Research Article
Austin J Anal Pharm Chem. 2014;1(1): 1002.
Simple and Flexible Microfabrication of Optical Biosensing Films Using Electrophoretic Deposition
Marta Marín-Suárez1, Santiago Medina-Rodríguez1,2, Jorge F Fernández-Sánchez1* and Alberto Fernández-Gutiérrez1
1Department of Analytical Chemistry, University of Granada, Spain
2Department of Signal Theory, Networking and Communications, University of Granada, Spain
*Corresponding author: :Jorge F Fernández-Sánchez, Department of Analytical Chemistry, University of Granada, Avd. Fuentenueva s/n, E-18071 Granada, Spain.
Received: June 06, 2014; Accepted: June 23, 2014; Published: June 25, 2014
Abstract
We propose a simple approach for the development of optical glucose sensing membranes adaptable to micro and nanofabrication technologies. The glucose sensitivity derives from the use of the enzyme glucose oxidase (GOx) and oxygen sensing polymeric particles. These two elements have been integrated onto gold coated chips in alternating layers using electrophoretic deposition (EPD). The oxygen sensing particles were obtained by precipitation-evaporation after covalently linking poly (styrene-co-maleic anhydride) and oxygen sensing Pt (II) porphyrin using click chemistry. The membrane is capable of determining glucose between 0.07mM and 3mM with a limit of detection of 0.02mM. The ability of EPD to adapt to substrates with different shapes and size allows its further implementation into different types of microdevices.
Keywords: Biosensing; Optical sensing; Glucose; Electrophoretic deposition; Click chemistry
Introduction
Science and Technology have always shown a great interest towards miniaturization [1]. Recent advances in modified semiconductor device fabrication has allow a fast growth in the development of micro and nanoelectromechanical systems (MEMS and NEMS) for a variety of applications, from miniaturized integrated systems, lab-on-a-chips or drug delivery systems [1-4]. Some of the potential of MEMS is their use as sensing elements [4]. Furthermore, there is a crescent interest in the integration of biosensors into miniaturized devices for a high-throughput screening, automation and optimization of microreactors, micro-fermentor arrays o microbiological assays kits [5,6]. The integration of optical sensors and biosensors in these devices offers a series of advantages towards the electrochemical detection, such as better sensitivity, non-consumption of the analyte, inertness to magnetic or electric fields, among others [2,3,7]. In addition they allow multi-analyte detection while in combination with electrochemical sensor can be used to obtain a richer set of data [8].
One problem arising from the use of micro machines is the integration of the sensing layer to small and sometimes irregular shaped surfaces [3], which is especially critical for luminescent optical sensors. Electrophoretic deposition (EPD) is considered as a suitable method for nanofabrication of devices with application in nanomedicine and drug delivery nano machines [9,10]. Our research group has recently demonstrated that this technique is useful for the implementation of optical oxygen sensing layers into a metal surface [11]. In addition, EPD of several types of biomaterials, including different types of enzymes, such as the enzyme glucose oxidase (GOx) [11,12], bacteria and cells has successfully been applied [9].
Different approaches have been developed to determine glucose with optical methods [7,12-14]. As a proof of concept we now report the use of EPD to produce biosensing films for the detection of glucose, using the enzyme glucose oxidase (GOx) and oxygen- sensitive particles for the optical transduction.
Material and Methods
Reagents
Poly(styrene-co-maleic anhydride) polymer (PSMA, 7% maleic anhydride, Mw =224000 g mol-1), tetrahydrofuran (THF), anhydrous N-N-dimethylformamide (DMF), triethanolamine (TEA), 2-aminoethanethiol (AET), glucose oxidase (GOx) from Aspergillus niger (100,000-250,000units/g solid), potassium phosphate dibasic and potassium phosphate monobasic were all purchased from Sigma Aldrich. Oxygen indicator Pt (II) meso-Tetra (pentafluorophenyl) porphine (PtTFPP) was purchased from Frontier Scientific. 1-Ethyl- 3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) crosslinker for enzyme immobilization was obtained from Piercenet.
Covalent attachment of the dye to the polymer
The covalent attachment of the dye PtTFPP to the polymer PSMA was carried out using anhydrous DMF and mild conditions by a two step reaction. In the first step, 10 mg of the PtTFPP dye in DMF were mixed with excess of TEA and 0.53 mg of AET during one hour. Subsequently, 100 mg of the polymer PSMA were added to the mixture and stirred overnight. The resulted PSMAcoPtTFPP in DMF was washed four times in methanol in order to remove the excess of TEA and PtTFFP and to confirm the covalent binding of the dye and the polymer. The modified polymer PSMAcoPtFP was subsequently dried under freeze-drying and storage in darkness.
Preparation and characterization of the oxygen sensitive nanoparticles
The oxygen sensitive nanoparticles (NP) were prepared using the precipitation-evaporation method [11]. Briefly, 5mg of PSMAcoPtTFPP were dissolved in 1mL of THF and subsequently dropped over 2mL phosphate buffer solution of pH 7.2 (50mM) under agitation. The mixture was exposed to blowing air for 20 minutes and THF was evaporated. The resulting polymeric particles (NP) formed stable dispersions.
Particle mean diameter (d, in nm), size distribution (PdI), zeta potential (ζ, in mV) and conductivity (C, in mS cm-1) of the dispersion were measured with a Zeta nanosizer (Malvern Instrument, model Zetasizer Nano ZS), which is equipped with a laser and a dynamic light scattering (DLS) detector.
Electrophoretic deposition
The cell used for EPD is described elsewhere [11]. It consists of a Titanium platinized plate sheet that acts as the cathode, an acryl glass box (polymethyl methacrylate) where the dispersion of NP or GOx (50 mg mL-1) is placed. A 1 cm2 gold coated silicon chip act as the anode for the deposition. Different cycles of potential and time were evaluated and the chips were washed with deionized water and dried with nitrogen between each deposition step.
Glucose sensing characterization
The characterization of the sensing layers was carried out by immersing the coated chips in a solution containing different amounts of glucose. The oxygen consumption of GOx during glucose oxidation was monitored during approximately 600 s by the change in the phase shift (Φ, in degrees) of PSMAcoPtTFPP. The chips were washed and the baseline recorded between each glucose concentration. The changes in the Φ were registered using a phase-resolved technique [15,16] with slight modifications from the setup described in reference [11].A home-made holder was prepared to place the excitation source and detector forming a 90° angle between them, and 45° from the sensing film surface. Briefly, the sensing phase was excited with an ultraviolet LED using a sinusoidal signal at a fixed frequency of 5145 Hz. The emission light collected by a photomultiplier tube and the phase shift was measured with a dual-phase lock-in amplifier.
Results and Discussion
Preparation of the oxygen sensing polymer and nanoparticles
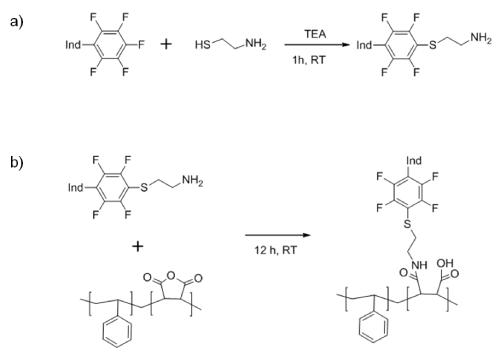
The oxygen sensitive dye PtTFPP was covalently attached to the PSMA polymeric chains, prior to produce the beads [17-20]. This approach solves problems associated to dye aggregation or leaching from the matrix, which eventually may decrease the sensitivity to oxygen [19]. For this aim, the reactivity of the para-fluorine of one of the PtTFPP was exploited using click chemistry [20]. In a first step aminoethanethiol was attached to the dye using very mild conditions in presence of TEA (Scheme 1a).In the second step, the anhydride maleic group of PSMA allows the crosslinking reaction with the amine group bonded to the PtTFPP, after 12 hours of reaction at room temperature (Scheme 1b).
Scheme 1: Reaction steps to covalently bond PSMA and PtTFPP. “Ind” denotes the oxygen indicator molecule.
The analysis of the absorption spectra of 0.5 g L-1 of PSMAcoPtFP in chloroform confirmed the presence on 1.59 % of PTTFFP attached to the polymer chains, considering an absorption coefficient of 250,000 L mol-1 cm-1. In addition, the spectrum for the PSMAcoPtTFPP showed no changes compared to a sensing layer of PtTFPP physically immobilized in polystyrene (PS), with the Soret band at 395 nm and the two Q bands between 508 and 541 nm.
Subsequently, beads were obtained by precipitation-evaporation with 0.1% w/w of this polymer, leading to particles with a size of 242 nm and PdI of 0.093, which are homogenous to provide uniform EPD. Table 1 shows the properties of the nanoparticles. The obtained particles provide very negative zeta potential (ζ =-50 mV), improving their electrophoretic mobility and therefore their deposition on the substrate [21].
Electrophoretic deposition
The properties of the media and the nature of the applied voltage play an important role in EPD. On one side, when high potential is applied in solutions with high conductivity, the decomposition of water and the heat generation can lead to enzyme denaturation, while disrupting the deposition of both the enzyme and the nanoparticles [22]. For this reason, the solution of NP was diluted with deionized water until reaching a conductivity of 7.96 mS cm-1(Table 1) in order to apply the same conditions of potential and time than GOx (with a conductivity of 8.81 mS cm-1).
Different approaches were accessed using unbalanced alternating fields of different magnitudes which are known to improve the electrophoretic deposition of enzymes [22,23]. However, co-deposition of both NP and GOx at the same time did not provide good results, probably due to the difference of charge between them (ζ of -50 towards -8.92 mV respectively; Table 1). Therefore, deposition protocols consisting of an increasing number of alternating layers of oxygen sensitive NP and GOx were evaluated. For a small number of alternating layers a lack of reproducibility was found, which suggest that the enzyme may be leaching from the surface. Better results were obtained when the number of layers is increased. The optimal response was found using four layers of NP and three layers of GOx, alternatively deposited via EPD. The optimal unbalanced potential applied for this deposition consisted of 9 cycles per layer, each one composed of an applied potential of 2V cm-1 during 45 seconds followed by 6V cm-1 during 25 seconds.
C (mS cm-1)
ζ (mV)
d (nm)
PdI
NP
7.96
-50
242.8
0.093
GOx
8.81
-8.92
-
-
Table 1: Properties of NP and GOx.
Response to glucose
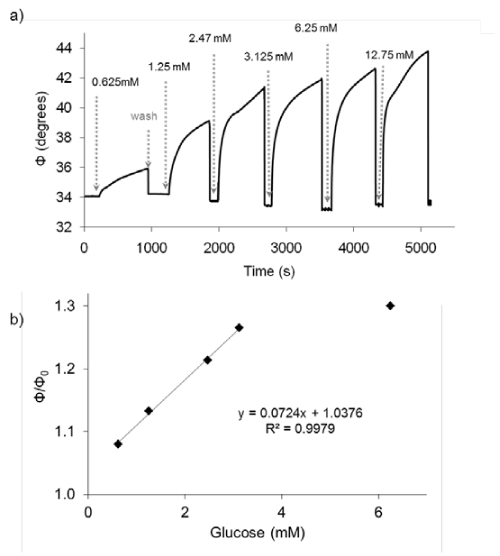
Figure 2 shows the change in the phase shift due to the oxygen consumption by GOx. A faster oxygen consumption is found when glucose concentration is increased, confirming its suitability to detect different amounts of glucose. The sensing layer showed a reversible behavior towards glucose, for at least more than 20 washing cycles and illumination during 2 hours.
Figure 2: a) Response in the phase shift (Φ) of the sensing film to different glucose concentrations; b) Calibration curve of the glucose sensing film.
The relative phase shift at 600 s can be directly correlated with the glucose concentration with a lineal interval between 0.07and 3.0mM. The detection limit, calculated as the concentration of glucose which produced an analytical signal three times the standard deviation of six injections of a blank (0% glucose) divided by the sensitivity was 0.02mM.
These results are in agreement to the values found for these types of sensing phases [7,12,13]. For example, in reference [12] a biosensor is obtained encapsulating GOx in silica based gels and integrating them into an oxygen sensing film of organically modified silicates and Ru (II) dye. The sensor had a linear response between 0.1 to 5mM with a limit of detection of 0.06mM. Other authors [13] obtained a linear range between 0.05mM to 5.0mM with a detection limit of 0.01mM by using a coordination polymer doped with Ir (III) dye and GOx encapsulated in hydrogel and immobilized on egg membrane using the layer-by-layer technique. Both approaches, although having similar analytical features, required several steps to obtain the sensing films and cannot be so easily implemented in microdevices as using EPD.
Conclusion
The suitability of EPD for the development of biosensors has been assessed by the development of glucose sensing layers with optical oxygen transduction. The oxygen sensing element consisted of PtTFPPcoPSMA, an oxygen sensitive polymer obtained using click chemistry. PSMAcoPtTFPP forms stable dispersion of particles that can be easily deposited onto a gold coated chip by EPD. The affinity element of the biosensor is based on electrophoretically deposited GOx, which confirms the compatibility of EPD to produce sensing films with optical transduction.
Different cycles of unbalanced potential were applied during EPD over the dispersion of oxygen sensing particles and GOx until a reproducibility in the response within the physiological range was found. Further optimization of the deposition procedure is expected to tune the analytical performance. Because EPD can be easily adapted to small size and irregular shapes, this approach can be utilized for the development of optical biosensors, especially as parts of microdevices.
References
- Fujita H. Micromachines as Tools for Nanotechnology. Springer Verlag. 2003.
- Sagmeister M, Tschepp A, Kraker E, Abel T, Lamprecht B, Mayr T, et al. Enabling luminescence decay time-based sensing using integrated organic photodiodes. Analytical and Bioanalytical Chemistry. 2013; 405: 5975-5982.
- Ergeneman O, Chatzipirpiridis G, Pokki J, Marín-Suárez M, Sotiriou GA, Medina-Rodríguez S, et al. In Vitro Oxygen Sensing Using Intraocular Microrobots. IEEE Transactions on Biomedical Engineering. 2012; 59: 3104-3109.
- Verpoorte E. Chip vision-optics for microchips. Lab Chip. 2003; 3: 42N-52N.
- Hegab HM, ElMekawy A, Stakenborg T. Review of microfluidic microbioreactor technology for high-throughput submerged microbiological cultivation. Biomicrofluidics. 2013; 7: 21502.
- Zhang Z, Perozziello G, Boccazzi P, Sinskey AJ, Geschke O, Jensen KF. Microbioreactors for Bioprocess Development. JALA - Journal of the Association for Laboratory Automation. 2007; 12: 143-151.
- Steiner MS, Duerkop A, Wolfbeis OS. Optical methods for sensing glucose. Chem Soc Rev. 2011; 40: 4805-4839.
- Eltzov E, Cosnier S, Marks RS. Biosensors based on combined optical and electrochemical transduction for molecular diagnostics. Expert Rev Mol Diagn. 2011; 11: 533-546.
- Boccaccini AR, Keim S, Ma R, Li Y, Zhitomirsky I. Electrophoretic deposition of biomaterials. J R Soc Interface. 2010; 7 Suppl 5: S581-613.
- Pokki J, Ergeneman O, Sivaraman KM, Ozkale B, Zeeshan MA, Lühmann T, et al. Electroplated porous polypyrrole nanostructures patterned by colloidal lithography for drug-delivery applications. Nanoscale. 2012; 4: 3083-3088.
- Marín-Suárez M, Medina-Rodríguez S, Ergeneman O, Pané S, Fernández-Sánchez JF, Nelson BJ, et al. Electrophoretic deposition as a new approach to produce optical sensing films adaptable to microdevices. Nanoscale. 2014; 6: 263-271.
- Chang G, Tatsu Y, Goto T, Imaishi H, Morigaki K. Glucose concentration determination based on silica sol-gel encapsulated glucose oxidase optical biosensor arrays. Talanta. 2010; 83: 61-65.
- Ho ML, Wang JC, Wang TY, Lin CY, Zhu JF, Chen YA, et al. The construction of glucose biosensor based on crystalline iridium (III)-containing coordination polymers with fiber-optic detection. Sensors and Actuators B: Chemical. 2014; 190: 479-485.
- Wang XD, Wolfbeis OS. Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem Soc Rev. 2014; 43: 3666-3761.
- Lakowicz JR. Principles of Fluorescence Spectroscopy. 2nd ed. New York: Kluwer Academic. 1999.
- Medina-Rodríguez S, de la Torre-Vega A, Fernández-Sánchez JF, Fernández-Gutiérrez A. An open and low-cost optical-fiber measurement system for the optical detection of oxygen using a multifrequency phase-resolved method. Sensors and Actuators B: Chemical. 2013; 176: 1110-1120.
- Waich K, Sandholzer M, Mayr T, Slugovc C, Klimant I. A highly flexible polymerization technique to prepare fluorescent nanospheres for trace ammonia detection. Journal of Nanoparticle Research. 2010; 12: 1095-1100.
- Zhou X, Su F, Tian Y, Johnson RH, Meldrum DR. Platinum (II) Porphyrin-Containing Thermoresponsive Poly (N-isopropylacrylamide) Copolymer as Fluorescence Dual Oxygen and Temperature Sensor. Sens Actuators B Chem. 2011; 159: 135-141.
- Koren K, Borisov SM, Klimant I. Stable optical oxygen sensing materials based on click-coupling of fluorinated platinum (II) and palladium (II) porphyrins: A convenient way to eliminate dye migration and leaching. Sensors and Actuators B: Chemical. 2012; 169: 173-181.
- Becer CR, Hoogenboom R, Schubert US. Click chemistry beyond metal-catalyzed cycloaddition. Angew Chem Int Ed Engl. 2009; 48: 4900-4908.
- Besra L, Liu M. A review on fundamentals and applications of electrophoretic deposition (EPD). Progress in Materials Science. 2007; 52: 1-61.
- Ammam M, Fransaer J. A study on electrodeposition of glucose oxidase from low conductivity solutions. ElectrochimicaActa 2010; 55: 9125-9131.
- Ammam M, Fransaer J. AC-electrophoretic deposition of glucose oxidase. Biosens Bioelectron. 2009; 25: 191-197.