergo the following reactions to be uptaken by the plants.

The soil urease enzyme catalyzes the reaction; one urea molecule gives rise to two ammonia molecules, as shown in equations 1, 2, and 3. Therefore the role of urease enzyme activity is very unique having the half-life of urease enzyme catalyzed acativity 20 ms at 25°C [78]. Further, the produced ammonium undergoes a nitrification process which is carried out by a very narrow range of bacteria, they convert ammonium to nitrite (NO2-) by Nitrosomonas spp. and then nitrate (NO3-) by Nitrosococcus spp. The ammonium can also be directly taken by the plants and some soil microorganisms. Inside the plant cells, the urease enzyme is produced due to the catabolism of purines and arginines, which actively convert the urea to ammonia which acts as an N source [52]. Both roots and shoots may reduce NO3-, although NO3- is reduced directly in the cytoplasm whereas NO2- is used in plastids and chloroplasts. The nitrate reductase enzyme converts NO3- to NO2- in the cytosol as shown in Figure 2. Nitrite is carried into the chloroplasts of leaves, where NO2- is then transformed by the enzyme nitrite reductase into ammonium ions.
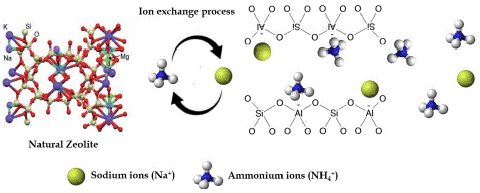
Figure 2: Mechanism of ion exchange process responsible for slow release of ammonium ions from zeolite.
Anas et al [8] reported that the ammonia byproducts glutamine and glutamate serve as N donors during the production of amino acids, chlorophyll, and nucleic acids. Three major ammonium assimilation processes, primary N assimilation, reassimilation of photorespiratory ammonia, and "recycled" N assimilation—have been identified, and their respective isoenzymes are glutamine synthetase, glutamate synthetase, and glutamate dehydrogenase. Glutamine and glutamate are converted to aspartate and asparagine in the presence of aspartate aminotransferase and asparagine synthetase respectively (Figure 1) [9].
Foliar Application of Nanofertilizer
The mechanism of uptake of N nanofertilizers through the foliar application (spraying the fertilizer directly onto the plant's leaves) is different from that of traditional fertilizer application methods, such as soil application. The mechanism of uptake of nanofertilizers through foliar application involves several steps:
1. Absorption of the nanofertilizer: The nanofertilizer particles are absorbed into the stomata, the small pores on the surface of the leaves, where they come into contact with the plant's cells. Many mechanisms were proposed for the intake of the nutrients, like pore formation, endocytosis, carrier protein-mediated absorption, and through plasmodesmata, as shown in Figure 1 [11].
2. Transport of the nanofertilizer: Once the nanofertilizer is inside the plant, it is transported through the plant's vascular system to the areas where it is needed. This allows the plant to receive the fertilizer's benefits quickly and efficiently [98].
3. Uptake of the nutrient: The nanofertilizer is broken down into its constituent parts, allowing the plant to absorb the N or other nutrients it contains. This process is similar to the way the plant absorbs fertilizer from the soil. The rate at which the nanofertilizer is absorbed by the plant through the foliar application is influenced by a variety of factors, including the size of the particles, the thickness and composition of the leaf cuticle (the waxy outer layer of the leaf), the stage of plant growth, and the presence of other substances in the fertilizer solution (such as surfactants or adjuvants that can enhance the uptake of the particles) [13].
4. Utilization of the nutrient: The plant uses the absorbed nutrients for various metabolic processes, including growth and reproduction, to help it thrive.
Enhancing the Fertilizer Delivery System
The nanofertilizers developed using nanotechnology can deliver the active ingredients of the fertilizer to the crop based on their biological demands. One of the important characteristics of nanourea in enhancing the delivery of nutrients is its size and surface area, high sorption capacity, and controlled release mechanism. The ordinary urea granule size ranges between 2.82 mm to 2.06 mm, whereas the nanourea’s size ranges from 20 nm to 50 nm. Nanourea has 10,000 times more surface area than ordinary urea [41]. This nanourea improves the absorption, nutrient-holding capacity, and bio-availability of crops and soil. Due to the smart delivery system of these nanofertilizers, it is also called ‘smart fertilizer’ [65,83]. The main mechanism involved in N nanofertilizer is that its ‘Smart delivery of the N’ means delivery to a specifically targeted place, by avoiding the barriers with multifunctional characteristics and also with remote regulation of these nutrients containing nanoparticles [81].
Controlled/ Slow Releasing of Nutrients
The thumb rule for applying synthetic fertilizers for the Indian agricultural system is given at the ratio of N:P:K is 4:2:1 to achieve optimum crop productivity, in reality, farmers are forced to apply NPK in the ratio of 10:2.7:1 [19]. So it is observed that our farmers are applying 2.5 times more N than the actual requirement of the crop. Still, we can see that the excess amount of fertilizer is also not able to fulfill the necessary N for the crop. Recent research studies on nanofertilizers have given us the hope of reducing the bulk usage of these N fertilizers as these nanostructured formulations have come up with a solution for the controlled and smart release of nutrients as and when required by the crop [48].
Hydroxyapatite Nanoparticles as Base Material
Hydroxyapatite is used in agriculture due to its bioactivity and biocompatibility properties. It is a rich source of phosphorous and calcium, and combining the urea with these hydroxyapatite nanostructures helps slow the release of N along with phosphorous and calcium. Tarafder et al [87] formulated a Hybrid Nano Fertilizer by taking a Nano urea modified hydroxyapatite nanoparticles and combining them with a few essential micronutrients and applying it to the Ladies’ finger (Abelmoschus esculentus) crop during a pot experiment. It was found that there was a slow and sustainable release of urea and other nutrients such as Ca2+, PO4 3-, Cu2+, Fe2+, and Zn2+. It was also reported that there was a considerable increase in the uptake of micronutrients due to slow release and increased nutrient use efficiency with a meager effect on the environment. In another study, a urea-modified hydroxyapatite nanohybrid fertilizer was synthesized using urea and hydroxyapatite in a ratio of 6:1 by weight. The slow release of N was tested in the Rice Research and Development Institute of Sri Lanka on the rice crop itself and found that the urea-modified hydroxyapatite nanohybrid released N 12 times slower than conventional urea and it reduced up to 50% of fertilizer application for rice crop [45,74]. An integrated nanofertilizer produced using hydroxyapatite containing NPK and also micronutrients (Zn, Fe, Cu, Co, and Ag) of size 20-30 nm in width and 80 nm in length, rod-shaped structures helped in improving the biomass production of medicinal crops like Ming aralia (Polyscias fruticosa) and vegetable crop like Asparagus (Asparagus officinalis) by 50% compared to non-nanofertilizer treated plots [49]. A urea-coated hydroxyapatite nanoparticles sized less than 90 nm containing 36.5% of the N when applied to aerobic paddy at the rate of 25% of recommended dose of N showed higher grain yield than 50% of RDN of UCH and 100% conventional urea [16].
Zeolite as Base Material
Zeolites are porous structures with a high absorption capacity to absorb and hold the N, P, and K nutrients. It is widely used because of its effective ion exchange capacity, high surface area of approximately, 900 m-2 g-1, 1-10 nm micropores, and higher water holding capacity as shown in Figure 2 [77]. It has a slow nutrient-releasing capacity, lasting up to 50 days, i.e., 5 times more release duration than conventional urea. Nanozeolite composite fertilizer was prepared by adding salts of macronutrients by simple co-precipitation method showed the long-term release of nutrients enhancing the nutrient availability and improving the soil’s physical, chemical, and biological properties [37,44]. A synthesized nano N fertilizer using zeolite as carrier material tested on Ipomoea aquatica (Kalmi) in a pot experiment showed higher N accumulation in plants with optimum pH, cation exchange capacity, soil moisture, and available N [69]. Few studies on the N-use efficiency of crops have increased due to the adsorbent nature of nano zeolites. The release of nutrients especially N from the conventional urea, NBPT-treated urea, zeolite amended urea, and nano zeolite-treated urea is 4 days, 10-15 days, 34 days, and 40-48 days respectively [76]. Rastogi et al. [73] mentioned that nano zeolites might be effectively used in agriculture to facilitate water filtration and preservation in the soil due to their porous and capillary properties, which act as a slow-release water source. Applying foliar-applied mixed Nanofertilizer and commercial fertilizer (NPK) significantly increased plant growth parameters, protein, fiber, Fe, Zn, and K compared to only commercial fertilizer-treated tomatoes [67]. Few studies on the N-use efficiency of crops have increased due to the adsorbent nature of nano zeolites. The release of nutrients, especially N from the conventional urea, NBPT-treated urea, zeolite amended urea, and nano zeolite-treated urea is 4 days, 10-15 days, 34 days, and 40-48 days respectively [76].
Montmorillonite as Base Material
Montmorillonite belongs to the smectite group of clay minerals (2:1), it has one octahedral sheet and two tetrahedral sheets with interlayer gaps after each reoccurring triple-layered sheet [92]. The specific surface area of normal montmorillonite clay has increased from 13-17 m2 g-1 to 1000-1200 m2 g-1 when it is fabricated to nano dimensions [28]. Thus, retaining the unique feature of a high surface mass ratio helps slow and consistent release of nutrients [86]. A nanohybrid developed by inculcating the urea-hydroxyapatite nanoparticles has proved to be a structurally and functionally valuable system in the slow and sustainable release of N fertilizer. A significant yield improvement in Rice crops during the field trials taken up at the Rice Research and Development Institute, Srilanka has promised the reduction of the use of conventional fertilizers by 50% [51].
Chitosan Derived Nanofertilizer
Chitosan-derived nanofertilizers are a type of fertilizer that is made by modifying chitosan, which is a biopolymer derived from chitin, a natural polymer that is found in the shells of crustaceans like shrimp and crabs [79]. Chitosan is known for its biocompatibility, biodegradability, and ability to interact with plant tissues, making it a promising material for use in agriculture. To create a chitosan-based nanofertilizer, chitosan is typically modified through chemical or physical methods to create nanoscale particles that can be applied to crops as a fertilizer [63]. These particles can be designed to release N and other essential nutrients slowly and consistently over time, providing plants with a steady supply of nutrients as they grow. A study conducted on engineering chitosan-based hydrogen in combination with the montmorillonite nano clays exhibited improved control release of fertilizers also it has increased the swelling nature thereby increasing water retention. Degradability was also improved, 57% of the applied engineered nanoparticles were degraded after swelling in water for 20 days [24].
Role of Nitrogen Nanofertilizer in Nitrogen dynamics
Nitrogen dynamics refers to how N moves and cycles through different components of the environment, such as the soil, water, air, and plants [20,25]. One of the key ways that N nanofertilizers impact N dynamics is by reducing the loss of N from the soil. According to Subramanian and Rahale [85], nutrients released from nanofertilizers can last for more than 50 days, in contrast to the 10–12 days that nutrients released from traditional fertilizers (urea) last. They also speculated that nanofertilizers could be used as a method for controlling the smart release of nutrients that are appropriate for crop needs. Leaching, denitrification, and volatilization account for 50–70% of the N losses (Figure 1) from the soil, while crop N Utilization Efficiency (NUE) seldom reaches 30–35% [70,89]. The small particle size and protective coating of the fertilizer particles help to keep the N in the soil, reducing the risk of loss through processes like volatilization, denitrification, and leaching. This allows for a more consistent supply of N to the plants over an extended period, reducing the need for frequent reapplication of fertilizer [90]. Additionally, there are two different types of N used by plants NH4-N and NO3-N (aerobic systems, such as those in maize and wheat) (rice and aquatic plants). Unlike NH4 + -N, which is attracted to soil organic matter and soil particles, nitrate-N is a negatively charged ion. Because it is soluble in water and therefore can travel below the crop's root system, nitrate-N may contribute to the pollution of groundwater [50]. The slow release of N from the nanofertilizer particles can reduce the risk of N leaching into groundwater and other water sources, which can help to protect the environment. This also can result in lower emissions of N oxides (NOx) and nitrous oxide (N2O), which are potent greenhouse gases and can also reduce the risk of N contamination in water bodies.
Role of Nitrogen Nanofertilizer in Mitigating Climate Change
The use of N nanofertilizers can play a role in mitigating climate change by improving the efficiency of N use in agriculture and reducing greenhouse gas emissions. First, by delivering N to the plant in a controlled and sustained manner, N nanofertilizers can reduce the amount of N lost to the environment through processes like volatilization, denitrification, and leaching [91]. This can result in lower emissions of N oxides (NOx) and nitrous oxide (N2O), which are potent greenhouse gases. By reducing these emissions, N nanofertilizers can help to mitigate the impact of agriculture on climate change. Second, the slow release of N from the nanofertilizer can also help to reduce the amount of fertilizer needed to achieve the same yield, which can lead to cost savings for farmers and reduced pressure on natural resources [65,71]. This can help to promote sustainable agriculture practices that are less reliant on fossil fuels and other resources that contribute to greenhouse gas emissions. Finally, the improved efficiency of N use in agriculture can lead to higher crop yields, which can help to feed a growing global population while reducing the need for additional land to be cleared for agriculture [80]. This can help to mitigate the impact of deforestation, which is a significant contributor to greenhouse gas emissions [18,93]. Overall, the use of nano N fertilizers has the potential to play a role in mitigating climate change by improving the efficiency of N use in agriculture and reducing greenhouse gas emissions. However, it is important to carefully evaluate the long-term effects of using these fertilizers and to consider their impact on soil health, plant health, and the environment as a whole. Additionally, reducing emissions and mitigating the impacts of climate change will require a comprehensive approach that incorporates multiple strategies, including the use of efficient and sustainable agricultural practices, reducing emissions from other sectors, and transitioning to low-carbon energy sources.
Impact of Nitrogen Nanofertilizers on Human Health in Agricultural Systems
Despite the undeniable benefits of nitrogen fertilizers, the neglect of their environmental and health ramifications has led to alarming levels of pollution, soil degradation, and greenhouse gas emissions [6]. High dietary nitrate intake contributes to various health conditions, including thyroid disorders and cancers, primarily through contaminated drinking water and excessive consumption of nitrate-rich foods as depicted in Figure 3 [39]. While nanofertilizers offer potential benefits in improving plant growth and reducing health risks associated with traditional fertilizers [29,42,62], they may also introduce new risks due to the release of nanomaterials into the environment, impacting human health adversely.
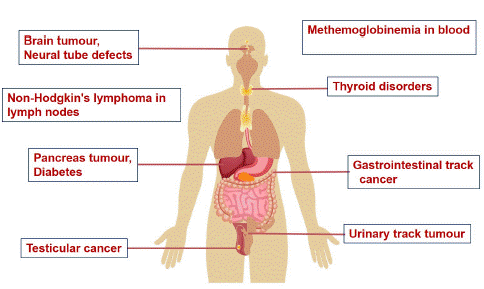
Figure 3: Adverse effects of nitrate and nitrite in drinking water and food on human health reach through nitrogenous fertilizers.
Some nanostructured fertilizers, such as water-zeolite suspension, exhibit promising environmental and health properties like no mutagenic activity and significant antimutagenic effects against certain mutagens [23]. Zeolite/Fe2O3 nanocomposites are generally non-toxic to human fibroblast cells and significantly pernicious to human malignant melanoma cells [36]. However, certain base nanomaterials used in synthesizing nitrogen nanofertilizers pose threats to human health. While zinc oxide nanoparticles show antimicrobial activity and enhance plant stress responses in some studies [38,43], others indicate potential risks such as reduced plant biomass and toxicity concerns in soil microorganisms and humans [5,94,96]. The adoption of nitrogen nanofertilizers presents a complex balance between agricultural benefits and potential health and environmental risks, necessitating thorough evaluation and risk management strategies. As the production scale of nanofertilizers (NFs) increases, unit costs decrease, fostering affordability and wider adoption among farmers. This cost reduction is driven by economies of scale, advancements in production efficiency, and enhanced crop yields, ultimately benefiting both farmers and the supply chain [27,82].
Conclusions
Overall, the use of N nanofertilizers can result in improved plant growth and yields, reduced N loss to the environment, and more efficient use of fertilizer resources. However, it is important to note that more research is needed to fully understand the long-term effects of using these fertilizers and their impact on soil health and the environment. By improving fertilizer products, nanotechnology has the potential to have a huge impact on energy, the economy, and the environment. It is crucial to look at new possibilities for combining nanotechnologies into fertilizers in light of any potential harm to the environment or human health. With focused efforts from governments and research institutions in developing such enabled agri-products, we believe that nanotechnology will change this market.
References
- Abd El-Azeim MM, Sherif MA, Hussien MS, Tantawy IA, Bashandy SO. Impacts of nano-and non-nanofertilizers on potato quality and productivity. Acta Ecol Sin. 2020; 40: 388-97.
- Abdel-Aziz HM, Hasaneen MN, Omer AM. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span J Agric Res. 2016; 14: e0902.
- Abdel-Aziz HM, Soliman MI, Abo Al-Saoud AM, El-Sherbeny GA. Waste-derived NPK nanofertilizer enhances growth and productivity of Capsicum annuum L. Plants. 2021; 10: 1144.
- Abhiram G. Contributions of Nano-Nitrogen Fertilizers to Sustainable Development Goals: A Comprehensive Review. Nitrogen. 2023; 4: 397-415.
- Ahmed B, Rizvi A, Syed A, Elgorban AM, Khan MS, Al-Shwaiman HA, et al. Differential responses of maize (Zea mays) at the physiological, biomolecular, and nutrient levels when cultivated in the presence of nano or bulk ZnO or CuO or Zn2+ or Cu2+ ions. J. Hazard. Mater. 2021; 419: 126493.
- Ahmed M, Rauf M, Mukhtar Z, Saeed NA. Excessive use of nitrogenous fertilizers: an unawareness causing serious threats to environment and human health. Environ Sci Pollut Res Int. 2017; 24: 26983-7.
- Al-Juthery HW, Lahmod NR, Al-Taee RA. Intelligent, nano-fertilizers: A new technology for improvement nutrient use efficiency (article review). InIOP Conference Series: Earth and Environmental Science. 2021; 735: 012086.
- Anas M, Liao F, Verma KK, Sarwar MA, Mahmood A, Chen ZL, et al. Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020; 53: 1-20.
- Ashihara H, Crozier A, Ludwig IA. Plant nucleotide metabolism: Biosynthesis, degradation, and alkaloid formation. John Wiley & Sons. 2020.
- Aslam AA, Aslam MS, Aslam AA. An overview on green synthesis of nanoparticles and their advanced applications in sustainable agriculture. Int J Chem Appl Biol Sci. 2022; 3: 70-99.
- Avila-Quezada GD, Ingle AP, Golinska P, Rai M. Strategic applications of nano-fertilizers for sustainable agriculture: Benefits and bottlenecks. Nanotechnology Reviews. 2022; 11: 2123-40.
- Azeem B, KuShaari K, Man ZB, Basit A, Thanh TH. Review on materials & methods to produce controlled release coated urea fertilizer. J Control Release. 2014; 181: 11-21.
- Babu S, Singh R, Yadav D, Rathore SS, Raj R, Avasthe R, et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere. 2022; 292: 133451.
- Batool A, Bashir S, Sudan J, Nazir M, Yatoo GN, Ranjan A, et al. Synthesis of Nanofertilizers and Nanopesticides: Current Understanding and Future Prospects. In The Nanotechnology Driven Agriculture 2024; 28-49.
- Bhaskar KA, Al-Hashimi A, Meena M, Meena VS, Langyan S, Shrivastava MS, et al. Conservation agricultural practices for minimizing ammonia volatilization and maximizing wheat productivity. Environ Sci Pollut Res Int. 2022; 29: 9792-9804.
- Bhavani P, Prakash SS, Harinikumar KM, Thimmegowda MN, Benherlal PS, Yoganand SB. Performance of slow release hydroxyapatite coated urea nanofertilizer on aerobic paddy. Int J Curr Microbiol App Sci. 2020; 91: 320-30.
- Byrappa K, Adschiri T. Hydrothermal technology for nanotechnology. Progress in crystal growth and characterization of materials. 2007; 53: 117-66.
- Carlson KM, Gerber JS, Mueller ND, Herrero M, MacDonald GK, Brauman KA, et al, West PC. Greenhouse gas emissions intensity of global croplands. Nature Climate Change. 2017; 7: 63-8.
- Compendium on Soil Health, Ministry of Agriculture, Department of Agriculture and Cooperation. 2012.
- Darjee S, Shrivastava M, Langyan S, Singh G, Pandey R, Sharma A, et al. Integrated nutrient management reduced the nutrient losses and increased crop yield in irrigated wheat. Arch. Agron. Soil Sci. 2023; 69: 1298-309.
- Darr JA, Zhang J, Makwana NM, Weng X. Continuous hydrothermal synthesis of inorganic nanoparticles: applications and future directions. Chem. Rev. 2017; 117: 11125-238.
- de Silva M, Siriwardena DP, Sandaruwan C, Priyadarshana G, Karunaratne V, Kottegoda N. Urea-silica nanohybrids with potential applications for slow and precise release of nitrogen. Mater Lett. 2020; 272: 127839.
- Degtyareva IA, Babynin EV, Prishchepenko EA. Nanostructured minerals developed to be used as fertilizers: biosafety evaluation. 2022; 12: 438-446.
- Dou Z, Bini Farias MV, Chen W, He D, Hu Y, Xie X. Highly degradable chitosan-montmorillonite (MMT) nano-composite hydrogel for controlled fertilizer release. Front. Environ. Sci. Eng. 2023; 17: 53.
- El-Ghamry A, Mosa AA, Alshaal T, El-Ramady H. Nanofertilizers vs. biofertilizers: new insights. Environment, Biodiversity and Soil Security. 2018; 2: 51-72.
- FAO F. The future of food and agriculture: alternative pathways to 2050. Food and Agriculture Organization of the United Nations Rome. 2018; 228.
- Gajraj Yadav KK, Awasthi KK. Synthesis of Nano Fertilizers via Physical and Chemical Approaches. Int J Chem Biol Sci. 2023.
- Golbashy M, Sabahi H, Allahdadi I, Nazokdast H, Hosseini M. Synthesis of highly intercalated urea-clay nanocomposite via domestic montmorillonite as eco-friendly slow-release fertilizer. Arch Agron Soil Sci. 2017; 63: 84-95.
- Ha NM, Nguyen TH, Wang SL, Nguyen AD. Preparation of NPK nanofertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Rev. Chem. Intermed. 2019; 45: 51-63.
- Hassan A, Gulzar S, Nawchoo IA. Role of nitrogen in photosynthesis. In Advances in Plant Nitrogen Metabolism. 2022; 86-95.
- Hong J, Wang C, Wagner DC, Gardea-Torresdey JL, He F, Rico CM. Foliar application of nanoparticles: mechanisms of absorption, transfer, and multiple impacts. Environ. Sci. Nano. 2021; 8: 1196-210.
- Hunter MC, Smith RG, Schipanski ME, Atwood LW, Mortensen DA. Agriculture in 2050: recalibrating targets for sustainable intensification. Biosci. 2017; 67: 386-91.
- Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev. 2020; 13: 223-45.
- Ingle AP, editor. Nanotechnology in plant growth promotion and protection. John Wiley & Sons, Incorporated. 2021.
- International Fertilizers Association (IFA). Market Intelligence and Agriculture Services. Fertilizer Outlook 2020–2024. 2020.
- Jahangirian H, Rafiee-Moghaddam R, Jahangirian N, Nikpey B, Jahangirian S, Bassous N, et al. Green synthesis of zeolite/Fe2O3 nanocomposites: toxicity & cell proliferation assays and application as a smart iron nanofertilizer. Int J Nanomedicine. 2020; 15: 1005-20.
- Jakhar AM, Aziz I, Kaleri AR, Hasnain M, Haider G, Ma J, Abideen Z. Nano-fertilizers: A sustainable technology for improving crop nutrition and food security. Nano Impact. 2022; 27: 100411.
- Ji H, Guo Z, Wang G, Wang X, Liu H. Effect of ZnO and CuO nanoparticles on the growth, nutrient absorption, and potential health risk of the seasonal vegetable Medicago polymorpha L. PeerJ. 2022; 10: e14038.
- Jones RR, Weyer PJ, DellaValle CT, Inoue-Choi M, Anderson KE, Cantor KP, et al. Nitrate from drinking water and diet and bladder cancer among postmenopausal women in Iowa. Environ. Health Perspect. 2016; 124: 1751-8.
- Kale AP, Gawade SN. Studies on nanoparticle induced nutrient use efficiency of fertilizer and crop productivity. Green Chem Technol Lett. 2016; 2: 88-92.
- Kantwa S, Yadav LR. Nano urea: Applications and significance. Just Agriculture. 2022; 2: 1-6.
- Karooki AK, Yavarzadeh M, Akbarian MM, Askari AA. Effects of Nanofertilizers (Mg and Fe) and Planting Data on Productivity and Quality of Potato Tubers in Cold Desert Climate. Rev. Agrogeoambiental. 2021; 13: 107-16.
- Keerthana P, Vijayakumar S, Vidhya EV, Punitha VN, Nilavukkarasi M, Praseetha PK. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti-infection and growth promotion in plants. Ind Crops Prod. 2021; 170: 113762.
- Khan MZ, Islam MR, Nahar N, Al-Mamun MR, Khan MA, Matin MA. Synthesis and characterization of nanozeolite based composite fertilizer for sustainable release and use efficiency of nutrients. Heliyon. 2021: 7.
- Kottegoda N, Sandaruwan C, Priyadarshana G, Siriwardhana A, Rathnayake UA, Berugoda Arachchige DM, et al. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS nano. 2017; 11: 1214-21.
- Kumar A, Yadav N, Bhatt M, Mishra NK, Chaudhary P, Singh R. Sol-gel derived nanomaterials and it’s applications: a review. Res J Chem. 2015; 5: 98-105.
- Kumar N, Manuja S, Sankhyan NK, Kumar P, Kumar A, Sharma T. 7.21 Effect of application of Nano-DAP and conventional fertilizers on rice yield. Sustainable Agricultural Innovations for Resilient Agri-Food Systems. 2022; 373.
- Kumar Y, Tiwari KN, Singh T, Raliya R. Nanofertilizers and their role in sustainable agriculture. Ann plant soil res. 2021; 23: 238-55.
- Le TT, Mai TT, Phan KS, Nguyen TM, Tran TL, Dong TN, et al. Novel Integrated Nanofertilizers for Improving the Growth of Polyscias fruticosa and Asparagus officinalis. J Nanomater. 2022: 2022.
- Li Z, Wen X, Hu C, Li X, Li S, Zhang X, Hu B. Regional simulation of nitrate leaching potential from winter wheat-summer maize rotation croplands on the North China Plain using the NLEAP-GIS model. Agric. Ecosyst. Environ. 2020; 294: 106861.
- Madusanka N, Sandaruwan C, Kottegoda N, Sirisena D, Munaweera I, De Alwis A, et al. Urea–hydroxyapatite-montmorillonite nanohybrid composites as slow-release nitrogen compositions. Appl. Clay Sci. 2017; 150: 303-8.
- Matiz A, Mioto PT, Mercier H. Urea in plants: metabolic aspects and ecological implications. Progress in Botany. 2020; 81: 157-87.
- ME Trenkel T. Slow-and controlled-release and Stabilized Fertilizers: an option for enhancing nutrient use effiiency in agriculture. International Fertilizer Industry Association (IFA). 2021.
- Mejias JH, Salazar F, Pérez Amaro L, Hube S, Rodriguez M, Alfaro M. Nanofertilizers: A cutting-edge approach to increase nitrogen use efficiency in grasslands. Front Environ Sci. 2021; 9: 635114.
- Moring A, Hooda S, Raghuram N, Adhya TK, Ahmad A, Bandyopadhyay SK, et al. Nitrogen challenges and opportunities for agricultural and environmental science in India. Front. sustain. food syst. 2021; 5: 505347.
- Mousavand T, Takami S, Umetsu M, Ohara S, Adschiri T. Supercritical hydrothermal synthesis of organic-inorganic hybrid nanoparticles. J Mater Sci. 2006; 41: 1445-8.
- Naseem F, Zhi Y, Farrukh MA, Hussain F, Yin Z. Mesoporous ZnAl2Si10O24 nanofertilizers enable high yield of Oryza sativa L. Sci Rep. 2020; 10: 10841.
- Nisar S, Sadique S, Kazerooni EG, Majeed U, Shehzad MR. Physical and chemical techniques to produce nano fertilizers. Int J Chem Biochem Sci. 2019; 15: 50-7.
- Nongbet A, Mishra AK, Mohanta YK, Mahanta S, Ray MK, Khan M, et al. Nanofertilizers: A smart and sustainable attribute to modern agriculture. Plants. 2022; 11: 2587.
- Pabodha D, Abeywardana L, Sandaruwan C, Herath L, Priyadarshana G. Urea-Hydroxyapatite-Polymer Nanohybrids as Seed Coatings for Enhanced Germination. 2022: 25.
- Pierre K. The Effects of Zinc Nanofertilizers on Tomato Plants. 2019.
- Ponce-García CO, Soto-Parra JM, Sánchez E, Muñoz-Márquez E, Piña-Ramírez FJ, Flores-Córdova MA, et al. Efficiency of nanoparticle, sulfate, and zinc-chelate use on biomass, yield, and nitrogen assimilation in green beans. Agron. 2019; 9: 128.
- Prajapati D, Pal A, Dimkpa C, Singh U, Devi KA, Choudhary JL, et al. Chitosan nanomaterials: A prelim of next-generation fertilizers; existing and future prospects. Carbohydr. Polym. 2022; 288: 119356.
- Priyam A, Das RK, Schultz A, Singh PP. A new method for biological synthesis of agriculturally relevant nanohydroxyapatite with elucidated effects on soil bacteria. Sci rep. 2019; 9: 15083.
- Qureshi A, Singh DK, Dwivedi S. Nano-fertilizers: a novel way for enhancing nutrient use efficiency and crop productivity. Int J Curr Microbiol App Sci. 2018; 7: 3325-35.
- Raguraj S, Wijayathunga WM, Gunaratne GP, Amali RK, Priyadarshana G, Sandaruwan C, et al. Urea–hydroxyapatite nanohybrid as an efficient nutrient source in Camellia sinensis (L.) Kuntze (tea). J. Plant Nutr. 2020; 43: 2383-94.
- Rahman MH, Hasan MN, Nigar S, Ma F, Aly Saad Aly M, et al. Synthesis and characterization of a mixed nanofertilizer influencing the nutrient use efficiency, productivity, and nutritive value of tomato fruits. ACS omega. 2021; 6: 27112-20.
- Rajemahadik VA, Chavan SA, More VG, Chavan VG, Chavan AP, Shetye VN. Nanotechnology: Innovative approach in crop nutrition management. Int J Agr Sci. 2018.
- Rajonee AA, Nigar F, Ahmed S, Huq SI. Synthesis of nitrogen nano fertilizer and its efficacy. Canadian Journal of pure and Applied sciences. 2016; 10: 3913-9.
- Ramalingappa PL, Shrivastava M, Dhar S, Bandyopadhyay K, Prasad S, Langyan S, et al. Reducing options of ammonia volatilization and improving nitrogen use efficiency via organic and inorganic amendments in wheat (Triticum aestivum L.). Peer J. 2023; 11: e14965.
- Rameshaiah GN, Pallavi J, Shabnam S. Nano fertilizers and nano sensors–an attempt for developing smart agriculture. Int J Eng Res Gen Sci. 2015; 3: 314-20.
- Ramírez-Rodríguez GB, Dal Sasso G, Carmona FJ, Miguel-Rojas C, Pérez-de-Luque A, Masciocchi N, et al. Engineering biomimetic calcium phosphate nanoparticles: a green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS App. Bio Mat. 2020; 3: 1344-53.
- Rastogi A, Tripathi DK, Yadav S, Chauhan DK, Živcák M, Ghorbanpour M, et al. Application of silicon nanoparticles in agriculture. 3 Biotech. 2019; 9: 90.
- Sampathkumar K, Tan KX, Loo SC. Developing nano-delivery systems for agriculture and food applications with nature-derived polymers. Iscience. 2020; 23: 101055.
- Sebastian A, Devika PS, Nair PP, Devadas VS. Green synthesis of potassium-infused nitrogenous nano-fertilizer for enhanced plant growth. Chem pap. 2024; 78: 1481-92.
- Sharma S, Kumar A, Choudhary A, Harish BM, Karmakar P, Sharma P, et al. Recent developments in smart nano-agrochemicals: A promise for revolutionizing present-day agriculture. Materials Today: Proceedings. 2022; 69: 530-4.
- Sharma S, Sahu BK, Cao L, Bindra P, Kaur K, Chandel M, et al. Porous nanomaterials: Main vein of agricultural nanotechnology. Prog Mater Sci. 2021; 121: 100812.
- Sigurdarson JJ, Svane S, Karring H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev Environ Sci Biotechnol. 2018; 17: 241-58.
- Singh A, Kumar H, Kumar S, Dutta PK. Role of chitosan and chitosan-based nanoparticles in antioxidant regulation of plants. Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences. 2022: 321-41.
- Singh R, Machanuru R, Singh B, Shrivastava M. Climate-resilient agriculture: enhance resilience toward climate change. InGlobal climate change. Elsevier. 2021; 45-61.
- Solanki P, Bhargava A, Chhipa H, Jain N, Panwar J. Nano-fertilizers and their smart delivery system. Nanotechnologies in food and agriculture. 2015: 81-101.
- Stolarski RR, Wasilewski M. The relation of operating costs and the economic productivity of assets according to the type and scale of activity in food enterprises. Zeszyty Naukowe Malopolskiej Wyzszej Szkoly Ekonomicznej w Tarnowie. 2022; 53: 1528.
- Subramanian KS, Manikandan A, Thirunavukkarasu M, Rahale CS. Nano-fertilizers for balanced crop nutrition. Nanotechnologies in food and agriculture. 2015: 69-80.
- Subramanian KS, Rahale CS. Ball milled nanosized zeolite loaded with zinc sulfate: a putative slow release Zn fertilizer. Int. J. Innov. Hortic. 2012; 1: 33-40.
- Subramanian KS, Rahale CS. Synthesis of nanofertiliser formulations for balanced nutrition. Proceedings of the Indian society of Soil Science-Platinum Jubilee Celebration. 2009: 22-5.
- Subramanian KS, Thirunavukkarasu M. Nano-fertilizers and nutrient transformations in soil. Nanoscience and Plant–Soil Systems. 2017: 305-19.
- Tarafder C, Daizy M, Alam MM, Ali MR, Islam MJ, Islam R, et al. Formulation of a hybrid nanofertilizer for slow and sustainable release of micronutrients. ACS omega. 2020; 5: 23960-6.
- Tripathi DK, Singh VP, Chauhan DK, Sharma S, Prasad SM, Dubey NK, et al. editors. Plant Life Under Changing Environment: Responses and Management. Academic Press. 2020.
- Umar W, Ayub MA, Rehman MZ, Ahmad HR, Farooqi ZU, Shahzad A, et al. Nitrogen and phosphorus use efficiency in agroecosystems. Resources use efficiency in agriculture. 2020: 213-57.
- Vejan P, Khadiran T, Abdullah R, Ahmad N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J Cont Rel. 2021; 339: 321-34.
- Verma KK, Song XP, Joshi A, Tian DD, Rajput VD, Singh M, et al. Recent trends in nano-fertilizers for sustainable agriculture under climate change for global food security. Nanomater. 2022; 12: 173.
- Wang A, Wang W, editors. Nanomaterials from clay minerals: a new approach to green functional materials. Elsevier. 2019.
- Wang F, Harindintwali JD, Yuan Z, Wang M, Wang F, Li S, et al. Technologies and perspectives for achieving carbon neutrality. The Inn. 2021; 2: 100180.
- Wang H, Wick RL, Xing B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Envi pol. 2009; 157: 1171-7.
- Yadav A, Yadav K, Abd-Elsalam KA. Exploring the potential of nanofertilizers for a sustainable agriculture. Plant Nano Biol. 2023: 100044.
- Yang Z, Chen J, Dou R, Gao X, Mao C, Wang L. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. Public Health. 2015; 12: 15100-9.
- Yoon HY, Lee JG, Esposti LD, Iafisco M, Kim PJ, Shin SG, et al. Synergistic release of crop nutrients and stimulants from hydroxyapatite nanoparticles functionalized with humic substances: Toward a multifunctional nanofertilizer. ACS omega. 2020; 5: 6598-610.
- Yuvaraj M, Subramanian KS. Novel slow release nanocomposite fertilizers. InNanotechnology and the Environment. 2020.
- Zahra Z, Habib Z, Hyun H, Shahzad HM. Overview on recent developments in the design, application, and impacts of nanofertilizers in agriculture. Sustainability. 2022; 14: 9397.