Research Article
Austin J Allergy. 2014;1(2): 4.
Evaluation of Ige Sensitization Profiles in a Pediatric Population with Wheat Allergy
Calamelli E, Ricci G* and Pession A
Pediatric Unit, Department of Medical and Surgical Sciences, University of Bologna, Italy
*Corresponding author: Ricci G, Pediatric Unit, Department of Medical and Surgical Sciences - S. Orsola- Malpighi Hospital, University of Bologna, Via Massarenti 9, 40138 Bologna, Italy.
Received: July 10, 2014; Accepted: September 02, 2014; Published: September 04, 2014
Abstract
Introduction: IgE-mediated reactions to wheat can occur after ingestion, inhalation, contact or exercise. Among wheat allergens, Tri a14 and Tri a19 not only cause food allergy, but also baker’s asthma (Tri a4) and exercise-induced anaphylaxis (Tri a19). Despite the prevalence of adverse reactions, few studies have been conducted in children.
Aim: To evaluate the pattern of sensitization to wheat allergenic components in a group of pediatric patients with sIgE to wheat referring to the Pediatric Allergy Unit of University of Bologna.
Materials and Methods: Patients were assessed by skin prick-test and serum specific IgE against pollens, wheat, gluten and the molecular allergens rTri a19 and rTri a14. The diagnosis was confirmed with open food challenges.
Results: The diagnosis of wheat allergy was confirmed in 7 patients (64%), of whom 2 (29%) suffered also from grass pollen allergy. The levels of specific IgE (geometric mean) to wheat and gluten were 5 times higher in allergic patients compared to tolerant ones. The comparison between the patterns of sensitization showed a higher prevalence of sensitization against gluten (100% vs. 75% in tolerant patients) and the molecular components rTri a14 (71% vs. 25%) and rTri a19 (71% vs. 0%) in the wheat-allergic group. Positive predictive value for rTria a19 was higher than rTri a14 (100% vs. 83%).
Conclusion: Patients with wheat allergy have different profiles of sensitization than the tolerant ones; in particular rTri a19 showed a higher positive predictive value than rTri a14. These findings need to be confirmed in a larger population.
Keywords: Food allergy; Gliadin; Molecular allergens; Wheat
Abbreviations
A/G: Albumins and Globulins; I-PAN: Italian Pediatric Allergy Network; nsLTP: Non-specific Lipid Transfer Protein; NPV: Negative Predictive Value; p values: Probability Values; PPV: Positive Predictive Value; sIgE: Specific IgE; SPTs: Skin Prick-tests; WDEIA: Wheat-dependent Exercise-induced Anaphylaxis.
Introduction
Wheat grain (Triticum aestivum) is a major staple of our diet and wheat-based foods are widespread consumed especially in western countries. Therefore, gluten-related disorders are largely diffused health diseases and have been classified in three clinical entities, named autoimmune (celiac disease, gluten ataxia and dermatitis herpetiformis), allergic (wheat allergy) and not-autoimmune/not allergic (gluten sensitivity) [1].
IgE-mediated reactions to wheat (wheat allergy) affect about 0.5% of the worldwide population and can occur after ingestion (food allergy), inhalation (occupational asthma/rhinitis; e.g. baker’s asthma), contact (contact urticaria) or physical exercise after eating wheat-based foods [wheat-dependent exercise-induced anaphylaxis (WDEIA)] [1,2]. Meanwhile, the prevalence of wheat sensitization and of the clinical manifestations related to wheat changes according to age and personal habits. Food-induced wheat allergy typically arises early in life and in most cases resolves by 3 to 5 years of age [3]. In contrast, as shown by birth cohort longitudinal studies, the prevalence of wheat sensitization is about 4% in pre-school children and progressively increases with age from 2 to 9 % from 2 to 10 years old, mainly due to the secondary sensitization in subjects with grass pollen allergy [4,5]. The cross-reactivity between wheat flour and grass pollen has been proved and is due to the presence of common pan-allergens both in pollens and wheat: indeed almost 65% of patients with grass pollen allergy show specific IgE (sIgE) against wheat and up to 40% of wheat allergic patients have sIgE against grass pollen [6,7]. Moreover, the nationwide survey promoted by the Italian Pediatric Allergy Network (I-PAN) has recently confirmed a relevant prevalence of wheat sensitization (18.8%) among Italian children and adolescents with pollen-induced allergic rhinitis [8].
Up to now, twenty-one allergenic components have been identified in wheat grain and classified into two groups, according to their solubility in different solvents: the water/dilute salt-soluble proteins [albumins and globulins (A/G)], and the gluten fraction composed by gliadins (soluble in acqueous alcohol) and glutenins (soluble in dilute alkali or acid) [9- 11]. Among these, wheat gliadins are considered as markers of genuine wheat sensitization; in particular the ω-5 gliadin Tri a 19 is the major trigger in WDEIA and a significant allergen in young children with immediate allergic reactions to ingested wheat [12]. Meanwhile, the non-specific lipid transfer protein (nsLTP) Tri a 14, which belongs to the A/G fraction, is a relevant food allergen in wheat allergic patients from the Mediterranean area and is also associated with baker’s asthma [13-15]. Despite this, the specific role of the different allergenic components of wheat as elicitors of different clinical reactions and the cross-reactivity with pollens still need to be clarified.
The aim of this study is to investigate the different patterns of wheat sensitization in patients with sIgE to wheat and to assess the prevalence of sensitization to grass pollens (Phleum pratense) among subjects with confirmed wheat allergy.
Materials and Methods
Study population and design
For this cross-sectional study, we consecutively enrolled 11 pediatric patients (8 males mean age 8 years’ olds) with suspected allergy to wheat referring to the Pediatric Allergy Outpatient Unit of S. Orsola- Malpighi Hospital - University of Bologna (Italy) from October 2013 to May 2014. Inclusion criteria were: 1) age from 3 months to 18 years old and 2) serum sIgE to wheat >0.35 kU/L. A clinical history of suspected immediate (within 1 hour) adverse reaction after wheat ingestion was investigated in all patients. Symptoms suggestive of atopic dermatitis, food and respiratory allergy (asthma and/or rhino-conjunctivitis), data on parental history of atopy and the age of onset of the above mentioned allergic diseases were collected.
Allergometric evaluation
Skin prick-tests (SPTs) were performed in all patients using commercial extracts (Lofarma, Milan; Italy) for a standardized panel of inhalant and food allergens: grass pollen, birch, hazel-tree, olive-tree and wheat. Histamine 0.1 mg/ml and saline solution were used as a positive and negative control respectively. Skin reaction was assessed 15 minutes after the prick-test; the average diameter of each wheal was established by measuring the longest diameter and the diameter perpendicular to it. A SPT result was considered positive if wheal diameter was ≥ 3 mm compared with the negative control.
In addition, total and sIgE against a panel of inhalant and food allergens (Phleum p., Betula v., Corylus a., Olea e., wheat, gluten) and molecular components (rPhl p 1, rPhl p 5, rPhl p 12, rTri a 14 and rTri a 19) were determined in all patients’ sera by ImmunoCAP 1000 (Thermo Fisher Scientific, Uppsala, Sweden). SIgE levels greater than 0.35 kU/L (>0.10 kU/L for rTri a 14 and rTri a 19) were considered positive.
Oral provocation test
The diagnosis of wheat allergy was confirmed with an open oral food challenge.
Statistical methods
Data were stored by means of customized databases. The Chi-square test and non-parametrical tests were applied when appropriate. In particular, proportions were compared by Chi-square test; geometric mean levels of total and sIgE were compared by Student’s t-test. Probability (p) values of less than 0.05 were considered significant. Sensitivity, specificity, positive and negative predictive values (PPV and NPV) were calculated for sIgE to rTri a 14 and rTri a 19 with the cutoff values of 0.10 kU/l. Statistical analyses were carried out by means of MedCalc statistical software (Version 12.5.0, MedCalc Software, Ostend, Belgium).
Ethical approval
The research was conducted according to the principles expressed in the Declaration of Helsinki, and approved by the Ethic Committee of S. Orsola-Malpighi Hospital - University of Bologna (protocol name: Wheat Allergy; protocol number 194/2013/O/OssN). Written informed consent was obtained from all the parents or caregivers of the minors involved in the study.
Results and Discussion
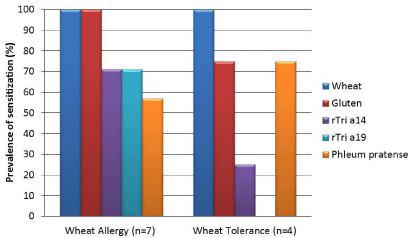
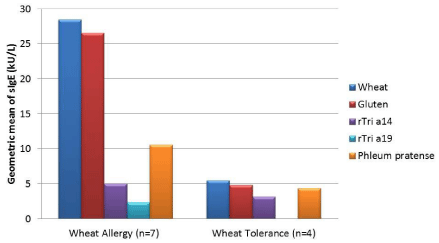
The demographical and clinical characteristics of the study population with suspected wheat allergy are resumed in Table 1. The diagnosis of wheat allergy was confirmed with oral food challenge in 7 patients (64%) and two of them (29%) suffered also from grass pollen allergy (asthma and rhino-conjunctivitis). Patients were allocated into two groups named “Wheat allergy” and “Wheat tolerance” according to the outcome of the wheat challenge. Wheat allergic children were younger (mean age 6 years vs. 12 years old, not significant) and had a higher prevalence of atopic dermatitis (6/7, 86% vs. 1/4, 25%, not significant) than the tolerant ones. The mean wheal diameter of SPTs with wheat was greater in wheat allergic patients, while no differences were found in SPTs with grass pollen extracts. All subjects with wheat allergy had symptoms after wheat ingestion, while one patient showed symptoms also after contact and inhalation of wheat flour (Table 2). This patient, a girl aged 14 years old with a history of multiple food and respiratory allergies, had a peculiar patter of wheat allergen sensitization with sIgE to wheat and gluten > 100 kU/L, sIgE to the nsLTP rTri a 14 of 70 kU/L and low levels of sIgE against rTri a 19 (1.13 kU/L). The main clinical manifestations of the adverse reactions to wheat involved the respiratory tract (rhinitis and/or bronchospasm, 3/7, 43% of patients) followed by skin and gastrointestinal symptoms. The three subjects (43%) who experienced anaphylaxis was sensitized to both rTria a 14 and rTri a 19. The patterns of sIgE sensitization against inhalant and food allergens (prevalence and geometric mean) are shown in Figures 1 and 2. Subjects with wheat allergy showed a higher prevalence of sensitization against gluten (7/7, 100% vs. 3/4, 75%) and against the two wheat molecular components rTri a 14 (5/7, 71% vs. 1/4, 25%) and rTri a 19 (5/7, 71% vs. 0/4, 0%), while the prevalence of sensitization to Phleum p. was higher among the tolerant children (3/4, 75% vs. 4/7, 57% respectively). The levels of serum sIgE (geometric mean) against both wheat and gluten were 5 folds higher in allergic patients. The frequency of sensitization against Phleum p. in wheat allergic patients was 57% (4/7).
Figure 1: Prevalence of specific IgE reactivity against wheat and grass pollen (Phleum pratense) allergens in a population of children with wheat sensitization (n=11). Patients were allocated into two groups named “Wheat allergy” and “Wheat tolerance” according to the outcome of the oral food challenge.
Figure 2: Geometric means of specific IgE levels against wheat and grass pollen (Phleum pratense) allergens in a population of children with wheat sensitization (n=11). Patients were allocated into two groups named “Wheat allergy” and “Wheat tolerance” according to the outcome of the oral food challenge.
Characteristics
Wheat Allergy
N=7
Wheat Tolerance
N=4
Sex, M (%)
5 (71%)
3 (75%)
Age, years (mean)
6
12
Parental atopy, n (%)
2 (29%)
0 (0%)
Atopic dermatitis, n (%)
6 (86%)
1 (25%)
Allergy to other foods, n (%)
5 (71%)
4 (100%)
Pollinosis (asthma/ rhinoconjunctivitis), n (%)
2 (29%)
2 (50%)
Total IgE. kU/L (geometric mean)
552
228
Skin Prick Test to Wheat
- wheal diameter (mm)
- n (%)
4.5
7 (100%)
2
2 (50%)
Skin Prick Test to Grass Pollen
- wheal diameter (mm)
- n (%)
4.5
4 (57%)
4.5
2 (50%)
Table 1: Demographical and clinical data of the study population with wheat sensitization.
Clinical manifestations
Number of Patients
(total N=7)
Symptoms, n(%)
Urticaria/Angioedema
2 (29%)
Gastrointestinal symptoms
2 (29%)
Respiratory symptoms
3 (43%)
Anaphylaxis
3 (43%)
Route of exposure, n (%)
Ingestion
7 (100%)
Inhalation
1 (14%)
Contact
1 (14%)
Table 2: Clinical manifestations occurred in patients with confirmed wheat allergy.
Furthermore, we calculated the diagnostic accuracy (sensitivity, specificity, PPV and NPV) of the two wheat molecules rTri a 14 and rTri a 19 (cutoff value of 0.10 kU/L) in predicting the outcome of oral food challenge. As shown in Table 3, the two components showed similar sensitivity (71%), while rTri a 19 showed a higher specificity (100% vs. 75%). A 100% PPV was obtained only for rTri a 19, but low NPV were calculated for both rTri a 14 and rTri a 19. Taking into account the presence of IgE against at least one of the two molecules we obtained a higher sensitivity (86%) and a better NPV (75%).
Sensitivity
Specificity
PPV
NPV
rTri a 14
71%
75%
83%
60%
rTri a 19
71%
100%
100%
67%
rTri a 14 ± rTria a19
86%
75%
86%
75%
Table 3: Sensitivity, specificity, PPV and NPV of specific IgE to rTri a 14 and rTria 19 in a population of children with wheat sensitization (n=11). A cutoff value of 0.10 kU/L was considered.
Our data revealed a relevant prevalence of sIgE reactivity both to the wheat LTP and the ω-5 gliadin (71%) in a group of Italian children with food allergy to wheat. Previous studies aimed at identifying the molecular patterns of sIgE reactivity in wheat allergic patients and to evaluate the role of wheat allergenic components as diagnostic tools in both children and adults. In contrast with our findings, Battais et al. [16] found an overall lower IgE reactivity to both the purified wheat LTP and the ω-5 gliadin (respectively of 28% and 37%) in a population of French children and adults with food allergy to wheat. In addition, also a more recent study by Nam et al. described a very low rate of sensitization against the wheat LTP (4.8%) among adults with wheat allergy from Korea, while the prevalence of sensitization against the wheat gliadin (70%) was comparable to our population [17]. The discrepancies with our findings concerning the rates of sensitization against the wheat LTP find an explanation in the different geographical areas of the study populations [18]. Indeed the so-called “LTP syndrome” is strongly influenced by geographic aspects and largely depends on differences in the patterns of pollen exposure and foods habits and LTPs are relevant allergens above all in patients from the Mediterranean area [13,15].
Our data on the diagnostic accuracy and of the ω-5 gliadin rTri a 19 reflect those previously reported by Palosuo et al. on a population of children from Finland [19]. In contrast with our findings, no correlation with the outcome of food challenges was found in two other patient populations with suspected wheat allergy (one from the United States and one from Germany) [20].
Conclusion
Children with food challenge-confirmed wheat allergy show different profiles of sensitization than those who tolerate wheat grain and both the ω-5 gliadin rTri a 19 and the nsLTP Tri a 14 behave as major wheat allergens in our population. In particular the ω-5 gliadin rTri a 19 showed a higher specificity and PPV than rTri a14 and could be useful as marker in the follow-up of wheat allergic children. However, a larger-sized patient population is needed to confirm these findings.
References
- Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012; 10: 13.
- Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, et al. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008; 121: 1210-1218.
- Inomata N. Wheat allergy. Curr Opin Allergy Clin Immunol. 2009; 9: 238-243.
- Ostblom E, Lilja G, Ahlstedt S, van Hage M, Wickman M. Patterns of quantitative food-specific IgE-antibodies and reported food hypersensitivity in 4-year-old children. Allergy. 2008; 63: 418-424.
- Matricardi PM, Bockelbrink A, Beyer K, Keil T, Niggemann B, et al. Primary versus secondary immunoglobulin E sensitization to soy and wheat in the Multi-Centre Allergy Study cohort. Clin Exp Allergy. 2008; 38: 493-500.
- Constantin C, Quirce S, Poorafshar M, Touraev A, Niggemann B, et al. Micro-arrayed wheat seed and grass pollen allergens for component-resolved diagnosis. Allergy. 2009; 64: 1030-1037.
- Freidhoff LR, Ehrlich-Kautzky E, Grant JH, Meyers DA, et al. A study of the human immune response to Lolium perenne (rye) pollen and its components, Lol p I and Lol p II (rye I and rye II). I. Prevalence of reactivity to the allergens and correlations among skin test, IgE antibody, and IgG antibody data. J Allergy Clin Immunol. 1986; 78: 1190-1201.
- Dondi A, Tripodi S, Panetta V, Asero R, Businco AD, et al. Pollen-induced allergic rhinitis in 1360 Italian children: comorbidities and determinants of severity. Pediatr Allergy Immunol. 2013; 24: 742-751.
- Allergen nomenclature.
- Battais F, Richard C, Jacquenet S, Denery-Papini S, Moneret-Vautrin DA. Wheat grain allergies: an update on wheat allergens. Eur Ann Allergy Clin Immunol. 2008; 40: 67-76.
- Baar A, Pahr S, Constantin C, Scheiblhofer S, Thalhamer J, et al. Molecular and immunological characterization of Tri a 36, a low molecular weight glutenin, as a novel major wheat food allergen. J Immunol. 2012; 189: 3018-3025.
- Palosuo K, Varjonen E, Kekki OM, Klemola T, Kalkkinen N, et al. Wheat omega-5 gliadin is a major allergen in children with immediate allergy to ingested wheat. J Allergy Clin Immunol. 2001; 108: 634-638.
- Egger M, Hauser M, Mari A, Ferreira F, Gadermaier G. The role of lipid transfer proteins in allergic diseases. Curr Allergy Asthma Rep. 2010; 10: 326-335.
- Malo JL, Chan-Yeung M. Agents causing occupational asthma. J Allergy Clin Immunol. 2009; 123: 545-550.
- Palacin A, Bartra J, Mu&nTilde;oz R, Diaz-Perales A, Valero A, et al. Anaphylaxis to wheat flour-derived foodstuffs and the lipid transfer protein syndrome: a potential role of wheat lipid transfer protein Tri a 14. Int Arch Allergy Immunol. 2010; 152: 178-183.
- Battais F, Courcoux P, Popineau Y, Kanny G, Moneret-Vautrin DA, et al. Food allergy to wheat: differences in immunoglobulin E-binding proteins as function of age or symptoms. J Cereal Sci. 2005; 42: 109-117.
- Nam YH, Hwang EK, Jin HJ, Lee JM, Shin YS, et al. Comparison of specific IgE antibodies types of wheat component allergens in two phenotypes of wheat allergy. J Korean Med Sci. 2013; 28: 1697-1699.
- Pastorello EA, Farioli L, Conti A, Pravettoni V, Bonomi S, et al. Wheat IgE-Mediated Food Allergy in European Patients: a-Amylase Inhibitors, Lipid Transfer Proteins and Low-Molecular-Weight Glutenins. Int Arch Allergy Immunol. 2007; 144: 10-22.
- Palosuo K, Varjonen E, Kekki OM, Klemola T, Kalkkinen N, et al. Wheat omega-5 gliadin is a major allergen in children with immediate allergy to ingested wheat. J Allergy Clin Immunol. 2001; 108: 634-638.
- Beyer K, Chung D, Schulz G, Mishoe M, Niggemann B, et al. The role of wheat omega-5 gliadin IgE antibodies as a diagnostic tool for wheat allergy in childhood. J Allergy Clin Immunol. 2008; 122: 419-421.