Case Report
Austin J Allergy. 2014;1(3): 3.
Gelatin Allergy as Cause for Repeated Severe Anaphylaxis after Administration of a Rabies Vaccine
Mylène Niclou, Cornelia S L Müller, Hedwig Stanisz, Thomas Vogt and Claudia Pföhler*
Department of Dermatology, Saarland University Medical School, Homburg/Saar, Germany
*Corresponding author: Prof. Dr. Claudia Pföhler, Saarland University Medical School, Department of Dermatology, Kirrbergerstrasse, 66421 Homburg /Saar, Germany.
Received: August 22, 2014; Accepted: September 20, 2014; Published: September 22, 2014
Abstract
Since the pioneering work of Jenner, Koch and Pasteur, vaccines have represented the most effective method of preventing the spread of many infectious diseases. Unfortunately, vaccines can cause side effects or adverse events. In the majority of these cases the reactions are limited to the site of the injection; they usually result from a non-specific activation of the immune system and do not need further investigation. If however an allergy is suspected there is a need for further diagnostic exams. In theory every component of a vaccine can cause an adverse event or an allergic reaction; in general the reactions are due to the additional elements in the vaccine, such as egg protein, antibiotics, phenol red, polysorbate or polygelin. Reactions caused by the active or non-active viral or bacterial particles are rare. We are reporting the case of a 21-year old soldier, who suffered from repeated allergic reactions after the administration of a rabies vaccine that contained polygelin.
Keywords: Anaphylaxis; Gelatin; Rabies; Vaccination
Background
Periodically, there is general public concern about possible reactions to vaccines and from time to time worried parents do not want their children to be vaccinated. However the rate of reported vaccine-induced adverse events is low. It ranges from 4.8 to 83.0 events per 100 000 doses of the most frequently used vaccines. The number of true allergic reactions to routine vaccines is not yet known, but is estimated to range from 1 per 500 000 to 1 per million doses for most vaccines [1]. When the vaccine contains allergens such as gelatin or egg proteins the rate for true allergic reactions might be higher. But potentially life-threatening anaphylactic reactions to vaccines are a rare event (around 1 per 1.5 million doses) [1]. Gelatin is added to vaccines as a preservative and stabilizer that conserves the vaccine from adverse conditions such as freeze-dying or heat and maintains the stability and efficacy of the vaccine.
Case Presentation
We are reporting the case of a 21-year old German soldier, who presented himself in our allergy department in November 2013 for further investigation after repeatedly suffering allergic reactions following the administration of the rabies vaccine Rabipur® in October 2013. The indication for the application of the rabies vaccine was a pre-exposure prophylaxis before a military mission in Afghanistan.
10 minutes after the administration of the first dose of the three-part vaccine the patient suffered from dizziness, cold sweats and pruritus of the skin on the scalp and in the armpits, followed by nausea and vomiting, hypotension and tachycardia. After the administration of steroids (500 mg prednisolone i.v.), antihistamines (4mg dimetinden and 50mg ranitidine i.v.) and adrenalin (300μg epinephrine i.m.) the patient could be stabilised. However, this reaction was rated as a psychovegetative reaction and not as an allergic reaction, so a week later the same vaccine was administered again. 5 minutes after the administration of the second dose, the patient suffered from similar but less pronounced symptoms. In addition he suffered from an urticarial rash. Again, after the administration of steroids (500 mg prednisolone i.v.) and antihistamines (4mg dimetinden and 50mg ranitidine i.v.) the symptoms regressed. The use of adrenalin was not necessary.
Before these two events the patient had never suffered from any allergic reaction. All types of medication that had been administrated since early childhood and all kinds of food (especially fish, egg, peanut, treenuts, shellfish and even jelly teddies) had been tolerated without any problem. History for atopic or allergic disposition and family history for atopy were negative.
Vaccination documents from the past and the present were available and showed vaccinations against measles-mumps-rubella in 1998 (no name of the product, unclear if it contained gelatin), against hepatitis B in 1998 (no name of the product, unclear if it contained gelatin), against hepatits A and B (Twinrix® and Havrix®) in 05/2013 and 10/2013, against tetanus-pertussis-diphtheria-poliomyelitis (Repevax®) in 04/2014, and Japanese encephalitis in 01/2014 (Ixiaro®). None of the clearly named vaccines contained gelatin or polygelin.
For the third dose a polygelin-free vaccine (Tollwut-Impfstoff HDC® from sanofi pasteur MSD) was administered. This dose was given 109 days after the second dose. After this application the patient had no allergic reaction.
Rabipur® vaccine is produced using chicken fibroblast cells. In addition to the inactivated rabies virus, it contains the following additional components: trometamol, sodium chloride, disodium- EDTA, potassium glutamate, polygelin, sucrose and water.
To track down the possible allergen cause of the symptoms we used the following diagnostic methods: prick testing and blood analysis.
Blood analysis
Tryptase was normal, Total IgE was elevated with 244U/ml (normally <100U/ml).
Specific IgE antibodies for egg yolk, egg white and egg albumin and for the allergens nGal d2 and nGal d1 from chicken egg were negative.
Specific IgE antibodies for gelatin were positive (18 kU/l; CAP class 4).
Specific IgE antibodies for pollen and for dust and storage mites were positive for grass in both the early- and late-flowering categories (CAP class 4) and for dust and storage mites (CAP class 2).
Specific IgE antibodies for milk protein, fish, and galactose-alpha- 1,3-galactosidase were negative.
Prick testing
Prick testing for grass, tree and weed pollen, dust mites, ragweed pollen, cat, hazelnut, almond, chicken, beef, pork, different flours and fish (sardine and cod) showed a histamine equivalent reaction to grass pollen, tree pollen and dust mites and negative reactions for all the other allergens (all skin prick solutions from Bencard Allergie GmbG, Munich, Germany). The test was read after 20 minutes.
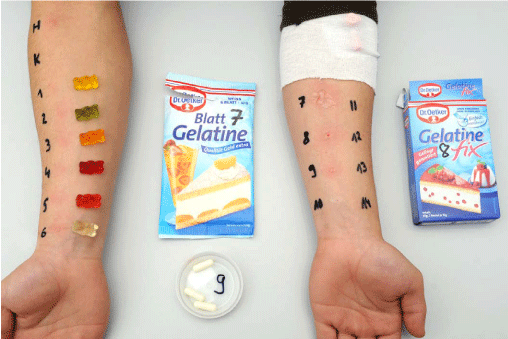
Prick-to-prick testing for all current colours of jelly teddies (Haribo®), gelatin sheets, gelatin capsules, and Gelatine Fix (Dr. Oetker®) showed a histamine equivalent reaction for all the jelly teddies and the gelatin sheets, gelatin capsules and Gelatine Fix (Figure 1). Prick-to-prick testing was performed with either one jelly teddy or one teaspoonful of the other substances diluted in 5ml 0.9% sodium solution. The test was read after 20 minutes.
Figure 1: Figure shows positive skin prick testing with different colours of jelly teddies (skin test numbers 1-6) and other gelatin-containing substances (skin test numbers 7-9); skin prick testing with beef, pork, chicken and fish (skin test numbers 10-14) was negative. H = histamine served as positive control; K = negative control performed with sodium chloride 0.9%.
We diagnosed a type-I sensitization against polygelin and clinically silent type-I sensitizations against grass pollen, tree pollen and dust mites.
As the reaction occurred immediately after the first administration of the polygelin-containing vaccine we assume a pre-sensitisation against gelatin/polygelin, possibly acquired via consumption or by the administration of gelatin/polygelin-containing medication. It is thinkable that the measles-mumps-rubella vaccination in 1998 contained gelatin and lead to the gelatin-sensitisation. We suspect that the second reaction was not as severe because of the former administration of steroids and antihistamines during the first allergic reaction.
Conclusion
As the patient did not suffer from any allergic reaction after eating food containing gelatin the following possibilities must be discussed:
On one hand the allergy could be a so-called “compartment allergy” with a different response to the allergen depending on the method of administration (as known for heparines, for example). On the other hand food containing gelatin may be tolerated because of the denaturing of the gelatin occurring in the gastrointestinal tract, resulting in loss of the potential allergenicity. In such a case in particular, it is imaginable that the consumption of food containing gelatin after the administration of medication to reduce the acidity in the stomach could cause an allergic reaction [2,3].
As the exact pathway of the allergic reaction is not yet known, we recommended to the patient to avoid any food or medication containing gelatin/polygelin.
Cases of allergic reactions to polygelin-containing vaccines have been reported in the past. In 1997, after the widespread use of the first paediatric tick-borne encephalitis (TBE) vaccination, there were reports of scattered cases of immediate allergic reactions in children [4,5]. The consequence was a voluntarily withdrawal of the drug by the manufacturer in 1998. Serological tests indicated that the gelatin-based vaccine stabiliser was the major cause of immediate allergic reaction after administration of this vaccine. A high percentage (>30%) reacted after the first administration, indicating a pre-sensitization against gelatin. In the following years a new vaccination formulation was developed, excluding any proteineous stabilizers such as gelatin-based compounds or human serum albumin commonly used in former or present TBE vaccines. Clinical studies demonstrated a good tolerability of the new paediatric TBE vaccine [5,6]. The situation with our patient was similar, as there was no adverse reaction to the administration of the third dose of a then polygelin-free rabies vaccine.
A similar vaccine reformulation took place in Japan when an increased number of children suffered allergic reactions to live measles vaccines in the nineties [7]. Laboratory tests for gelatin-specific antibodies led to the conclusion that the reactions were due to a sensitisation against gelatin [7,8]. The change in gelatin content of vaccines led to a reduced number of allergic reactions following vaccination [9,10].
Our case report and the past reports of allergic reactions against polygelin-containing vaccines show that after the occurrence of an allergic reaction following the administration of a polygelin-containing vaccine the allergen polygelin must be borne in mind in the subsequent diagnostic testing. Whenever possible, gelatin/ polygelin-free vaccines should be favoured. To our knowledge expect of Rabipur® there are no other licened polygelin-containing rabies vaccines worldwide. Modern, cell culture- derived rabies vaccines are usually well tolerated without allergic reactions as they do not contain ingredients with a high anaphylactic potential except of traces of antibiotics such as neomycine or traces of phenol red that is used as a pH-indicator.
Acknowledgements
We thank Eva Janssen for the photos of skin prick testing. There was no funding of this work.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Authorīs Contributions
MN, CSLM, HS and CP performed clinical investigation and allergy testing of the patient. MN, CSLM, HS and CP acquired, analyzed and interpreted laboratory results. All authors were involved in drafting of the manuscript and revising it critically for important intellectual content. All authors gave final approval of the version to be published.
References
- Fritsche J, Helbling A, Ballmer-Weber B. Vaccine hypersensitivity – update and overview. Swiss MED Wkly 2010; 140: 238-246.
- Pali-Schöll I, Herzog R, Szalai K, Brunner R, Lukschal A, et al. Antacids and dietary supplements with an influence on the gastric pH increase the risk for food sensitization. Clin Exp Allerg 2010; 40: 1091-1098.
- Pali-Schöll I, Jensen-Jarolim E. Anti-acid medication as a risk factor for food allergy. Allergy 2011; 66: 469-477.
- Zent O, Henning R. Post-marketing surveillance of immediate allergic reactions: polygeline-based versus polygeline-free pediatric TBE vaccine. Vaccine 2004; 23: 579-584.
- Zent O, Banzhoff A, Hilbert AK, Meriste S, Sluzewski W, et al. Safety, immunogenicity and tolerability of a new pediatric tick-borne encephalitis (TBE) vaccine, free of protein-derived stabilizer. Vaccine 2003; 21: 3584-3592.
- Zent O, Beran J, Jilg W, Mach T, Banzhoff A. Clinical evaluation of a polygeline-free TBE vaccine for adolescents and adults. Vaccine 2003; 21: 738-741.
- Sakaguchi M, Nakayama T, Fujita H, Toda M, Inouye S. Minimum estimated incidence in Japan of anaphylaxis to live virus vaccines including gelatin. Vaccine 2000; 19: 431-436.
- Sakaguchi M, Nakayama T, Inouye S. Food allergy to gelatin in children with systemic immediate-type reactions, including anaphylaxis, to vaccines. J Allergy Clin Immunol 1996; 98: 1058-1061.
- Nakayama T, Aizawa C. Change in gelatin content of vaccines associated with reduction in reports of allergic reactions. J Allergy Clin Immunol 2000; 196: 591-591.
- Kuno-Sakai H, Kimura M. Removal of gelatine from live vaccines and DtaP –an ultimate soliztion for vaccine related gelatine allergy. Biologicals 2003; 31: 245-249.