
Mini Review
Austin J Allergy. 2016; 3(1): 1022.
Peanut Allergy and Strategies for Allergenicity Reduction
Bavaro SL and Monaci L*
Institute of Sciences of Food Production, National Research Council of Italy (ISPA-CNR), Italy
*Corresponding author: Linda Monaci, Institute of Sciences of Food Production, National Research Council of Italy (ISPA-CNR), Italy
Received: May 07, 2016; Accepted: May 18, 2016; Published: May 20, 2016
Abstract
Peanut allergy is a typical IgE mediated immune disease and has become a major health concern worldwide. Even a low intake of peanuts or peanut containing foods can cause severe and sometimes fatal allergic reactions in sensitive individuals. Differences in the preparation of peanuts before consumption could contribute to whether an individual will eventually display an allergic reaction. Several thermal and non thermal treatments might account for this change in allergenicity due to an alteration of the allergenic proteins therein contained. Such paper will report and review the different strategies aimed to reduce peanuts allergenicity.
Peanut Allergy and Allergens Modification
Peanut is a seed crop legume widely employed for human consumption thanks to its high nutritional value and sensory qualities [1]. It is characterized by a high oil (44-56%) and protein content (22- 30%) and represents a good source of energy and proteins. On the other hand peanut is also very rich in allergenic proteins capable of triggering allergic reactions in sensitive individuals. It is well known that even few milligrams of peanuts ingested might provoke allergic reactions in predisposed individuals that can be sometimes lifethreatening. Evidence of peanuts specific IgE can be established by allergy skin-prick test (SPT) or by in vitro determinations although negative tests to IgE do not always exclude an allergic reaction. Symptoms of IgE mediated disorders are typically related to the skin, the gastrointestinal tract, and the respiratory tract. Anaphylaxis, a systemic allergic response to allergen ingestion, can include other additional cardiovascular symptoms such as hypotension and dysrhythmia. It has been estimated that about two thirds of all deaths are due to anaphylaxis caused by accidental exposure to peanuts [2]. About the prevalence of peanut allergy worldwide, recent data suggest that the incidence of allergic reactions has increased in the North America and in several European countries during the past decades. In a self-reported population survey it was calculated that the prevalence of peanut allergy in US children was around 1.4% in 2008 compared to 0.8% in 2002 [3], whereas in Europe accounts for 1.3% in 4–18-years-old youngsters [4]. It is in Figure 1 reported a graphical representation of the prevalence of clinical peanut allergy worldwide according to the studies carried out in different countries. Data reported a prevalence ranging between 1.2 and 3% of the adult population calculated in the different geographical areas.
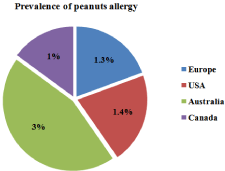
Figure 1: Prevalence of peanut allergy in the different geographical areas.
Data are referred to self reported and prevalence of “clinical peanut allergy”,
according to sIgE-based criteria reported in literature [3,4,49].
According to the Allergen Nomenclature Sub-Committee of the International Union of Immunological Societies, 17 Ara h proteins were identified as capable of inducing allergic reactions and that are recognized by IgE allergic patients upon peanut ingestion [5]. Despite the identification and characterization of various peanut allergens, to date no cure exist and a diet based on the strict avoidance of peanut containing foods is still the safest choice to protect allergic consumers from displaying undesired reactions. Given the constant increase of peanuts allergies mainly diagnosed in industrialized countries and given the focus placed in the recent years on consumers’ health, new challenges have been recently posed to the food industry and regulatory agencies in terms of food safety. An alternative strategy to the avoidance of the allergen from the diet relies on the availability of processed peanuts endowed with a reduced allergenic potential that could significantly lower consumer risk and industry liability factors [6,7]. The development of processing technologies aimed to inhibit allergen activity or to remove allergens, represents a common goal for obtaining low allergenic foods [8]. However, little is known about how food processing may affect allergic sensitization and subsequent elicitation of adverse reactions to peanut proteins. Depending on the conditions and the type of processing applied, an alteration of immunodominant epitopes can take place thus affecting the final protein allergenicity [9]. Processing may alter existing epitopes spread along a protein or may generate a change in protein conformation. In addition, an alteration of the food might also induce a masking or unmasking of the epitopes. As a result, allergenicity of the offending food can change, reduced or enhanced [9] and then increasing or decreasing IgE reactivity [10,11]. Considering that, the IgE binding capacity of proteins or foods is a prerequisite for observing a clinical reactivity, i.e. ability to elicit an allergenic reaction in sensitized populations, as well as the properties conferring to proteins the ability to induce sensitization [12]. Many types of processing reported in the literature are known to influencing peanut allergenic potential such as heating (thermal processing), fermentation including endogenous enzymatic hydrolysis, enzymatic and acid hydrolysis, physical treatments (such as high pressure processing or extrusion), the use of preservatives, changes in pH, or combinations of any, two or more of these [13-15]. In this paper the different types of processing, herein categorized in thermal and non-thermal treatments, and the impact on antigenic and allergenic integrity of peanuts proteins will be reviewed and discussed.
Strategies to Reduce Peanut Allergenicity
Peanuts can undergo different types of processing in order to decrease their allergenic potential. These can be divided into two categories: thermal and non thermal treatments here after described and resumed in Table 1.
Thermal treatments
Effects
References
Roasting
Denaturation; protein conformational change; Maillard reaction.
[6,17-30]
Boiling
Frying
Autoclaving
Non thermal treatments
High pressure
Denaturation; proteinconformationalchange.
[31-33]
Pulsedultravioletlight
Photothermal, photophysical, and photochemical.
[34-37]
Gamma Irradiation
Proteinfragmentation and aggregation.
[38,39]
Chemical and enzymatic
Protein crosslinking; protein modification; protein fragmentation.
[40-47]
Table 1: Summary of thermal and non thermal treatments on peanuts proteins.
Thermal Processing
Significant alterations in protein structure may occur during heat treatment, with the nature and the extent depending on the specific thermal treatment and the binary duration/temperature applied. In general, heat-treatments were found to significantly reduce the IgE reactivity of well known allergens, most likely as the result of unfolding mechanisms [16]. Several studies already demonstrated that heat treatments alter the conformation of the heat-labile proteins, with a loss of conformational epitope(s), and the reduction or abolition of allergenic potential for some of them [14,17]. Denaturation like boiling can cause a reduction of the IgE-binding capacity of peanut proteins in vitro. Beyer et al. showed that boiling peanuts for 20 minutes in water (100°C) caused a decrease of the IgEbinding capacity of all tested allergens (Ara h 1, 2 and 3) compared to roasted peanuts [17]. Another study concluded that IgE-binding capacity of proteins extracted from boiled peanuts (100°C, 30min) was significantly lower than that observed for raw peanuts [18]. In the same paper it was also reported that Low-Molecular-Weight (LMW) proteins were transferred into the cooking water during boiling. A similar decrease in allergenicity of peanuts allergens after boiling was documented by Visser et al. [19]. In this case, native forms of Ara h 2/6 purified from raw and heated peanuts and placed in aqueous solution at 100°C for 15min induced a significant loss in IgE-binding capacity and ability of the protein to elicit histamine release with a significant decrease of the mediator release. Turner et al., found that boiling peanuts for 6 hours resulted in a loss of Ara h 2, Ara h 6 and Ara h 7 proteins. Moreover, it was also demonstrated that oral food challenge with boiled peanuts induced desensitization in four peanuts allergic patients [20]. However, boiling treatment did not always produce a decrease in allergenicity as reported by Cabanillas et al. showing that one out of seven peanuts allergic patients elicited a positive reaction towards boiled peanut extracts in a SPT study [21].
Beyond boiling also other thermal processes can alter conformational epitopes, with a consequent production of linear epitopes available for the antibody binding [22]. In particular, roasting has been reported to induce important modifications consequent to the Maillard reactions, leading to the formation of stable Advanced Glycation End-Products (AGE) through the reaction of reducing sugars with the free amino groups on proteins [23,24]. It was documented that these Advanced Glycation End (AGE) adducts formed upon the roasting of purified Ara h 1 and Ara h 2 enhanced the IgE-binding capacity up to 90-fold, concluding that the high level of AGE adducts can be correlated with the level of IgE binding [25]. On the other hands, Maillard reaction does not always affect the allergenicity of peanut proteins, and a lot depends on the type of protein or the conditions under which the Maillard reactions take place. On this regards, Maleki et al. compared the proliferative ability and IgE production of both purified Ara h 1 and Ara h 2 extracted from raw and roasted peanuts, towards T-cells demonstrating the higher IgE reactivity recorded for roasted Arah1 compared to the raw protein; however a general decrease in T-cell proliferation was observed for roasted peanuts on five patients sera analysed [6]. Other studies accomplished on different types of roasting, in the presence or absence of glucose, resulted in an increased degranulation capacity in cellular Mediator Release Assays (MRAs), upon incubation with Ara h1. Conversely, a decrease was recorded when cells were incubated with Ara h 2/6 [26].
Few studies have been reported about sensitization of thermally treated peanuts (boiled in aqueous buffer) on animal models. Moghddam et al. demonstrated that extracts from roasted peanuts increased the elicitation capacity of peanut allergens compared to those obtained from blanched peanuts, and were also capable of inducing sensitization across mucosal and cutaneous routes in mice [27]. Similar results were obtained by Kroghsbo et al. although the roasted peanuts did not show to impact sensitization in rats [28].
Other authors investigated the effect of heat treatment on Ara h 2 immunogenicity using an oral food allergy mouse model reporting an increase of IgE levels when mice where immunized with heated Ara h 2 [29]. Data on the effects of frying are instead quite limited and controversial. It has been demonstrated that frying reduced the IgETable binding capacity of Ara h 1, Ara h 2 and Ara h 3 but did not reduce mediator release by SPT [17,21].
In the last years new strategies have been investigated and adopted to reduce peanuts allergenicity; in particular, the combination of heating and high pressure appeared to be a promising approach. In a recent study, Cabanillas et al. reported that intensive autoclaving on different forms of peanuts (raw, roasted and fried) showed a decreasing relative levels of the allergens Ara h 2, Ara h 6 and Ara h 1 in P three treated peanuts [30]. Moreover, the level of reduction of the major allergens was directly correlated with a decreased IgE-binding capacity, diminished basophiles activation and a reduced mediator release by skin prick test in allergic patients.
It has been hypothesized that the effects of autoclaving were related to an unfolding of some basic protein structures (α-helix and β-strand) and an increased formation of random coils that consequently produced and increased digestibility of different peanut proteins. However it was also reported that allergenic proteins such as Ara h 2 and Ara h 6, thanks to the highly stable protein structure, were extremely resistant to proteolytic digestion though the heat treatment applied [21].
Non Thermal Processing
Most processing technologies are still based on heat physical principles to alter the structure of food allergens. Although this technology is frequently applied in traditional food processing, the sensory quality and nutritional value of the processed food may be impaired. Therefore, the development of non thermal processing technologies has emerged in the last years.
Among the non thermal processing technologies many studies reported the importance of High Processing Pressure (HPP) capable of causing a reversible or irreversible structural modification of proteins, determining changes of non covalent bonds such as electrostatic and hydrophobic interactions, and leading to protein denaturation and/or aggregation. All these modifications may finally alter the allergenic potential of a certain food. Huang et al. reported that of HHP treatment reduced Ara h 2 antigenicity, with 73.3% of immunoreactivity reduction recorded in peanuts extracts treated with high pressure at 800 MPa for 10 min [31]. Similar results were obtained in other studies, where high-pressure micro-fluidization treatment demonstrated to change the secondary structure of Ara h 2 decreasing its antigenicity by nearly 50% [32]. By contrast, Johnson et al. showed that no changes in the secondary structure were observed following high-pressure treatment at 20°C as well as at 80°C, demonstrating a slight effect on the structure of the purified allergen [33]. The HPP treatment can be associated or not, to other physical treatments such as Pulsed Ultraviolet (PUV), or gamma irradiation. PUV light system, consists in a high-voltage electrical energy stored in a capacitor and released in a single burst, which passes through a lamp filled with inert gas, such as xenon [34]. It is speculated that PUV light has photothermal, photophysical, and photochemical effects on food systems, which could alter allergen conformation [35] or cause protein aggregation [36], resulting in the loss of conformational epitopes.
Chung et al. submitted peanuts and liquid peanut butter extracts to PUV treatment finding a decrease in IgE binding from six- to seven-fold compared to the control. PUV light treatment is likely to cause protein aggregation of the major peanut allergen Ara h 1, thus altering protein conformation and IgE binding epitopes [36]. The efficacy of Pulsed Ultraviolet Light (PUV) was evaluated in another study, where protein extracts from raw and roasted peanuts showed a reduction in the intensity of Ara h 1, Ara h 2, and Ara h 3 bands by using energy levels from 111.6 to 223.2 J/cm². A reduction in IgE binding up to 12.9- and 6.7-folds, was also observed in roasted peanuts [37].
Another non thermal processing method that can structurally alter the IgE binding epitopes creating free radicals and inducing protein fragmentation and aggregation is based on Gamma irradiation. The use of gamma irradiation on Ara h 6, caused alterations in its conformational and antigenic properties with an important impact on its secondary and tertiary structure [38]. In addition, Oh et al. evaluated the changes of allergenicity and cytokine production profiles after exposure of irradiated peanut extract to a peanut-allergy mouse model [39]. Results showed a general increase of Th1/Th2 ratio whereas a down-regulation of Th2 lymphocyte activity was recorded in peanut-sensitized mice.
In other works, the use of non heat processing was explored with the addition of chemical or enzymatical compounds, chemical or enzymatic, with the aim to produce hypoallergenic foods with a reduced sensitizing capacity. Molecules such as phenolic compounds or phytic acid can lead to the formation of soluble and insoluble complexes with modifications in the structure of the proteins and/ or polymerization of allergens into larger compounds, thus escaping IgE recognition [40,41]. In this regard, it has been shown that copper ions, peroxidase and hydrogen peroxide have the ability to crosslink peanuts proteins via oxidation of tyrosine residues, showing a reduction in the IgE binding [42]. On the other hand, the IgE binding capacity can be also reduced upon production of protein hydrolysates, that contain di- and tri-peptides which are absorbed more rapidly than free amino acids along the intestinal tract [43]. In this regards, the combination of enzymatic treatment coupled with post-hydrolysis food processing, such as heat treatment and ultrafiltration, are considered to be effective in obtaining protein products with a reduced allergenicity risk [44]. In particular, two studies demonstrated that peroxidase or digestive enzymes, such as a-chymotrypsin and trypsin, could hydrolyse and reduce the IgE response for Ara h 1 and Ara h 2 in roasted peanuts, but not in raw peanuts [24,45]. It has also been reported that boiling before hydrolysis enhanced the effectiveness of enzyme treatment in thermally treated peanuts, but not in raw products [45]. However, a combined approach based on the pre-treatment before hydrolysis and the correct choice of a proper enzyme(s), represents a fundamental prerequisite in order to highly influence the IgE-binding capacity. Interestingly, Shi et al. reported that although enzymatic hydrolysis could significantly reduce IgE-binding capacity in ELISA, IgE crosslinking capacity was still retained in the basophil activation tests, indicating that a reduction in IgE binding capacity by hydrolysis does not give a clear prediction of allergenicity reduction [46]. More recent studies carried out by Mihajlovic et al. in in vivo mouse models, showed that treating peanuts proteins with laccase did not produce an increase of allergenicity in in vivo systems, instead resulting in a modulated immune response in the animals, recorded through an increase of IgG2a [47].
Conclusion
Strategic approaches devoted to reducing peanut allergen reactivity represents the main goal of the scientific community due to the growing prevalence of peanut allergy worldwide. For this reason, this report focuses on the impact of processing on antigenic and allergenic integrity of proteins of the major recognized allergens. According to what already known, every processing may influence, but not completely abolish the allergenic potential of the major peanut allergens. Many approaches herein described, have been shown to modify peanuts reactivity by changes in protein structures and alteration of their IgE binding sites. However, the use of one treatment does not guarantee the total abolishment of allergenicity and sometimes the combination of different treatments might be the successful approach. The understanding of how the processing can influence peanut allergenicity could help mitigating the risks for allergic consumers reducing the likelihood to trigger allergic reactions.
References
- Oerise NL, Lau HA, Ritchey SJ, Murphy EW. Yield, proximate composition and mineral element content of three cultivars of raw and roasted peanuts. Food Sci. 1974; 36: 264-266.
- Cianferoni A, Muraro A. Food-induced anaphylaxis. Immunol Allergy Clin North Am. 2012; 32: 165-195.
- Sicherer SH, Muoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010; 125: 1322-1326.
- Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A. EAACI Food Allergy and Anaphylaxis Guidelines Group. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014; 69: 992-1007.
- Monaci L, De Angelis E, Bavaro SL, Pilolli R. High-resolution Orbitrap-based mass spectrometry for rapid detection of peanuts in nuts. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015; 32: 1607-1616.
- Maleki SJ, Schmitt DA, Galeano M, Hurlburt BK. Comparison of the digestibility of the major peanut allergens in thermally processed peanuts and in pure form. Foods. 2014; 3: 290-303.
- Allen KJ, Turner PJ, Pawankar R, Taylor S, Sicherer S, Lack G, et al. Precautionary labelling of foods for allergen content: are we ready for a global framework?. World Allergy Organ J. 2014; 7: 10.
- Huang HW, Hsu CP, Yang BB, Wang CY. Potential Utility of High-Pressure Processing to Address the Risk of Food Allergen Concerns. Compr Rev Food Sci Food Saf. 2014; 13: 78-90.
- Sathe SK, Teuber SS, Roux KH. Effects of food processing on the stability of food allergens. Biotechnol Adv. 2005; 23: 423-429.
- Cabanillas MB, Crespo JF, Burbano C, Rodríguez J. Systemic IgE-mediated reaction to a dietary slimming bar. Ann Allergy Asthma Immunol. 2010; 104: 450.
- Cuadrado C, Cabanillas B, Pedrosa MM, Varela A, Guillamón E, Muzquiz M, et al. Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Mol Nutr Food Res. 2009; 53: 1462-1468.
- Verhoeckx KC, Vissers YM, Baumert JL, Faludi R, Feys M, Flanagan S, et al. Food processing and allergenicity. Food Chem Toxicol. 2015; 80: 223-240.
- Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA J. 2014; 12: 3894.
- Mills EN, Mackie AR. The impact of processing on allergenicity of food. Curr Opin Allergy Clin Immunol. 2008; 8: 249-253.
- Thomas K, Herouet-Guicheney C, Ladics G, Bannon G, Cockburn A, Crevel R, et al. Evaluating the effect of food processing on the potential human allergenicity of novel proteins: international workshop report. Food Chem Toxicol. 2007; 45: 1116-1122.
- Besler BA, Grichar WJ, Smith OD, Jaks AJ. Response of Peanut Cultivars to Full and Reduced Spray Programs of Tebuconazole for Control of Southern Stem Rot1. Peanut Science. 2001; 28: 5-8.
- Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001; 107: 1077-1081.
- Mondoulet L, Paty E, Drumare MF, Ah-Leung S, Scheinmann P, Willemot RM, et al. Influence of thermal processing on the allergenicity of peanut proteins. J Agric Food Chem. 2005; 53: 4547-4553.
- Vissers YM, Iwan M, Adel-Patient K, Stahl Skov P, Rigby NM, Johnson PE, et al. Effect of roasting on the allergenicity of major peanut allergens Ara h 1 and Ara h 2/6: the necessity of degranulation assays. Clin Exp Allergy. 2011; 41: 1631-1642.
- Turner PJ, Mehr S, Sayers R, Wong M, Shamji MH, Campbell DE, et al. Loss of allergenic proteins during boiling explains tolerance to boiled peanut in peanut allergy. J Allergy Clin Immunol. 2014; 134: 751-753.
- Cabanillas B, Maleki SJ, Rodríguez J, Burbano C, Muzquiz M, Jiménez MA, et al. Heat and pressure treatments effects on peanut allergenicity. Food Chem. 2012; 132: 360-366.
- Davis PJ, Williams SC. Protein modification by thermal processing. Allergy. 1998; 53: 102-105.
- Blanc F, Vissers YM, Adel-Patient K, Rigby NM, Mackie AR, Gunning AP, et al. Boiling peanut Ara h 1 results in the formation of aggregates with reduced allergenicity. Mol Nutr Food Res. 2011; 55: 1887-1894.
- Chung SY, Maleki SJ, Champagne ET. Allergenic properties of roasted peanut allergens may be reduced by peroxidase. J Agric Food Chem. 2004; 52: 4541-4545.
- Maleki SJ, Chung SY, Champagne ET, Raufman JP. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol. 2000; 106: 763-768.
- Vissers YM, Blanc F, Skov PS, Johnson PE, Rigby NM, Przybylski-Nicaise L, et al. Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. PLoS One. 2011; 6: 23998.
- Moghaddam AE, Hillson WR, Noti M, Gartlan KH, Johnson S, Thomas B, et al. Dry roasting enhances peanut-induced allergic sensitization across mucosal and cutaneous routes in mice. J Allergy Clin Immunol. 2014; 134: 1453-1456.
- Kroghsbo S, Rigby NM, Johnson PE, Adel-Patient K, Bøgh KL, Salt LJ, et al. Assessment of the sensitizing potential of processed peanut proteins in Brown Norway rats: roasting does not enhance allergenicity. PLoS One. 2014; 9: 96475.
- Starkl P, Krishnamurthy D, Szalai K, Felix F, Lukschal A, Oberthuer D, et al. Heating Affects Structure, Enterocyte Adsorption and Signalling, As Well as Immunogenicity of the Peanut Allergen Ara h 2. Open Allergy J. 2011; 4: 24-34.
- Cabanillas B, Cuadrado C, Rodriguez J, Hart J, Burbano C, Crespo JF, et al. Potential changes in the allergenicity of three forms of peanut after thermal processing. Food Chem. 2015; 183: 18-25.
- Huang HW, Yang BB, Wang CY. Effects of high pressure processing on immunoreactivity and microbiological safety of crushed peanuts. Food Control. 2014; 42: 290-295.
- Hu CQ, Chen HB, Gao JY, Luo CP, Ma XJ, Tong P. High-pressure microfluidisation-induced changes in the antigenicity and conformation of allergen Ara h 2 purified from Chinese peanut. J Sci Food Agric. 2011; 91: 1304-1309.
- Johnson PE, Van der Plancken I, Balasa A, Husband FA, Grauwet T, Hendrickx M, et al. High pressure, thermal and pulsed electric-field-induced structural changes in selected food allergens. Mol Nutr Food Res. 2010; 54: 1701-1710.
- Oms-Oliu G, MartÌn-Belloso O, Soliva-Fortuny R. Pulsed light treatments for food preservation. Food Bioprocess Technol. 2010; 3: 13-23.
- Shriver S, Yang W, Chung SY, Percival S. Pulsed ultraviolet light reduces immunoglobulin E binding to Atlantic white shrimp (Litopenaeus setiferus) extract. Int J Environ Res Public Health. 2011; 8: 2569-2583.
- Chung SY, Yang W, Krishnamurthy K. Effects of pulsed UV-light on peanut allergens in extracts and liquid peanut butter. J Food Sci. 2008; 73: 400-404.
- Yang WW, Mwakatage NR, Goodrich-Schneider R, Krishnamurthy K, Rababah TM. Mitigation of major peanut allergens by pulsed ultraviolet light. Food Bioprocess Tech. 2012; 5: 2728-2738.
- Luo C, Hu C, Gao J, Li X, Wu Z, Yang A, et al. A potential practical approach to reduce Ara h 6 allergenicity by gamma irradiation. Food Chem. 2013; 136: 1141-1147.
- Oh S, Jang DI, Lee JW, Kim JH, Byun MW, Lee SY. Evaluation of reduced allergenicity of irradiated peanut extract using splenocytes from peanutsensitized mice. Radiat Phys Chem. 2009; 78: 615-617.
- Chung SY, Champagne ET. Reducing the allergenic capacity of peanut extracts and liquid peanut butter by phenolic compounds. Food Chem. 2009; 115: 1345-1349.
- Chung SY, Champagne ET. Effects of phytic acid on peanut allergens and allergenic properties of extracts. J Agric Food Chem. 2007; 55: 9054-9058.
- Chung SY, Kato Y, Champagne ET, Singh VK, Govil JN, Ahmad K, et al. Reducing the allergenic properties of peanut allergens by copper/hydrogen peroxide. Nat Product. 2007; 15: 443-453.
- Pasquale D, Mauro G. Amino acids and proteins for the athlete: The anabolic edge. Boca Raton FL; CRC Press. 1997.
- Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci Tech. 2000; 11: 254-262.
- Yu J, Ahmedna M, Goktepe I, Cheng H, Maleki S. Enzymatic treatment of peanut kernels to reduce allergen levels. Food Chem. 2011; 127: 1014-1022.
- Shi X, Guo R, White BL, Yancey A, Sanders TH, Davis JP, et al. Allergenic properties of enzymatically hydrolyzed peanut flour extracts. Int Arch Allergy Immunol. 2013; 162: 123-130.
- Mihajlovic L, Radosavljevic J, Nordlund E, Krstic M, Bohn T, Smit J, et al. Peanut protein structure, polyphenol content and immune response to peanut proteins in vivo are modulated by laccase. Food Funct. 2016.
- Ben-Shoshan M, Harrington DW, Soller L, Fragapane J, Joseph L, St Pierre Y, et al. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J Allergy Clin Immunol. 2010; 125: 1327-1335.
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using populationbased sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011; 127: 668-676.