
Research Article
Austin J Allergy. 2017; 4(2): 1028.
Periorbital Dermatitis: The Role of Type 1 and Type 4 Hypersensitivity as Contributing Factors
Ornek SA*, Kuteyla Can P, Degirmentepe EN, Kiziltac K, Gungor S, Oguz Topal I and Kocaturk E
Department of Dermatology, Okmeydani Training and Research Hospital, Istanbul, Turkey
*Correspoing author: Ornek Sinem Ayse, Department of Dermatology, Okmeydani Training and Research Hospital, Istanbul, Turkey
Received: May 26, 2017; Accepted: July 04, 2017; Published: July 11, 2017
Abstract
Background: Periorbital dermatitis is common and may be a manifestation of either allergic or non-allergic diseases.
Objectives: In this study, we investigated the role of type 1 and type 4 hypersensivity as etiologic factors on periorbital dermatitis and we explored whether the prognosis was associated with the etiology.
Methods: Data of sixty patients who had referred with eyelid dermatitis and had undergone patch testing between January 2014 and January 2017 were retrospectively analyzed.
Results: Patients have been diagnosed as Allergic Contact Dermatitis (ACD) (28,3%), Irritant Contact Dermatitis (ICD) (51,6%), atopic dermatitis (8,3%), Seborrheic Dermatitis (8,3%), Periocular Rosacea (3,3%), and Discoid Lupus Erythematosus (DLE) (1,6%). Atopy history was present in 21,7% of patients. Positive prick test reactions were found in 9 patients (15%). The most prevalent allergens were house dust mites, grass mix, and cat dander. Patch test positivity for at least one allergen was found in 27 patients (45%). The most common clinically relevant allergens were detected as isothiazolinone (methylchloroisothiazolinone and methylisothiazolinone), thiomersal, formaldehyde, p-phenylenediamine and dispers blue.
Conclusion: Physicians should take into account the patients’ use of cosmetics and hygiene products as well as atopy history and should perform a general skin examination not to miss other dermatological conditions that may present as periorbital dermatitis.
Keywords: Periorbital dermatitis; Eyelid eczema; Periocular dermatitis; Patch test; Prick test
Introduction
Periorbital dermatitis is common and may be a manifestation of either allergic or non-allergic diseases. The differential diagnosis includes endogenous causes like seborrheic and atopic dermatitis and exogenous causes like contact dermatitis [1]. Contact dermatitis may be irritant or allergic; the former develops secondary to the contact of an irritating substance and occurs by a direct local toxic effect. The personal and environmental factors such as atopy, age, sweat and heat may exacerbate this process. However, allergic contact dermatitis is a reaction of delayed hypersensitivity and occurs after previous sensitization [2]. Important sources of eyelid contact allergy include cosmetics, fragrances, topical medications, cleaning products and metals. In addition, the thin skin of the eyelids is susceptible to air borne allergens such as pollen, dust mites, animal dander and volatile chemicals [3].
In this study, we investigated the role of type 1 and type 4 hypersensivity as etiologic factors on periorbital dermatitis and we explored whether the prognosis was associated with the etiology.
Materials and Methods
We performed a retrospective analysis of 60 patients who had a complaint of eyelid dermatitis and had undergone patch testing at Okmeydani Training and Research Hospital between January 2014 and January 2017. All patients’ important demographic and clinical information’s like age, sex, profession, presence or history of atopy (atopic dermatitis, allergic rhinitis and asthma), duration of complaints, localization, the results of prick and patch tests and diagnosis had been recorded. Patch tests with standard series were performed with all the patients. After 48 hours, the patches were removed and assessed and readings were repeated 96 hours after tests began. For all the positive test results, the clinical relevance was investigated regarding the association between exposure and dermatitis. Prick tests included inhalant and food allergen panel and latex. Histamine was used as a positive control and saline was used as a negative control. The skin was evaluated after 20 min, and any wheal =3mm than the negative control was considered to indicate positivity. For the long term follow up, the patients were given a telephone call visit and evaluated for the regression of symptoms. Statistical analyses were performed with IBM SPSS version 22. Non parametric tests were used for the analysis of data, and the chi-square test and fisher exact test were used for the comparison of the data. A p-value <0,05 was considered to be statistically significant.
Results
During the 3-year period from January 2014 to 2017, of all 715 patch-tested patients, 60 (8,3%) had a complaint of periorbital dermatitis. There were 44 (73,3%) female and 16 (26,7%) male patients. The patients’ ages ranged from 9 to 72 with a mean age of 34 years. In terms of professions, housewives predominated among patients with periorbital dermatitis (41,7%), followed by office workers (20%), high risk jobs for occupational contact dermatitis (18,3%), students (15%), and healthcare workers (5%).
In most of the patients, periorbital dermatitis was the only clinical manifestation (83,3%) (Figure 1,2). The remaining 16,7% had also hand dermatitis. The duration of symptoms ranged from 1 to 180 months with mean time of 29 months. Atopy history was found in 21, 7% of patients with periorbital dermatitis. For demographic data, see Table 1.
Sex
N
%
Female
44
73,30
Male
16
26,70
Age
Min-Max (Median)
9-72 (35)
Mean ± SD
34,20 ± 12,74
N
%
<40 years
44
73,30
>40 years
16
26,70
Occupation
N
%
Housewives
25
41,70
Office workers
12
20,00
High risk jobs for
11
18,30
occupational contact dermatitis
Students
9
15,00
Healthcare workers
3
5,00
Atopy history
N
%
No
47
78,34
Yes
13
21,66
Duration of symptoms
Min-Max (Median)
1-180 (12)
Mean ± SD
29,50 ± 41,29
Table 1: Demographic data.
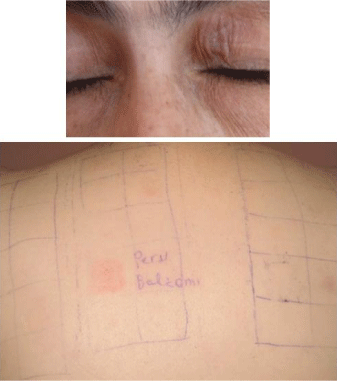
Figure 1: A&B: Periorbital dermatitis with positive reaction to balsam of peru.

Figure 2: A, B&C: Periorbital dermatitis with positive reactions to thiomersal
and disperse blue.
One or more positive prick test reactions were found in 9 patients (15%). The most prevalent allergens were house dust mites, grass mix, and cat dander.
Patch tests positivity for at least one allergen was found in 27 patients (45%). The remaining 33 patients (55%) obtained negative results. Among patients who tested positive, 11 were positive for more than one allergen (40,7%) and 16 tested positive to only one allergen (59,2%). Although the most frequently observed allergen in the patch tests was nickel sulfate (11 patients, 18, 30% of those which indicated positive), only one of them was clinically relevant. Of all patients with positive patch test results, clinical relevance was observed in 28, 3% of all patch tested patients and 63% of positive patch test reactions. The most common clinically relevant allergens were detected as isothiazolinone (methylchloroisothiazolinone and methylisothiazolinone), thiomersal, formaldehyde, p-phenylenediamine and dispers blue. For further clinically relevant allergens, see Table 2-4.
Periorbital dermatitis (N=60)
Patch test positivity (N=27, 45%)
Prick test positivity (N=9, 15%)
Contact allergy
(n=17, 28,3%)
Contact sensitization
(n=10, 16,6%)
Atopy history
(n=4, 6,6%)
No atopy history
(n=5, 8,3%)
Table 2: Classification of patients according to allergy test results.
Allergens
No. Patients n
(n=60) Relevance %
No. n
Nickel sulfate
11
18,3%
1
Isothiazolinones
4
6,7%
4
Thiomersal
2
3,3%
2
Formaldehyde
2
3,3%
2
Phenylenediamine
2
3,3%
2
Disperse blue
2
3,3%
2
Wool alcohols
2
3,3%
1
Thiuram mix
2
3,3%
1
Gold sodium thiosulfate
2
3,3%
1
1,2-dibromo-2,4-dicyanobutane
1
1,7%
1
Peru balsam
1
1,7%
1
Fragrance mix I-II
1
1,7%
1
Lyral
1
1,7%
1
Colophony
1
1,7%
1
Diazolidinyl urea
1
1,7%
1
Budesonide
1
1,7%
1
Potassium dichromate
1
1,7%
0
Cobalt chloride
1
1,7%
0
total
39
65,1%
24
Table 3: Contact allergens detected in patients with periorbital dermatitis.
Allergens
No. Patients (n=60) N
%
D. pterynissinus
7
11,6%
D. farinae
7
11,6%
Grass mix
3
5%
Cat dander
2
3,3%
Cow milk
1
1,7%
Total
20
33,2%
Table 4: Prick test allergens detected in patients with periorbital dermatitis.
Whilst 11,1% of patients with positive patch test results had also positivity in prick tests, 18,2% of patients with negative patch test results had positivity in prick tests. The results were compared using Fisher exact test and there were no statistically significant difference (p=0,495).
Personal atopy history was present in 18,5% of patients with positive patch test results and in 24,2% of patients with negative patch test results. The results were compared using Pearson Chi-Square test and there were no statistically significant difference (p=0,592).
Prick test positivity was present in 30,8% of patients who had atopy history compared to 10,6% of patients who had no atopy history. Although the former group was three times higher in prick positivity, this difference had no statistical significance (Fishers exact test; p=0,092).
Patients with clinically relevant positive patch tests have been diagnosed as Allergic Contact Dermatitis (ACD) (28,3%). The remaining patients were considered as having non-allergic periorbital dermatitis which consisted of Irritant Contact Dermatitis (ICD) (51,6%), atopic dermatitis (8,3%), seborrheic dermatitis (8,3%), periocular rosacea (3,3%), and Discoid Lupus Erythematosus (DLE) (1,6%).
The complaints of the patients in their long-term follow up continued in 21 patients (35%) and disappeared in 39 patients (65%). In terms of diagnosis, 52,9% of ACD cases, 77,4% of ICD cases, 20% of atopic dermatitis cases, 60% of seboreic dermatitis cases and all of rosacea cases healed. On the other hand, the complaints of 47% of ACD cases, 22, 5% of ICD cases, 80% of atopic dermatitis, 40% of seboreic dermatitis cases and all of DLE continued.
Discussion
Periorbital dermatitis is a common disease that affects a significant portion of population. The reported prevalence of patients with periorbital dermatitis in the literature ranges between 3% and 21% [5-7]. In our study, 8,3% of patients patch tested presented with periorbital dermatitis.
Our results demonstrated that periorbital dermatitis affects mainly women (73,3%). This significantly higher female predominance amongst periorbital dermatitis cases is similar in all studies and has been attributed to the more frequent cosmetic and hygiene products use in women [4,5,7-11].
Although the majority of patients in earlier reports are elderly [4-9], the age distribution of our study population showed that periorbital dermatitis affects all age groups, especially middle aged adults.
Like epidemiological study of periocular dermatitis of Rojo-España et al. [9], housewives predominated among patients with periorbital dermatitis (41,7%). These results are associated to the frequent use of irritative cleaning agents such as detergents. Office workers were the second most frequent occupation group with periorbital dermatitis (20%). This is probably associated to more cosmetic use of people with high socio-economical status. The third occupation group consisted of high risk occupations such as hairdressers, textile workers, carpenters and construction workers (18,3%). The occupational sensitizers and irritants might be responsible from the occurrence of eyelid contact dermatitis via direct or airborne mechanisms [12].
Eyelids are in contact with hands that are constantly exposed to many substances. These allergenic and irritant agents may be transmitted by direct hand contact to eyelids [13,14]. On the other hand, our study results showed that the vast majority of cases have periorbital dermatitis without hand involvement (83,3%).
In our study, atopy history was present in 21, 7% of patients with periorbital dermatitis. A study of Tomar et al. showed that atopy history of their patients was similar to us (20%) [14]. Herbst et al. observed that less than 30% of patients with periorbital dermatitis were atopics [8] of all our patients with periorbital dermatitis, 9 (15%) had positive prick test reactions and the most prevalent allergens were house dust mites, grass mix, and cat dander. In the study of Dirschka and Tronnier, atopy history was found in 17 of 23 patients who were diagnosed with periorbital dermatitis. They reported that 15 of 23 patients had positive prick test results with aeroallergens [15]. These results are higher than ours but this difference might be linked to the genetic differences of German and Turkish patients and also the small sample size of the mentioned study.
On the basis of our results, patients who have atopy history had three times frequent positivity in prick tests compared to non-atopics, even though the results could not reach statistical significance. This might be linked to the low number of patients and should be further analysed in larger sample sized studies.
In the vast majority of studies about periorbital dermatitis, the most frequent cause was found as ACD [4,8]. But, ICD was found as leading cause of periorbital dermatitis in our study (56,6%). In 25% of our patients, ACD was responsible for periorbital dermatitis. Similar to our results, Cooper and Shaw reported that 28,9% of 232 patients patch tested for periorbital dermatitis had ACD [11]. The other diagnoses have been observed in our study were seborrheic dermatitis (8,3%), atopic dermatitis (5%) and periocular rosacea (3,3%). One patient had been diagnosed as ACD on pre-existing DLE lesion(1.6%). Frequencies of causes may vary in different studies. Feser et al. observed that other causes of periorbital dermatitis were atopic dermatitis (25%), airborne allergic dermatitis (10,2%), ICD (9,1%) and periorbital rosacea (4,5%) [4]. Herbst et al. diagnosed their patients with non-allergic periorbital dermatitis as atopic dermatitis (55,6%), ICD (29,7%) and seborrheic dermatitis (4,1%) [8]. The low rate of ACD in our series might be linked to patch testing limited to baseline series. Unlike us, Feser et al. and Herbst et al. used patients’ own products to identify relevant contact allergens and they diagnosed ACD in the majority of patients with periorbital dermatitis [4,8]. This is a limitation of our study.
In our study, 27 of patients patch tested showed positive reactions to at least one allergen (45%). The remaining 33 patients (55%) obtained negative results. In accordance with most studies [4,8- 10], the most frequently observed allergen in our study was nickel sulfate (11 patients, 18,30% of those which indicated positive). But nickel sulfate might not be established to be relevant in most cases of periorbital dermatitis. Besides, the positive reactions to nickel sulfate are not different between in patients with or without periorbital dermatitis.8 Similar to the literature, only one of nickel sulfate positivity was clinically relevant in our study. Still, nickel sulfate may be relevant in individual cases as presence in cosmetics and eye pencils has been reported by Travassos et al. [16].
In our study, the leading clinically relevant allergens were detected as isothiazolinone, thiomersal, formaldehyde, p-phenylenediamine and dispers blue.
Preservatives like isothiazolinone, thiomersal and 1,2-dibromo- 2,4-dicyanobutane are used to decrease contamination of the cosmetics [3]. Isothiazolinone is one of the common preservatives used in cosmetics, shampoos and other hygiene products [17]. Rojo- España et al. found that 5,6% of positive allergens and 13,6% of positive relevant allergens were isothiazolinone in their periorbital dermatitis cases. They mentioned that hypersensitivity to isothiazolinone in the periorbital area is common as such products frequently come into contact with this area [9]. However, its incidence was found lower by Temesvari et al. (0,7%) [13]. Our study results showed 6,7% of patients with periorbital dermatitis had positive relevant patch test results to isothiazolinone. Thiomersal is another preservative found in cosmetic products as well as contact lenses solutions [8]. Rojo-España et al. reported that 6,6% of positive allergens and 11,3% of positive relevant allergens were thiomersal [9]. In our study, 2 of all patients had positive relevant patch test results to thiomersal (3,3%) similar in to the study of Temesvari et al. (3,5%) [13]. On the basis of the results of Landeck et al. study, patch test positivity to isothiazolinone and thiomersal were similar between inpatients with or without eyelid dermatitis [10]. 1,2-dibromo-2,4-dicyanobutane is also a preservative found in cosmetic and hygiene products [18]. Herbst et al. found positivity to 1,2-dibromo-2,4-dicyanobutane in 2,1% of patients with allergic contact periorbital dermatitis whereas 4,2 % of patients with non-allergic periorbital dermatitis [8]. We also found 1,2-dibromo- 2,4-dicyanobutane positivity in one of patients.
Sensitization to formaldehyde may occur with both formaldehyde and Formaldehyde-Releasing Products (FRPs) like imidazolidinyl urea and diazolidinyl urea. Eye cosmetics may contain formaldehyde or FRPs and these products may cause periorbital dermatitis either directly or with hand contact.7Two of our patients had positive relevant patch test results to formaldehyde (3,3%) and one to diazolidinyl urea (1,7%) and the source of allergen was considered as eye cosmetics. Herbst et al. reported 1,3% of patients had formaldehyde sensivity [8]. In the study of Amin&Belsito, three patients had positive relevant patch test results to formaldehyde (6,5%) and two to diazolidinyl urea (4,3%) [5].
Paraphenylenediamine is the most frequent allergen that is found in hair dyes.3Two male of our patients had positive relevant patch test results to paraphenylenediamine (3,3%) and the occupation of both were hairdresser. The incidence of positive relevant patch test results has been reported 2,8% by Rojo-España et al. and 3,7% by Temesvari et al. [9,13].
Dispers blue dyes are used to color the textile and may cause ACD [19]. Bosco et al. reported that patch test results to disperse blue dye were statistically more frequent in periorbital dermatitis comparing with non-periorbital dermatitis (3,1% vs 0,7%) [20]. There were positive relevant patch test results to disperse blue in 2 patients of our study (3,3%).We think this is a relevant allergen because one of our dispers blue positive patients was a textile worker and the other was making toys from textiles.
Fragrance mix, balsam of Peru, lyral and wool alcohols are used as fragrance in cosmetics. Fragrances are known as a frequent cause of ACD and the relevance of fragrances in periorbital dermatitis depends on exposure time [21]. The study of Herbst et al. showed that one of the leading allergens was fragrance mix (9,4%) in patients with allergic contact periorbital dermatitis but they found the proportion of patch test positivity significantly lower when compared to the control group which had no periorbital dermatitis (11,5%) [8]. The incidence of hypersensivity to balsam of Peru was obtained 6,6% in the study of Feser et al., 0,94% in Rojo-España et al.’s study and 4% in Temesvari et al.’s. [4,9,13]. In our study, positive relevant patch test results to fragrance mix and balsam of Peru were found in 1,7% for each of the allergens. The other fragrance allergens we detected in our study were lyral and wool alcohols (1,7% for each). The source of allergens was also linked to use of cosmetics and hygiene products.
The North American Contact Dermatitis Group (NACDG) had reported that twenty-two patients of eyelid dermatitis caused by gold (8,2%) and it was the most common allergen in patients with pure eyelid dermatitis [22]. In previous study of NACDG, 9,5% of 4,101 patch-tested patients had positive reactions to gold and the most common sites of dermatitis in gold-allergic patients were the hands (29.6%), face (19.3%), and periorbital areas (7.5%) [23]. In Guin’s study, 33 patients were allergic to gold; he noted that some cases were relevant [24]. Ehrlich and Belsito mentioned that periorbital dermatitis of 7 of 15 gold-allergic patients healed by not wearing gold jewelry [25]. Our two patients had patch test positivity to gold sodium thiosulfate and only one of them had clinical relevance.
Periorbital dermatitis may occur with eye medications containing topical corticosteroids used in the treatment of eye disease [23]. One of our patients had been treated with ophtalmic corticosteroid solutions for macular degeneration and after a while she presented with periorbital dermatitis. Positive patch test results for budesonide and hydrocortisone-17-butyrate had been observed in this case. After cessation of her medicines, the symptoms decreased. Corticosteroid hypersensitivity in periorbital dermatitis has been described in the literature previosly. Herbst et al. reported that patch test positivity in two cases with betamethasone- 17-valerate, one case with amcinonide and one case with hydrocortisone- 21-butyrate [8].
The complaints of the patients in their long-term follow up continued in 21 patients (35%) and disappeared in 39 patients (65%). In terms of diagnosis, 52,9% of ACD cases, 77,4% of ICD cases, 20% of atopic dermatitis cases, 60% of seboreic dermatitis cases and all of rosacea cases healed. On the other hand, the complaints of 47% of ACD cases, 22,5% of ICD cases, 80% of atopic dermatitis, 40% of seboreic dermatitis cases and all of DLE continued. It would be expected that ACD cases would disappear by avoiding the allergen that cause the dermatitis, but this was not the case for our patients, this could be linked to providing insufficient information to the patient or difficulty of avoiding allergens which are very common in daily living.
It is important to evaluate all the cosmetics and medical treatments used by the patients and to identify the relevant allergens which might be responsible for ACD in patients with periorbital dermatitis. Therefore baseline series as well as cosmetic series and patients’ own products should be included in patch testing allergens for patients presenting with periorbital dermatitis.
Conclusion
Periorbital dermatitis is a very common condition in the dermatology clinics. Physicians should take into account the patients’ use of cosmetics and hygiene products as well as atopy history and should perform a general skin examination not to miss other dermatological conditions that may present as periorbital dermatitis.
References
- Wolf R, Orion E, Tuzun Y. Periorbital (eyelid) dermatides. Clin Dermatol. 2014; 32: 131-140.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009; 129: 320-322.
- Peralejo B, Beltrani V, Bielory L. Dermatologic and allergic conditions of the eyelid. Immunol Allergy Clin North Am. 2008; 28: 137-168.
- Feser A, Plaza T, Vogelgsang L, Mahler V. Periorbital dermatitis – a recalcitrant disease: Causes and differential diagnoses. Br J Dermatol. 2008; 159: 858-863.
- Amin KA, Belsito DV. The aetiology of eyelid dermatitis: a 10-year retrospective analysis. Contact Dermatitis. 2006; 55: 280-285.
- Landeck L, Schalock PC, Baden LA, Gonzale E. Periorbital contact sensitization. Am J Ophthalmol. 2010; 150: 366-370.
- Herro EM, Elsaie ML, Nijhawan RI, Jacob SE. Recommendations for a screening series for allergic contact eyelid dermatitis. Dermatitis. 2012; 23: 17-21.
- Herbst RA, Uter W, Pirker C, Geier J, Frosch PJ. Allergic and non-allergic periorbital dermatitis: patch test results of the Information Network of the Departments of Dermatology during a 5-year period. Contact Dermatitis. 2004; 51: 13-19.
- Rojo-España R, Tomas-Mallebrera L, Gimeno-Clemente N, Marquina-Vila A, Morales-Suarez-Varela M. Epidemiological study of periocular dermatitis in a specialised hospital department. Iran J Allergy Asthma Immunol. 2011; 10: 195-205.
- Landeck L, John SM, Geier J. Periorbital dermatitis in 4779 patients – patch test resuts during a 10-year period. Contact Dermatitis. 2013; 70: 205-212.
- Cooper SM, Shaw S. Eyelid dermatitis: an evaluation of 232 patch test patients over 5 years. Contact Dermatitis. 2000; 42: 291-293.
- Handa S, De D, Mahajan R. Airborne contact dermatitis-current perspectives in etiopathogenesis and management. Indian J Dermatol. 2011; 56: 700-706.
- Temesvari E, Ponyai G, Nemeth I, Hidvegi B, Sas A, Karpati S. Periocular dermatitis: a report of 401 patients. J Eur Acad Dermatol Venereol. 2009; 23: 124-128.
- Tomar JY, Jain VK, Aggarwal K, Dayal S, Gupta S. Contact allergies to cosmetics: Testing with 52 cosmetic ingredients and personal products. J Dermatol. 2005; 32: 951-955.
- Dirschka T, Tronnier H. [Periocular dermatitis]. J Dtsch Dermatol Ges. 2004; 2: 274-278.
- Travassos AR, Bruze M, Dahlin J, Goossens A. Allergic contact dermatitis caused by nickel in a green eye pencil. Contact Dermatitis. 2011; 65: 307- 308.
- Scherrer MA, Rocha VB, Andrade AR. Contact dermatitis to methylisothiazolinone. An Bras Dermatol. 2015; 90: 912-914.
- Zachariae C, Johansen J D, Rastogi S C, Menne T. Allergic contact dermatitis from ’ methyldibromo glutaronitrile – clinical cases from 2003. Contact Dermatitis. 2005: 52: 6–8.
- Isaksson M, Ryberg K, Goossens A, Bruze M. Recommendation to include a textile dye mix in the European baseline series. Contact Dermatitis. 2015; 73: 15-20.
- Bosco L, Schena D, Girolomoni G. The aetiology of eyelid dermatitis in a series of 191 patients. Clinical Dermatology. 2016; 4: 1-7.
- Feser A, Mahler V. Periorbital dermatitis: causes, differential diagnoses and therapy. J Dtsch Dermatol Ges. 2010; 8: 159-166.
- Rietschel RL, Warshaw EM, Sasseville D, Fowler JF, DeLeo VA, Belsito DV, et al. Common contact allergens associated with eyelid dermatitis: data from the North American Contact Dermatitis Group 2003-2004 study period. Dermatitis. 2007; 18: 78-81.
- Fowler J Jr, Taylor J, Storrs F, Sherertz E, Rietschel R, Pratt M, et al. Gold allergy in North America. Am J Contact Dermat. 2001; 12: 3–5.
- Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002; 47: 755–765.
- Ehrlich A, Belsito DV. Allergic contact dermatitis to gold. Cutis. 2000; 65: 323–326.