
Special Article: Legumes
Ann Agric Crop Sci. 2024; 9(4): 1160.
Prebiotic and Antiproliferation Effects of Arabinogalactan Isolated from Green Gram (Vigna radiata) and Its Hydrolysates
Kiranmayi Ketha; Muralikrishna Gudipati*
Department of Biochemistry, CSIR- Central Food Technological Research Institute, Mysuru, Karnataka, India
*Corresponding author: G Muralikrishna FAFST (I), FACCT(I) MNASc, Formerly Chief Scientist (Scientist-G) & Formerly HOD, Professor AcSIR, Department of Biochemistry, CSIR-Central Food Technological Research Institute, Mysuru 570020, Karnataka, India. Tel: +91-821-2516531 (R) Mobile (0) +91-9480382199 Email: krishnagm2002@yahoo.com
Received: May 30, 2024 Accepted: June 25, 2024 Published: July 02, 2024
Abstract
An Arabinogalactan (AGP) derived from green gram and its hydrolysates (AGO, Arabinogalacto oligosaccharides) were screened for their prebiotic potential and antiproliferation effects on colon cancer cells (in vitro). Three lactobacilli strains namely L. acidophilus, L. delbrucki and L. fermentum showed relatively better response towards AGP and AGO as compared with other tested strains with respect to defined prebiotic characteristics. The activities of a-L-arabinofuranosidase (39 to 89.5 mU/mL) and β-D-galactopyranosidase (19 to 49.5 mU/mL) in the culture broth indicated the breakdown of AGP and AGO into constituent sugars and their subsequent utilization for the growth of bacteria. AGP and AGO exhibited dose dependent cytotoxic and anti-proliferative effects on (p <0.001) Caco-2 cells with IC50 values of 150 and 500 μg/mL, respectively as estimated by MTT assay. The present study demonstrated the prebiotic properties of AGP and AGO as well as their ability to provide natural defence against colon cancer.
Keywords: Arabinogalactan; green gram; prebiotic activity; anti-proliferation; Caco- 2 cell line
Introduction
Plants are one of the rich sources of bioactive molecules which can act as prebiotics that include proteins, glycosides, Non-Starch Polysaccharides (NSP) and Non-Digestible Oligosaccharides (NDO) [1,2]. Prebiotic oligosaccharides are known to maintain the population of beneficial bacteria in the gut particularly lactic acid bacteria (Lactobacilli and Bifidogenic). NSP are natural antitumour agents which can act as immunomodulators [3], and also can cause direct cytotoxic effect on the tumour cells, but very little is known about their exact mechanism [4]. In addition to NSP, NDO also were proven to have anti-cancer properties [5]. Fermentation of NSP and NDO by lactic acid bacteria produces Short Chain Fatty Acids (SCFA) i.e. lactate, butyrate, propionate and acetate which have shown anti proliferation effects on colon cancer [6].
Green gram [Vigna radiata (L.)] commonly called mung bean, is used in several cuisines either with or without hull. Mung bean extracts showed various health benefits including anticancer [7], antidiabetic [8] and anti-inflammatory activities [9]. Structural features of cell wall polysaccharides from green gram cotyledons and antioxidant activity of polysaccharides extracted from mung bean hull have been reported previously [10-12]. Mung bean polysaccharides isolated with water and alkali were demonstrated to activate macrophages [13,14].
There are limited reports regarding prebiotic and anti-cancer properties of dietary fibre components of green gram. We have purified an Arabinogalactan (AGP) from green gram and proved its immunomodulatory activity [15,16]. The present study is envisaged to study the 1) Prebiotic activity of AGP and its hydrolysates (AGO) and 2) Antiproliferation effect of AGP, AGO and the SCFA (produced by lactobacilli upon fermentation of AGP and AGO) on colon cancer cell line (Caco- 2 cell line).
Materials and Methods
Preparation of Hydrolysates of AGP
AGP (1 mg) was suspended in lowest volume of acetate buffer (2 mL, 0.1 M, pH 4.5) followed by addition of 0.1 M TFA (20 μL) and the resultant mixture was incubated in boiling water bath for 12 h. Hydrolysis of polysaccharide was monitored by estimating the content of reducing sugar for every 1h duration. The above solution was allowed to attain room temperature and was neutralized with alkali followed by the addition of ethanol at 4 oC for 6 h for precipitation. The supernatant obtained after centrifugation at 8000 rpm for 10 min. was concentrated and freeze dried to obtain the liberated oligosaccharides [17]. The hydrolysis and release of oligosaccharides were verified by running the sample on TLC plate (100 μ thick silica gel adsorbent, Eastman Kodak Co., Rochester, NY) in the solvent system of ethylacetate, n-propanol, acetic acid and water (4:2:2:1). The TLC chamber was saturated for 3h with the solvent system. Samples were loaded and allowed to run on TLC plate till the solvent front reached the top rim of the plate. The plate was air dried followed by spraying with Orcinol-H2SO4 (250 mg Orcinol in 5 mL H2SO4 and 95 mL absolute ethanol) reagent and was dried in the hot air oven at 100 °C until the characteristic bluish-purple spots developed on the chromatogram. The oligosaccharides derived from acid hydrolysis of AGP were designated as AGO.
Microorganisms
Lactobacillus sp. (L brevis 01, L delbrueckii10, L acidophilus 011, L casei 017, Lfermentum 156) and Bifidobacterium sp. (B bifidum 235 and B adolescentis 236) cultures were acquired from National Dairy Research Institute (NDRI), Karnal, Haryana India. The cultures were maintained in lactobacillus MRS broth medium at 6°C (for Bifidobacteria- the broth was supplemented with 0.05% cysteine HCl) and sub cultured for every 30 days. Prior to experimentation, sub-culturing of the cultures was done thrice in respective MRS broths at 37°C for 24 h.
Prebiotic Activity
Filter sterilized samples (0.22 μm membrane, Millipore) were added (0.25, 0.5, 1 % w/v) to MRS broth media (formulated excluding beef extract, dextrose, sodium acetate, yeast extract, and replaced peptone with tryptone) which were inoculated with culture suspension aliquots (100 μL) having a pre requisite cell number (1 × 106 CFU/mL) and incubated for 24, 48, 72 and 96 h at 37°C. The broth color change from colorless to deep yellow was considered as positive test. The increase in microbial growth utilizing the given samples was examined by measuring the pH and turbidity of the culture broth. Turbidity of broth was measured at 600 nm using UV-visible spectrophotometer. The lactobacillus sp. and bifidobacterium sp. were screened for their positive response towards the test samples. To determine the dry cell mass incubated cultures were centrifuged (3000 × g) for 20 min and pellets were oven dried (80°C) and the resultant supernatant was analyzed for SCFA and enzymes [18]. All the experimental values are averages of three independent experiments.
Enzyme Assays
Culture broth incubated for 48 h, containing test sample (0.5%) was analyzed for the presence of various enzyme activities such as a-L- arabinofuranosidase, a-D-galactopyranosidase, β-D- galactopyranosidase and acetyl esterase. The activities of a-L-arabinofuranosidase, a-D-galactopyranosidase and β-D- galactopyranosidase were determined by incubating p-nitro phenol glycosides (0.5 ml of 2 mM substrate in sodium phosphate buffer, 0.1 M, pH 5.7) with culture broth (0.1 ml) for 1 h at 37 °C followed by estimation of the release of p-nitro phenol from respective substrates [18]. Acetyl esterase activity was estimated by incubating culture supernatant (0.1 ml) with saturated solution of p-nitro phenyl acetate (1 ml in sodium potassium phosphate buffer 0.2 M, pH 6.5) for 30 min at 25 °C. All the above-mentioned reactions were stopped by the addition of saturated sodium tetra borate solution (0.5 mL) and absorbance was read at 400 nm. One unit of enzyme activity was defined as the amount of enzyme required for the liberation of p-nitro phenol (1 μM) per minute under assay conditions [19].
SCFA Analysis
SCFAs in the culture supernatant were extracted using diethyl ether after acidifying it to pH 2.0 with sulphuric acid (50%) [20]. The diethyl ether containing SCFAs was analyzed by Gas Liquid Chromatography on carbowax-20 M column, temperatures of column, injector and detector were maintained at 120, 220 and 230°C, respectively and nitrogen was used as the carrier gas (40 ml/min). Acetate, propionate, butyrate and lactate (10 μM in diethyl ether) were used as standards. Individual SCFA in the sample was quantified by determining the peak area of respective standard [21].
Antiproliferation Activity
Cell Culture: Caco-2 cells were acquired from NCCS Pune, India and maintained in DMEM medium supplemented with 10 % Fetal Bovine Serum and 2.5 % antibiotic incubating at 37 °C in an atmosphere of 5% CO2–95% air mixture. The cells were dispersed with 0.05% trypsin and 0.02 % EDTA for cell counting and sub-culturing.
Analysis of Cell Viability by MTT Assay
To evaluate cell viability, colorimetric MTT assay [22] was carried out. Briefly, cells (1 x 105) were seeded in 96-well plates and incubated for 24 h followed by treatment with serial concentrations of AGP, AGO (25, 50, 100 and 200 μg/mL) and SCFAs (2- 50μM) for 24 and 48 h. MTT (5 mg/mL, 10 μL) in PBS solution was added to each well at a final concentration of 0.5 mg/mL followed by incubating the plate for 4 h. After incubation, MTT-containing media was removed from wells, followed by addition of DMSO (150 μL) to each well to solubilize the formazan crystals and plate was shaken on a rotary shaker for 10 min. Finally, A550 was measured and growth inhibition was calculated as follows: ([Acontrol–Atest] / Acontrol) X 100 %.
Neutral Red Assay
Cells were incubated with samples for 72 h, followed by addition of freshly prepared neutral red solution (150 μL, 3.3 g/L diluted 1/100 in cell culture medium) to each well and incubation of all plates at 37 °C for additional 4 h. After incubation, the neutral red solution was removed and the cells were rinsed two times with PBS and followed by adding extracting solution (150μL; 50% ethanol and 1% acetic acid in distilled water) in each well and plates were shaken for 15 min. The optical density at 540 nm using a Microplate Reader was recorded [23].
LDH Assay
The cytotoxic effects of test samples on Caco-2 cells were evaluated by determining LDH enzyme released from damaged cells into the medium [24]. LDH kit (Thermoscientific Pierce LDH Cytotoxicity Assay Kit) was used to determine cell membrane damage due to AGP, AGO and SCFAs treatments according to manufacturer’s instructions. Briefly, after the incubation (48 h) culture supernatant (50 μL) was pipetted into test plate, reaction mixture (Substrate mix and assay buffer) was added and incubated for 30 min at room temperature after which Stop solution (50 μL) was added and absorbance was measured at 490 nm.
ATP Assay
ATP levels in cells were determined using ATP assay kit [25]. Briefly, caco-2 cells were harvested, lysed, centrifuged and culture supernatant was deproteinized. Thus, obtained cell lysate (50μL) was taken in test plates and reaction mixture (50μL., Assay buffer, ATP probe, ATP converter and developer mix) was added. After the plate was incubated for 30 min under dark, absorbance was read. ATP levels were expressed as ATP % = (O.D)Expt x 100/ (O. D)Control.
Results
Hydrolysis of Polysaccharide Isolated from Green Gram
Arabinogalactan (AGP) isolated from green gram was subjected to acid hydrolysis using TFA (0.1 M) and the release of oligosaccharides was determined by estimation of the reducing sugar (DNS method). The graph plotted for release of oligosaccharides was shown in Figure 1a. The minimum time taken for complete liberation of oligosaccharide was found to be 9 h. The oligosaccharides were identified (Figure 1b). The supernatant obtained after neutralization and ethanol evaporation consisted of ~95 % sugar, out of which uronic acid content was ~10%.
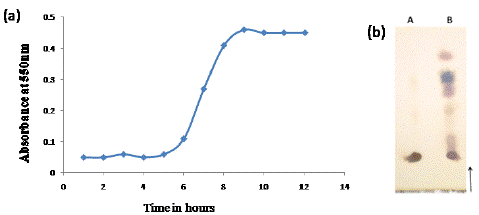
Figure 1: Liberation of oligosaccharides by TFA hydrolysis of AGP determined using DNS method (a), TLC of oligosaccharides liberated by acid hydrolysis (b A- AGP, B- AGO).
In vitro Fermentation
Various strains of Lactobacillus [L. brevis (NDRI 01), L. delbrueckii (NDRI 10), L. acidophilus (NDRI 11), L. casei (NDRI 17) and L. fermentum (NDRI 156)] and strains of Bifidobacterium [B. bifidum (NDRI 235) and B. adolescentis (NDRI 236)] were screened for their response (in vitro fermentation effect) towards AGP and its hydrolysates. The results of the study are summarized in Table 1. FOS at 0.5% concentration showed positive effect on all the used strains at 48 h incubation. AGP and AGO showed selective positive response towards L. acidophilus, L. delbrueckii and L. fermentum with respect to optical density, change in media color to yellow, reduction in pH and increase in dry cell mass. AGO showed relatively significant effect on the above strains as compared to AGP and AGO showed marginal effect on the other strains however the effect was not significant as compared to the control. Therefore, the above three selective strains were chosen for further experimentation.
Microorganism
Carbon source -0.5%
Optical density at 600 nm
pH
Cell mass (mg/mL)
L. brevis 01
Control
0.08 ±0.02
6.9± 0.4
0.09± 0.02
FOS
0.86 ± 0.03
5.7 ± 0.2
1.12± 0.05
AGP
0.12 ± 0.03
6.7 ± 0.4
0.23± 0.04
AGO
0.25 ± 0.04
6.2 ± 0.3
0.32± 0.06
L. delbrueckii 10
Control
0.1 ±0.03
7.0± 0.4
0.11± 0.04
FOS
1.2 ± 0.02
5.5 ± 0.2
1.02± 0.03
AGP
0.39 ± 0.02
6.0 ± 0.3
0.42± 0.02
AGO
0.87 ± 0.03
5.4 ± 0.4
0.92± 0.05
L. acidophilus 11
Control
0.09 ±0.04
6.9± 0.6
0.09± 0.04
FOS
0.89 ± 0.02
5.7± 0.3
1.0± 0.03
AGP
0.41 ± 0.03
6.8 ± 0.3
0.57± 0.04
AGO
0.95 ± 0.04
5.1 ± 0.2
1.12± 0.06
L. casei 17
Control
0.12 ±0.03
6.8± 0.4
0.08± 0.02
FOS
0.89 ± 0.02
5.5 ± 0.4
1.2± 0.04
AGP
0.14 ± 0.05
6.8 ± 0.1
0.09± 0.07
AGO
0.29 ± 0.03
6.0 ± 0.3
0.42± 0.05
L. fermentum 156
Control
0.11 ±0.02
6.9± 0.4
0.11± 0.02
FOS
0.92 ± 0.01
5.5 ± 0.4
1.1± 0.04
AGP
0.37 ± 0.02
5.6±0.3
0.4± 0.04
AGO
0.85 ± 0.07
5.2 ± 0.2
0.90± 0.03
B. bifidum 235
Control
0.09 ±0.06
7.1± 0.2
0.12± 0.04
FOS
0.66 ± 0.02
5.4 ± 0.1
0.72± 0.06
AGP
0.14 ± 0.03
6.7 ± 0.3
0.22± 0.04
AGO
0.33 ± 0.01
6.1 ± 0.2
0.52± 0.02
B. adolescentis 236
Control
0.09 ±0.04
6.9± 0.4
0.09± 0.04
FOS
0.71 ± 0.05
5.7 ± 0.2
0.82± 0.03
AGP
0.11 ± 0.02
6.8 ± 0.4
0.12± 0.04
AGO
0.38 ± 0.04
6.8 ± 0.4
0.39± 0.06
Table 1: Screening of Lactobacilli Sp and Bifidobacteria Sp for the utilization of AGP and AGO, FOS (Fructo oligosaccharides) served as positive control.
Growth Characteristics of Selected Microorganisms
The growth pattern of all the three strains was same when treated with AGP. AGP at concentrations of 0.5 and 1 % showed slow stimulation of growth from 24 h, reached maximum at 48 h and reached constant thereafter whereas at 0.25 % concentration did not show significant effect on any of the selected strains (Figure 2a,c,e). The strains treated with AGO at all the concentrations (0.25, 0.5, 1 %) showed rapid increase in growth at 24 h, reached maximum at 48 h and became constant thereafter (Figure 2b, d, f). The change in growth rate of the above microorganisms from 24 to 48 h was not significant.
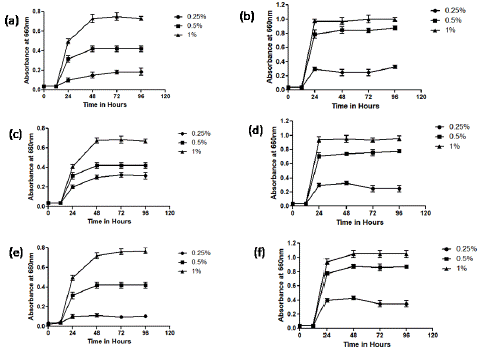
Figure 2: Growth Charecteristics of L. acidophilus (a, b), L. delbricki(c,d) and L. fermentum(e,f)in response to AGP (left panel ) and AGO (right panel).
SCFA Analysis
Acetate was the major SCFA produced due to fermentation of both AGP and AGO by the microorganisms after 48 h incubation (Table 2). In addition to acetate, L. acidophilus produced small amounts of propionate (1 %) grown on both AGP and AGO, and butyrate (1.5 %) when grown on AGO. Acetate (100%) was the only end product identified in culture broth of L. delbrueckii fermenting both the samples. Acetate, propionate and butyrate were the SCFAs detected in culture broth of L. fermentum grown on both the samples. Lactate was not detected in any of the test samples.
Micro-organism
Carbon Source
a- L-arabinofuranosidase
a- D-Galactopyranosidase
β- D-Galactopyranosidase
Acetyl esterase
SCFA*
AA
PA
BA
LA
L. delbrueckii
AGP
43.7±0.04
4.6±0.06
24.0±0.03
3.9±0.02
100
ND
ND
ND
(NCDC 10)
AGO
89.3 ± 0.02
9.5 ± 0.01
49.5 ± 0.01
4.4 ± 0.05
100
ND
ND
ND
L. acidophilus
AGP
39.0±0.03
4.9±0.02
20.6±0.05
2.8±0.05
98.7
1.2
ND
ND
(NCDC 11)
AGO
78.4 ± 0.03
6.8± 0.06
46.4 ± 0.06
3.3 ± 0.05
97.5
1
1.5
ND
L. fermentum
AGP
40.2±0.02
5.9±0.04
19.4±0.02
3.5±0.06
97
1.5
1.5
ND
(NCDC 156)
AGO
88.5 ± 0.04
8.1 ± 0.05
42.1 ± 0.05
7.3 ±0.04
98
1
1
ND
*AA: Aceteic Acid, PA: Propionic Acid, BA: Butyric Acid, LA: Lactic Acid, ND: Not Detected
Table 2: Enzyme activities and production of SCFA by selected lactobacilli sp upon utilizing AGP and AGO at 48 h incubation.
Enzyme Assays
The presence of the hydrolytic enzymes in the culture supernatants after 48 h incubation (with sample 0.5%) were determined and the activity is expressed as mU/min/mL (Table 2). a-L-arabinofuranosidase (39 to 89.5 mU/mL) and β-D-galactopyranosidase (19 to 49.5 mU/mL) were the major enzymes found in culture filtrates as compared to a-galactopyranosidase and acetyl esterase. The activity of these enzymes was more in culture broths of microbes grown on AGO as compared to those grown on AGP.
Antiproliferation Activity
MTT assay: AGP isolated from green gram inhibited growth of Caco-2 cells in a time and dose dependent manner (Figure 3a). The growth of Caco-2 cells was significantly (p <0.001) inhibited at 24 and 48 h by AGP at 50- 500 μg/mL concentrations (Figure 3a). AGO significantly (p <0.001) inhibited the cell growth at higher concentrations 100 to 500 μg/mL after 48 h incubation (Figure 3b). The IC50 values of AGP and AGO against Caco- 2 cells were found to be 150 and 500 μg/mL. SCFAs failed to show any effect on Caco-2 cells and showed only marginal effect at 50 μM/ mL after 48 h (p <0.05, Figure 3c).
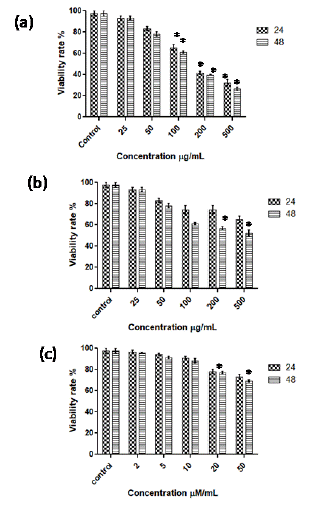
Figure 3: MTT assay to determine cell viability of Caco-2 cells treated with AGP (a), AGO (b) and SCFA (c) at 24 and 48 h incubation. *p <0.001 vs control.
Neutral red assay
The results of neutral red assay were same as MTT assay (Figure 4). The viability rate (%) of Caco-2 cell line in response to AGP and AGO according to neutral red assay was less as compared to MTT assay. However, SCFAs’ effect on cell growth with this assay was found to be the same as MTT assay. The IC50 values of AGP and AGO against Caco-2 cells were found to be 125 and 450 μg/mL respectively.
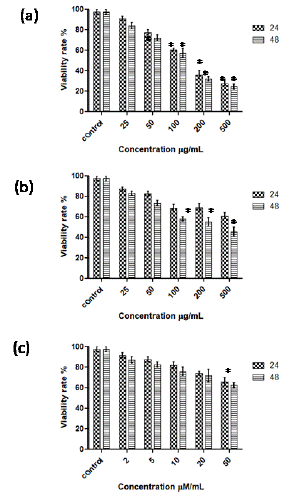
Figure 4: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
LDH activity Assay
As shown in figure 5a, the exposure of AGP (25- 500 μg/mL) to Caco-2 cells caused 1.7 to 9.2-fold and 2-to-10-fold increase in release of LDH as compared to the control cells at 24 h and 48 h incubations respectively. AGO at lower concentrations (25, 50, 100 μg/mL) did not show significant effect on LDH release whereas at higher concentrations (200 and 500 μg/mL) showed 1.8- and 3.3-fold increase in the release of LDH by Caco-2 cells (Figure 5b). SCFAs exhibit marginal effect on LDH release by Caco-2 cells (Figure 5b).
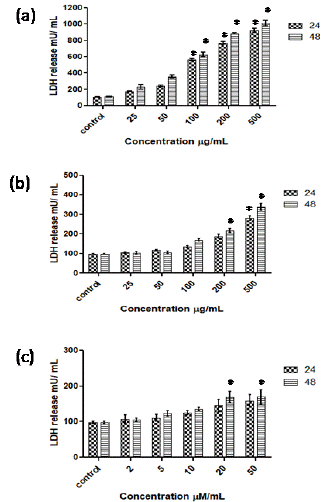
Figure 5: Effect of AGP (a), AGO (b) and SCFA (c) on the increase in release of LDH by Caco 2 cells at 24 and 48 h incubation. *p <0.001 vs control.
ATP assay
The effect of AGP, AGO and SCFAs on intracellular ATP levels in Caco-2 cells is shown in Figure 6. In Caco-2 cells exposed to AGP at 50 to 500 μg/mL concentration, ATP levels decreased from 73 to 25 % at 24 h. Cells exposed to lesser concentration of AGP (25 μg/mL) did not show significant decrease in the ATP levels as compared to control at 24 or 48h (Figure 6a). AGO at concentrations of 25 and 50 μg/mL did not decrease ATP levels in cancer cells whereas at 100, 200 and 500 μg/mL concentrations, AGO showed 75 to 50 % decrease in ATP levels in Caco-2 cells (Figure 6b). SCFAs showed marginal effect on intracellular ATP levels in colon cancer cells at 48 h (Figure 6c).
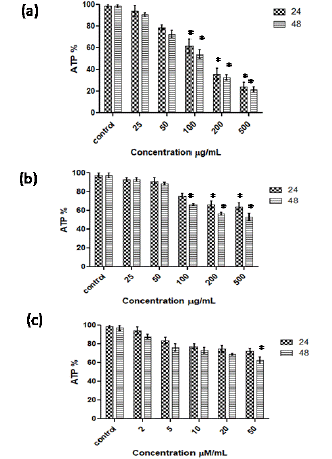
Figure 6: Effect of AGP (a), AGO (b) and SCFA (c) on the intracellular ATP levels of Caco- 2 cells at 24 and 48 h incubation. *p <0.001 vs control.
Discussion
In vitro experiments carried out using AGP and AGO demonstrated their prebiotic nature with respect to Bifidobacteria and Lactobacilli sp. in terms of the growth characteristics pattern. Out of seven strains screened for their ability to utilize AGP and AGO isolated from green gram, three (L. acidophilus, L. delbruckiand L. fermentum) strains were found to utilize these samples for their growth effectively. The above-mentioned strains utilized AGO better as compared to AGP. It was previously reported that Bifidobacteria and lactobacilli utilize polysaccharides where as readily utilize their hydrolysates [26]. There was reduction in pH of culture broth of bacteria that utilized AGP and AGO which was due to the SCFAs produced as a result of the fermentation of non-digestible carbohydrates. Such decrease in pH can be considered as an indication of the prebiotic effect of the polysaccharides and oligosaccharides incorporated in the culture broth [27]. The bacteria produced different proportion of SCFA in all the culture tubes inoculated with AGP and AGO as carbon source. The concentration of total SCFA produced varied for individual organism and carbon source. Acetate was the major SCFA produced by all the three strains and its quantity varied from 97.5% to 100% (Table 2). The results were almost similar to the ones reported for human fecal bacteria [28].
The presence of various enzyme activities such as a -L-arabinofuranosidase, a -D-galactopyranosidase, β-D-galactopyranosidase and acetyl esterase activities were identified in 48 h old cultures. All the cultures showed maximum a -L-arabinofuranosidase activity which could possibly be due to the presence of high arabinose content in AGP [16]. The relatively higher activities of a -L-arabinofuranosidase and β-D-galactopyranosidase indicated the hydrolysis of arabinogalactan into its constituent sugars arabinose and galactose which were utilized by the bacteria for their growth. The hydrolytic enzymes produced by the microorganisms facilitate the digestion of non-digestible carbohydrates which cannot be digested in the upper gastrointestinal tract and produce SCFA resulting in the decrease of pH of the media. Reduction in pH in bowel generates acidic environment, which consecutively decreases the number of pathogenic microorganisms [29].
In addition to prebiotic activity, the anti-Caco-2 cell proliferation effects of green gram AGP, AGO and SCFA produced by the lactobacilli (utilizing AGP and AGO) were studied in the present study. Polysaccharides and oligosaccharides isolated from variety of sources were shown to have anti proliferation effects towards colon cancer cell lines [24,30] SCFA especially butyrate is known to induce growth inhibition in human colon cancer cells [31,32]. Viability of Caco-2 cells was evaluated after treating with AGP, AGO and SCFA using the MTT and neutral red tests. AGP exhibited excellent antiproliferation effect against Caco-2 cells with an IC50 value 150 μg/mL according to MTT and 125 μg/mL according to neutral red assay. AGP did better when compared to AGO which showed IC50 value of 500 and 450 μg/mL as per MTT and neutral red assays. LDH liberation into the culture media is more often used as an index of the loss of cell membrane integrity or necrosis caused by antitumor activity [33]. The release of LDH into media by Caco-2 cells in response to AGP increased up to 10-fold as compared to control where as an increase up to 3-fold was observed in response to AGO. These results suggest that AGP and AGO inhibition of Caco-2 cell growth was accompanied by the disruption of cellular membrane thereby releasing LDH into the culture medium. ATP levels decreased to 25% and 50 % when cancer cells exposed to AGP and AGO after 48 h respectively.
The ATP levels of a cell can be directly related to metabolic activity of the cell, as cell injury or death is causes reduced ATP activity [25]. A decrease in ATP was associated with a decrease in cancer cell viability exposed to increasing concentration of AGP and AGO.
SCFA produced by L. fermentum due to the fermentation of AGP and AGO containing acetate: propionate: butyrate in the ratio 98:1:1 was tested for antiproliferation activity towards Caco- 2 cells. SCFA tested did not show any significant effect on cell viability up to 20 μm/mL and showed only marginal effect towards cell viability at 50 μm/mL. The effect of SCFA on increase in release of LDH and decrease in ATP levels in Caco-2 cells was not significant. The inability of SCFA tested in the study might be because of high quantity of acetate (98%). It was previously proved by many research groups that acetate has the least antiproliferation effects on colon cancer cells [34,35,36]. The marginal effect of the SCFA in the present study must be due to the presence of butyrate which is known to have potential antiproliferation activity [37].
Conclusion
Arabinogalactan isolated from green gram and its hydralysates were screened for prebiotic activity on various Lactobacilli and Bifidobacteria. Among which, three strains (L. delbrucki, L. acidophilus and L. fermentum) were shown to have positive response towards AGP and AGO. The above three strains readily utilized the oligosaccharides but slowly utilized AGP. Acetate was the major SCFA produced upon fermentation of AGP and AGO. Propionate and butyrate were produced in very low quantity. AGP showed better antiproliferation activity when compared with AGO with respect to cell viability, LDH release and ATP levels in Caco-2 cells. SCFA obtained from the fermentation of AGP and AGO did not show significant anti proliferation towards caco-2 cells. In conclusion AGP isolated can be explored as antiproliferation agent towards colon cancer and AGO can be used as potential prebiotic for the growth of beneficial bacteria in the large intestine.
Author Statements
Conflict of Interest
Authors declare no conflict of interest.
Acknowledgment
The authors would like to thank the Director, Council of Scientific and Industrial Research-Central Food Technological Research Institute, Mysuru for the constant encouragement. Ms. Kiranmayi Ketha thank UGC, New Delhi for research fellowship.
References
- Philip CC, Kew S. The immune system: a target for functional foods?. Br J Nutr. 2002; 88: S165–S176.
- Kau AL, Ahem PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome, and immune system: envisioning the future. Nature. 2011; 474: 327–336.
- Yang X, Zhao Y, Wang H, Mei Q. Macrophage activation by an acidic polysaccharide isolated from Angelica sinensis (Oliv.) Diels. J Biochem Mol Biol. 2007; 40: 636–643.
- He NW, Yang XB, Jiao YD, Tian L, Zhao Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012; 133: 978-989.
- Taper HS, Delzenne NM, Roberfroid MB. Growth inhibition of transplantable mouse tumors by non-digestible carbohydrates. Int J Cancer. 1997; 71: 1109-1112.
- Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nnutr. 2002; 132: 1012-1017.
- Soucek J, Skvor J, Pouckova P, Matousek J, Slavik T, Matousek J. Mung bean sprout (Phaseolus aureus) nuclease and its biological and antitumor effects. Neoplasma. 2006; 53: 402-409.
- Yao Y, Chen F, Wang M, Wang J, Ren G. Antidiabetic activity of Mung bean extracts in diabetic KK-Ay mice. J Agric Food Chem. 2008; 56: 8869-8873.
- Lee SJ, Lee JH, Le HH, Lee S, Kim SH, Chun T, et. al. Effect of mung bean ethanol extract on pro-inflammtory cytokines in LPS stimulated macrophages. Food Sci Biotechnol. 2011; 20: 519-524.
- Gooneratne J, Needs PW, Ryden P, Selvendran RR. Structural features of cell wall polysaccharides from the cotyledons of mung bean Vigna radiata. Carbohydr Res. 1994; 265: 61- 77.
- Lai F, Wen Q, Li L, Wu H, Li XF. Antioxidant activities of water-soluble polysaccharide extracted from mung bean (Vigna radiata L.) hull with ultrasonic assisted treatment. Carbohydr Polym. 2010; 81: 323-329.
- Zhong K, Lin W, Wang Q, Zhou S. Extraction and radicals scavenging activity of polysaccharides with microwave extraction from mung bean hulls. Int J Biol Macromol. 20121; 51: 612-617.
- Yao Y, Zhu Y, Ren G. Antioxidant and immunoregulatory activity of alkali extractable polysaccharides from mung bean. Int J Biol Macromol. 2016; 84: 289-294.
- Yao Y, Zhu Y, Ren G. Immunoregulatory activities of polysaccharides from mung bean. Carbohydr Polym. 2016; 139: 61-66.
- Ketha K, Gudipati M. Immunomodulatory activity of non starch polysaccharides isolated from green gram (Vigna radiata). Food Res Int. 2018; 113: 269-276.
- Ketha K, Gudipati M. Purification, structural characterization of an arabinogalactan from green gram (Vigna radiata) and its role in macrophage activation. J Funct Foods. 2018; 50: 127-136.
- Hu K, Liu Q, Wang S, Ding K. New oligosaccharides prepared by acid hydrolysis of the polysaccharides from Nerium indicum Mill and their anti-angiogenesis activities. Carbohydr Res. 2009; 344: 198–203.
- Madhukumar MS, Muralikrishna G. Fermentation of xylo-oligosaccharides obtained from wheat bran and Bengal gram husk by lactic acid bacteria and Bifidobacteria. J Food Sci Technol. 2012; 49: 745–752.
- Beldman G, Osuga D, Whitaker JR. Some characteristics of β-D-xylopyranosidases, a-L-arabinofuranosidases and arabinoxylan_-a- L arabinofuranosidase from wheat bran and germinated wheat. J Cereal Sci. 1996; 23: 169–80.
- Karppinen S, Liukkonen K, Aura AM, Forssell P, Poutanen K. In vitro fermentation of polysaccharides of rye, wheat and oat brans and inulin by human faecal bacteria. J Sci Food Agric. 2000; 80: 1469–1476.
- Silvi S, Rumney CJ, Cresci A; Rowland IR. Resistant starch modifies gut micro flora and microbial metabolism in human flora associated rats inoculated with faeces from Italian and UK donors. J Appl Microbiol. 1999; 86: 521–530.
- Yasuda S, Yogosawa S, Izutani Y, Nakamura Y, Watanabe H, Sakai T. Cucurbitacin B induces G2 arrest and apoptosis via a reactive oxygen species dependent mechanism in human colon adenocarcinoma SW480 cells. Mol Nutr Food Res. 2010; 54: 559–565.
- Kouadio JH, Mobio TA, Baudrimont I, Moukha S, Dano SD, Creppy EE. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology. 2005; 213: 56-65.
- He NW, Zhao Y, Guo L, Shang J, Yang XB. Antioxidant, antiproliferative, and pro-apoptotic activities of a saponin extract derived from the roots of Panax notoginseng (Burk.) FH Chen. J Med Food. 2012; 15: 350-359.
- Losso JN, Bansode RR, Trappey A, Bawadi HA, Truax R. In vitro anti-proliferative activities of ellagic acid. J Nutr Biochem. 2004; 15: 672-678.
- Jaskari J, Kontula P, Siitone A, Jousimies-Somer H, Mattila-Sandholm T, Poutanen K. Oat b-glucan and xylan hydrolysates as selective substrates for Bifidobacterium and Lactobacillus strains. Appl. Microbiol. Biotechnol. 1998; 49: 175–181.
- Manisseri C, Gudipati M. Prebiotic activity of purified xylobiose obtained from Ragi (Eleusine coracana, Indaf-15) Bran. Indian J. Microbiol. 2012; 52: 251-257.
- Karppinen S, Liukkonen K, Aura AM, Forssell P, Poutanen K. In vitro fermentation of polysaccharides of rye, wheat and oat brans and inulin by human faecal bacteria. J Sci Food Agric. 2000; 80: 1469–1476.
- Morisse JP, Maurice R, Boilletot E, Cotte JP. Assessment of the activity of a fructooligosaccharide on different caecal parameters in rabbits experimentally infected with E. coli 0.103. Annal Zootechnol. 1993; 42: 1–87.
- Kang HJ, Jo C, Kwon JH, Son JH, An BJ, Byun MW. Antioxidant and cancer cell proliferation inhibition effect of citrus pectin-oligosaccharide prepared by irradiation. J Med Food. 2006; 9: 313-320.
- Augeron C, Laboisse CL. Emergence of permanently differentiated cell clones in a human colonic cancer cell line after treatment with sodium butyrate. Cancer Res. 1984; 44: 3961–3969.
- Whitehead RH, Young GP, Bhathal PS. Effects of short chain fatty acids on a new human colon carcinoma cell line (LIM 1215). Gut. 1986; 27: 1457–1463.
- Nzaramba MN, Reddivari L, Bamberg JB, Miller JC. Antiproliferative activity and cytotoxicity of Solanum jamesii tuber extracts on human colon and prostate cancer cells in vitro. J Agric Food Chem. 2009; 57: 8308–8315.
- Siavoshian S, Blottière HM, Le Foll E, Kaeffer B, Cherbut C, Galmiche JP. Comparison of the effect of different short chain fatty acids on the growth and differentiation of human colonic carcinoma cell lines in vitro. Cell Boil Int. 1997; 21: 281-287.
- Scheppach W, Bartram HP, Richter F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur J Cancer. 1995; 31: 1077-1080.
- Ogawa N, Satsu H, Watanabe H, Fukaya M, Tsukamoto Y, Miyamoto Y, et al. Acetic acid suppresses the increase in disaccharidase activity that occurs during culture of caco-2 cells. J Nutr. 2000; 130: 507-513.
- Ruemmele FM, Dionne S, Qureshi I, Sarma DS, Levy E, Seidman EG. Butyrate mediates Caco-2 cell apoptosis via up-regulation of pro-apoptotic BAK and inducing caspase-3 mediated cleavage of poly-(ADP-ribose) polymerase (PARP). Cell Death Differ. 1999; 6: 729.