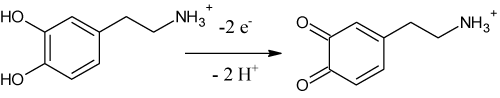Research Article
Austin J Anal Pharm Chem. 2014;1(1): 1004.
Electrochemical Behaviour Study and Sensitive Determination of Dopamine on Cathodically Pretreated Boron-doped Diamond Electrode
Sochr J, Cinkova K and Svorc L*
Department of Chemical and Food Technology, Slovak University of Technology, Slovak Republic
*Corresponding author: :Svorc L, Institute of Analytical Chemistry, Chemical and Food Technology, Slovak University of Technology in Bratislava, Radlinského 9, 812 37 Bratislava, Slovak republic.
Received: June 29, 2014; Accepted: July 08, 2014; Published: July 08, 2014
Abstract
An unmodified and cathodically pretreated boron-doped diamond (BDD) electrode was used as a sensitive electrochemical sensor for the determination of dopamine (DA) using differential pulse (DPV) and square-wave voltammetry (SWV). Cyclic voltammetric studies indicated a quasi-reversible behaviour of DA in acetate buffer solution at pH 3 with well-defined oxidation and reduction peak at +0.66 and +0.07 V vs. Ag/AgCl/3 M KCl, respectively. The electrode reaction of DA was shown to be a two-electron diffusion-controlled process. With optimized experimental parameters, the current response of DA was proportionally linear in the concentration range from 0.3 to 100μM and 0.7 to 100μM with low detection limits of 0.09 and 0.04μM as well as good repeatability (relative standard deviation of 3.5 and 3.3 %) for DPV and SWV, respectively. The influence of possible interfering compounds was also studied. The practical applicability of the developed method was demonstrated on the determination of DA in model human urine and water samples, with results similar to those obtained by a spectrophotometric method. The proposed electrochemical methodology is simple, inexpensive and rapid with no need of tedious sample pretreatment. In this way, BDD electrode may represent an efficient alternative to widely used modified electrodes in the determination of DA.
Keywords: Dopamine; Catecholamine; Boron-doped diamond electrode; Detection limit; Recovery
Introduction
Dopamine (3,4-dihydroxyphenethylamine, DA) is a biologically active compound belonging to the group of catecholamine. As a hormone it mediates a variety of the central nervous system functions including emotions, memory and endocrine regulation. DA is secreted in hypothalamus by regular physical activities such as eating, sports and especially during sex [1]. As a neurotransmitter, DA participates in the transfer of nervous signals between dendrites. Moreover, it allows the induction of some reactions (muscle contraction, emptying of the gland). Low concentration values of DA reflect mainly Parkinson’s and Alzheimer’s disease, rarely depression and anhedonia [2]. In contrary, higher concentrations are monitored in the case of long-term smoking, which may lead to dependency and taking hard drugs [3]. Therefore, a reliable determination of DA in the body fluids is important in clinical practice, particularly in diagnostics of the health state.
Instrumental analytical methods for the selective and sensitive detection and quantification of DA in mixtures containing also other compounds in various matrices (human urine, blood, vegetables, plants, etc.) are generally based on utilization of separation and spectral methods such as high-performance liquid chromatography coupled with UV [4], gas chromatography [5,6], capillary electrophoresis [7], fluorescence [8], UV/VIS [9,10] and NMR detection [11]. Nevertheless, these methods require sophisticated and expensive instrumentation, complicated sample pretreatment (preconcentration and/or purification step), long analysis time and extensive consumption of chemicals. In this sense, the development of novel, simple, low-cost and rapid analytical methods for the determination of DA in different matrices is still needed.
In the last decade, electrochemical methods may offer a useful alternative with favourable characteristics such as low expense of instrument, operation simplicity and lower sensitivity to matrix effects in comparison with chromatographic methods. Glassy carbon (GCE) and carbon paste electrodes (CPE) are commonly used in the determination of DA as the non-modified electrode surfaces [12,13]. Mahanthesha et al. [13] developed a method for the determination of DA with detection limit (LOD) of 0.2μM using DPV in phosphate buffer at pH 7. Cathodically pretreated CPE exhibited excellent selectivity in the presence of large excess of ascorbic (AA) and uric acid (UA). Various modifiers (carbon nanotubes (CNTs), graphenes, nanoparticles (NPs), polymer films and biocomponents) have been mainly applied to enhance sensitivity and in order to carry out the selective determination of DA in the presence of common urinary electroactive interferents such as AA, folic (FA) and UA. Shankar et al. [15] developed a method using CPE modified by do-decyl benzene sulfate (DDBS) for simultaneous determination of DA, AA and UA by DPV. The LOD was found to be 0.01μM for DA and the procedure was applied to the analysis of real samples with good recoveries. Voltammetric behaviour of DA on GCE modified by NiFe2O4 – MWCNTs was described by Ensafi et al. [16]. The oxidation of peak current was increased linearly in the wide concentration range of 0.05 – 6 and 6 – 100μM with LOD of 0.2μM using DPV. Applicability of the proposed method was evaluated on the analysis of the pharmaceuticals, human urine and blood serum samples. Golden nanoparticles (AuNPs) immobilized on a polycrystalline gold electrode (AuE) by Raj et al. [17] were used for the sensitive and selective determination of DA (LOD = 0.13 μM) in the presence of AA. The coexistence of AA did not interfere with the voltammetric sensing of DA. Moreover, modified AuE showed excellent antifouling properties.
Nowadays, the use of boron-doped diamond (BDD) electrode is very attractive in electroanalytical chemistry. This non-toxic electrode material has several unique properties such as high thermal conductivity, good mechanical and electrochemical stability in both alkaline and acidic media, low background current, wide potential range (up to 3.5 V) and low sensitivity on dissolved oxygen in aqueous solutions. Due to its high resistance to adsorption processes (presence of sp3 hybridized diamond carbon atoms) BDD differs from other conventional carbon electrodes [18,19]. Its properties can be influenced either by the structure (quantity of doping agents, presence of impurities) or by controllable (hydrogen or oxygen) surface termination. Consequently, BDD has been utilized as an effective alternative to traditional electrode materials in the determination of various biologically active compounds in the field of clinical [20,21] and environmental [22-24] trace analysis.
Electrochemical oxidation of DA and NADH was investigated by Fujishima et al. (1999) [25] using chronoamperometry (CA). Anodically pretreated BDD electrode was used to determine DA selectively with high sensitivity in the presence of large excess of AA in acidic media with a very low LOD = 50nM. BDD electrode was also applied in the investigation of DA oxidation in the study with surface modification based on the utilization of negatively charged AuNPs and polyelectrolyte. Multilayer sphere-modified electrodes showed high electrocatalytic activity and promote the oxidation of DA in the presence of AA with good selectivity. The peak current was linear for the concentration of DA in the range of 5 – 100μM and the LOD was found to be 0.8μM [26]. The BDD electrode modified with gold clusters [27] and AuNPs [28] were prepared and applied for the determination of DA by SWV with LOD = 0.1 and 0.03μM, respectively.
In this paper we describe the electrochemical behaviour of DA and the development of a novel, simple and sensitive voltammetric method for its determination using an unmodified and cathodically pretreated BDD electrode and its application to the analysis of model human urine and water samples. As stated above, the determination of DA has already been investigated by chronoamperometry using anodically pretreated BDD electrode [25]. However, the current work studies in detail the possibilities of a highly sensitive quantification of DA in voltammetric mode with the cathodic pretreatment of an electrode working surface. In this way, BDD offers an efficient modification-free alternative to widely used modified electrodes for a sensitive monitoring of DA.
Materials and Methods
Dopamine hydrochloride (p.a. purity ≥ 99.8 %) was obtained from Sigma-Aldrich Chemie GmbH (Germany) and used without any further purification. The acetate buffer solution (ABS) was prepared by mixing acetic acid (0.1 M) with sodium hydroxide (0.1 M) to the required pH value. A stock standard solution of DA (10mM) was prepared by dissolving 94.8 mg of its solid hydrochloride standard in 50 ml of deionized water and then stored in the refrigerator at +8OC. The working solutions of DA with lower concentrations were freshly prepared by dilution of respective volume of DA standard solution with supporting electrolyte. All other reagents were of analytical grade purity.
The electrochemical analyzer Autolab PGSTAT-302N (Metrohm Autolab B.V., The Netherlands) potentiostat/galvanostat was applied for all electrochemical measurements, controlled with the NOVA 1.10 electrochemical software. The three electrode cell system was used with BDD as a working electrode (Windsor Scientific Ltd, UK) with 3 mm inner diameter of an active surface, resistivity of 0.075 O cm and boron content of 1000ppm, a platinum wire as a counter electrode and an Ag/AgCl/3 M KCl as a reference electrode. All pH values were measured by pH meter Model 215 (Denver Instrument, USA) with a combined electrode (glass-reference electrode). The pH meter was calibrated weekly with standard buffer solutions. All potentials mentioned in this paper are referred against Ag/AgCl/3 M KCl reference electrode. The spectral measurements were made using 6715 UV/VIS Spectrometer Jenway (Bibby Scientific Limited, UK).
Cyclic voltammetry (CV), differential pulse voltammetry (DPV) and square-wave voltammetry (SWV) were used as electrochemical techniques for the purpose of this work. Before measuring, dissolved oxygen was eliminated by gaseous nitrogen for 10 min. prior to use of BDD electrode at the beginning of every work day, it was rinsed with deionized water. Subsequently, it was cathodically pretreated by applying -1.5 V for 30 s in 0.1 M H2SO4 solution in order to predominantly reach hydrogen terminated surface. After optimizing the instrumental parameters of DPV and SWV, calibration curves were obtained by successive addition of aliquots of the DA stock standard solution into the electrochemical cell already containing 25mL of supporting electrolyte; each concentration was measured in six replicate. The linear least-square regression in OriginPro 8 (OriginLab Corporation, USA) was used for the evaluation of calibration curve and the relevant results (slope and intercept) were reported with 95 % confidence level. The detection limit (LOD) was calculated as the three times standard deviation for the blank solution divided by the slope of the calibration curve.
Drug-free human urine sample was obtained from a healthy non-smoking volunteer (man, 27 years) immediately before the experiments. The water samples were obtained from communal source of drinking water and from the river. Aliquot volume of fresh urine (1mL) and water (1mL) was placed into the electrochemical cell with 20mL of supporting electrolyte. Subsequently, this solution was suitably fortified with DA standard solution to achieve a required concentration. Analysis of all samples was performed by the standard addition method with respective volumes of 20, 40 and 60μL (n = 6).
The samples for the spectrophotometric method were prepared similarly according to the above mentioned process. Before the addition of acidic medium (ABS, pH 3) the same volume K4[Fe(CN)6] and FeCl3 (1 mL), both of 15 mM was added . After the reaction time of 35 min, the solution was subsequently 50-times diluted and theabsorbance of prepared solutions was measured at 725 nm. This procedure was undertaken according to Guo et al. (2009) [9].
Results and Discussion
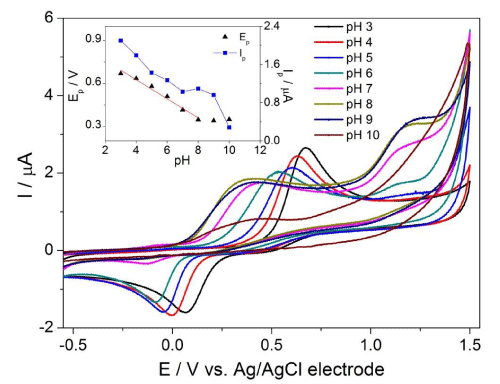
First, the electrochemical behaviour of DA was studied in various electrolytes such as Britton-Robinson (BRBS), phosphate (PBS) and acetate (ABS) buffer in the pH range of 3–10 by CV on the cathodically pretreated BDD electrode (results not shown). The best results with the highest magnitude, low background and good repeatability were obtained in ABS. Hence, it was chosen as an optimal medium for further voltammetric studies in this work. Generally, DA is an electrochemically active substance which can transfer electrons from solution to the electrode. Figure 1 shows the representative CV voltammograms in the presence of 0.1mM DA in ABS at pH 3 (black curve). The well-defined oxidation peak of DA was observed on the forward scan at the potential of +0.66 V. On the reverse scan the corresponding cathodic peak was recorded at +0.07 V indicating the quasi-reversible character of electrode reaction of DA on BDD electrode. The electrochemical behaviour of DA well coincides with those previously reported in literature [14,15].
Figure 1: CV voltammograms of 0.1 mM DA at various pH (3 – 10) of ABS on BDD electrode with scan rate of 100 mV·s-1. The effect of pH on the peak potential (Ep) and peak current (Ip) of DA appears in the inset.
The effect of pH on the peak potential of 0.1 mM DA was investigated by CV in the pH range from 3 to 10 using ABS as a supporting electrolyte with the scan rate of 100 mV s-1. It was found that the two oxidation peaks (first intensive one within potentials of 0.35 – 0.58 V, second ill-defined one at 1.14 – 1.25 V) were registered with no or smaller cathodic peaks within the range from 0 to -0.12 V on the reverse scan on BDD electrode (Figure 1). This phenomenon was observed in pH values in the range of 4 – 10. In pH = 3 of ABS, DA provided only one intensive oxidation peak at +0.66 V. Furthermore, this study also revealed that with increasing pH the peak potential of first oxidation peak (Ep) is shifted to less positive values (Figure 1); this dependence is linear over the pH range from 3 to 8 and can be expressed by following equation (Equation 1):
Above pH 8 the slope levels off and the peak potential for the first oxidation peak of DA is independent on the proton concentration. This fact relates with pKa value for DA which slightly differs from theoretical value of 8.89 pertaining to the monoprotonated/unprononated form of DA. Although the elucidation of the mechanism of the electrode reaction of DA has been beyond the main aim of this study, from our results, the proposed oxidation mechanism for DA (Figure 2) is believed to occur via two-electron and two-proton transfer to form dopamine-o-quinone (rate-determining step). Nevertheless, in our work, no further efforts have been performed to characterize or identify the final products formed, as it has been well established in the literature [29].
Figure 2: Oxidation mechanism of DA on BDD electrode.
The effect of pH on the peak current (Ip) was also investigated in the pH range of 3–10. The results indicated the highest peak current for DA oxidation at pH 3 with sharp decrease of magnitude as the pH of electrolyte was increased (Figure 1). Therefore, a pH 3 represented the most appropriate value for ABS in the next measurements.
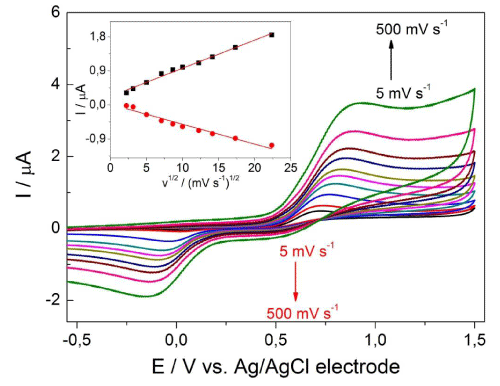
The valuable information concerning the electrode reaction mechanism (rate-determining step) may be acquired from the relationship between the peak current and scan rate (v). The effect of scan rate (v) on the anodic (Ipa) and cathodic (Ipc) peak current of 0.1mM DA in ABS at pH 3 was tested in the range from 5 to 500 mV s-1 (Figure 3). As can be seen in the inset of Figure 3, a shift of the peak potential towards more positive (in reverse scan to more negative) values was observed as the scan rate was increased. This behaviour is typical for irreversible and quasi-reversible systems. The linear relationships between the peak currents (Ipa, Ipc) and the square root of scan rate (v1/2) indicated a diffusion-controlled process. They are represented as the following equations (Equation 2 and Equation 3):
Figure 3: CV voltammograms of 0.1 mM DA in ABS at pH 3 on BDD electrode at various series of scan rate from 5 to 500 mV s-1. The dependence I = f(v1/2) appears in the inset.
DPV and SWV were applied as sensitive voltammetric techniques with good discrimination against the background current. In order to investigate the dependence between peak current and concentration of DA, DPV and SWV parameters were optimized using 10μM DA in ABS at pH 3 on the BDD electrode. Variation of (modulation) amplitude in the range of 5 to 500 mV showed that the current response of the oxidation peak of DA was increased. This effect was associated with a broadening of the peak and the slight shift towards more negative potentials. As for the modulation time (with modulation amplitude fixed at 100 mV), the peak current decreased with an increasing modulation time in the range from 10 to 300 ms (with fixed interval time at 500 ms). The magnitude of oxidation signal was constant with the interval time in the range from 5 to 500 ms (with fixed modulation time at 50 ms) and the background current declined with increasing interval time. In the case of frequency, the corresponding oxidation peak was increasing in the range of 1–100 Hz. above the value of 10 Hz; the peak current became unstable and shifted to more positive potentials. Overall, modulation amplitude of 100 mV, modulation time of 50 ms, interval time of 500 ms for the DPV and amplitude of 100 mV and frequency of 10 Hz for the SWV represent the most suitable values of studied parameters for the determination of DA using the BDD electrode.
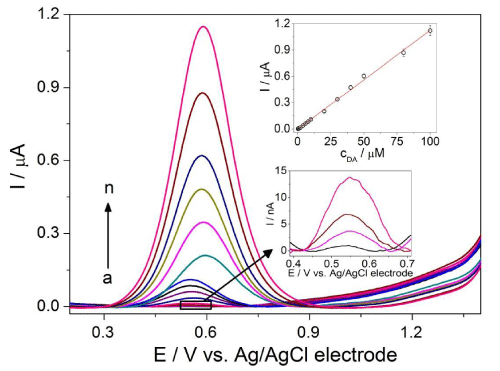
Optimized values were used to acquire DP (Figure 4) and SW (Figure 5) voltammetric profiles for the various concentrations of DA. The calibration curve was constructed by plotting peak current against DA concentration in the range from 0.3 to 100μM and from 0.7 to 100μM for DPV and SWV, respectively. The analytical parameters are summarized in Table 1. According to the slopes of calibration curves, the SWV technique appeared to be more sensitive than DPV (approximately 1.6-fold). Therefore, SWV was chosen for the next voltammetric measurements. The low LOD values were achieved as a consequence of high S/N ratio and without any chemical modification of BDD electrode surface. Moreover, they are comparable with the values reported previously in the literature [14,28,30,31]. Low RSD values characterizing intra-day repeatability confirmed minimal adsorption of the BDD electrode surface. The results demonstrate the suitability of the proposed method for the sensitive quantification of DA with good precision.
Figure 4: DP voltammograms for various concentrations of DA: (a) 0.3, (b) 0.7, (c) 1, (d) 2, (e) 4, (f) 6, (g) 8, (h) 10, (i) 20, (j) 30, (k) 40, (l) 50, (m) 80 and (n) 100 µM in ABS at pH 3 on BDD electrode with the optimized DPV parameters: modulation amplitude of 100 mV, modulation time of 50 ms and interval time 500 ms. The calibration curve I = f(cDA) appears in the inset of figure.
Figure 5: SW voltammograms for various concentrations of DA: (a) 0.7, (b) 2, (c) 4, (d) 8, (e) 10, (f) 20, (g) 30, (h) 40, (i) 50, (j) 80 and (k) 100 µM in ABS at pH 3 on BDD electrode with the optimized SWV parameters: amplitude of 100 mV and frequency of 10 Hz. The calibration curve I = f(cDA) appears in the inset of figure.
Analytical parameter
Value
DPV
SWV
Peak potential (V vs. Ag/AgCl/3 M KCl)
0.54
0.6
Intercept (nA)
-5.445
-4.245
Standard deviation of intercept (nA)
0.342
0.233
Slope (µA µM-1)
11280
18302
Standard deviation of slope (µA µM-1)
842
1232
Linear concentration range (µM)
0.3 – 100
0.7 – 100
Coefficient of determination (R2)
0.997
0.998
Detection limit* (µM)
0.09
0.04
Intra-day repeatability** (%)
3.5
3.3
Table 1: Analytical parameters for the determination of DA in ABS at pH 3 on the BDD electrode using DPV and SWV method (n = 6).
In order to evaluate the selectivity of proposed method, the effect of possible interfering compounds was investigated using SWV under the optimal conditions with fixed concentration of 10μM DA in ABS at pH 3. Some biomolecules typically occurring in human urine were studied in the concentration ratios to DA of 1:1, 1:2, 1:10 and 1:50 (data not shown). The criterion evaluating the serious interference effect was defined as the tolerance limit of ±10 % signal change of DA in its determination. In the case of glucose, sucrose and citric acid, no major effect on the peak current of DA was observed in a 50-fold excess. As for the AA, FA, UA, creatinine and urea, the peak current of DA was seriously affected in their 2-fold excess. AA, FA and UA are electrochemically active species with oxidation signals at potentials close to DA, thus lowering the selectivity in this way. In conclusion, the interference study revealed that the utilization of proposed method in the analysis of human urine samples containing DA could be limited in the presence of some urinary compounds.
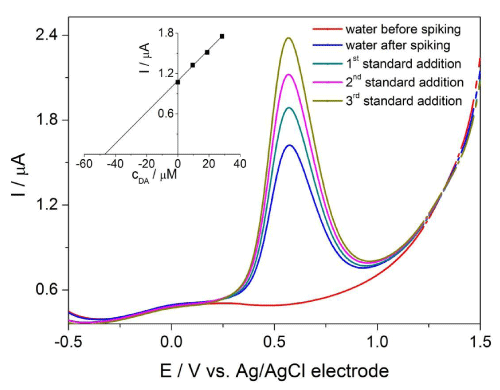
The developed procedure was subsequently applied for the determination of DA in human urine, tap and river water samples spiked with aliquots of DA standard solution. In order to eliminate the influence of matrix the standard addition method was used and the accuracy was controlled by the spectrophotometric method. The average results of six successive measurements under optimal experimental conditions are summarized in Table 2. The recovery values (ranged from 96.8 to 98.7 %) revealed the sufficient accuracy of the proposed method. These values indicated that there are no significant matrix interferences in the analyzed samples. Thus, the DA concentration can be quantitatively determined by the developed procedure. Typical SW voltammograms of DA in the tap water sample is illustrated in Figure 6. As can be seen, the observed oxidation peak at about +0.55 V increased after each addition of DA demonstrating that it can be assigned to the oxidation of DA in the sample.
Figure 6: SW voltammograms of analysis of tap water sample in ABS at pH 3 on BDD electrode. The respective aditions: 100 μL (spike), 20, 40 and 60 μL of 10 mM standard DA solution. Experimental conditions: amplitude of 100 mV, frequency of 10 Hz. The determination of DA by standard addition method is depicted in inset of figure.
Sample
Added (µM)
Found by
proposed method
(µM)
Recovery
(%)
Found by spectrophotometry
(µM)
Tap water
47.4
46.1 ± 1.2
97.3
47.2 ± 2.1
River water
47.4
45.9 ± 1.5
96.8
46.9 ± 1.3
Human urine
47.4
46.8 ± 4.2
98.7
48.1 ± 3.2
Table 2: Human urine and water samples analysis using proposed method (n=6).
The comparison between proposed method and so far reported electrochemical methods for the determination of DA is given in Table 3. Most of the reported papers declare higher LODs even using modifiers when compared with LOD in this work. In general, modifications were based on immobilization of polymer [32-36], carbon nanostructures [30], inorganic compounds [16], golden nanoparticles [31] and biocomponents [37] on various substrates such as CPE, GCE or AuE. The methods using CPE modified by calgamite [32] and DDBS [15] have been considered to be the most sensitive for the electroanalytical determination of DA with the lowest LODs of 0.01 and 0.03μM, respectively. An unmodified BDD electrode showed excellent results in the amperometric determination of DA with LOD = 0.05μM [25], thus representing the benefits in comparison with some chemically modified SWCNTs – GCE [39] and AuNPs – AuE [17]. Despite the fact that the modification of BDD electrode surface is relatively new phenomenon in electrochemical determination of DA, similar results were reached only by the use of AuNPs [28]. According to the Table 3, LOD obtained herein by unmodified BDD electrode (in voltammetric mode) is comparable with those reached by modified electrodes and previously applied BDD electrode in amperometric determination of DA [25]. Concerning the modification, the preparation of such electrode is sometimes time-consuming step with increasing possibility of measurement errors and may lead to the low reproducible results. Therefore, it is useless to modify the electrode especially in the case of routine analysis. Following to our experiments, low LOD, wide concentration range and intra-day repeatability was obtained without the BDD electrode surface modification. This fact confirms sufficient reliability of the proposed method with utilization of BDD electrode as a sensitive electrochemical sensor in drug analysis.
Electrode
Technique
Other analytes
LCR
(µM)
LOD
(µM)
Sample
Reference
AuNPs
Au
SWV
AA
n/a
0.13
n/a
[17]
5ADB
CNPE
SWV
PAR, FA, NAC
1.2 – 900
0.57
drugs
[40]
a-Co-SO4/SDS
CPE
DPV
AA
0.5 – 100
0.25
n/a
[41]
CoNSal
CPE
DPV
AA
1 – 100
0.5
human urine
[42]
DDBS
CPE
DPV
AA, UA
n/a
0.01
drugs
[15]
Graphite oxide
CPE
DPV
AA, UA
0.07 – 70
0.015
human blood serum
[43]
MWCNTs-TN
CPE
DPV
AA
0.1 – 80
0.08
drugs
[30]
Poly (calmagite)
CPE
DPV
AA, UA
9 – 35
0.01
drugs
[32]
TX-100
CPE
DPV
AA
0.7 – 500
0.03
drugs
[38]
n/a
CPE
DPV
AA, UA
2 – 10
0.2
human blood serum
[13]
NiFe2O4-MWCNTs
GCE
DPV
n/a
0.05 – 6
6 – 100
0.02
drugs, human urine and blood serum
[16]
Poly-CDDA
GCE
DPV
AA, UA
5 – 280
0.29
drugs, human urine
[36]
Solar graphene
GCE
DPV
AA, UA
20 – 400
2.8
n/a
[44]
SWCNTs
GCE
DPV
ADS
1 – 100
7
human urine and blood plasma
[39]
AuNPs
MWCNTs
DPV
AA, UA
0.06 – 8
0.04
drugs
[31]
Peroxidase (Curcubita pepo L.)
MWCNTs
SWV
n/a
32 – 44
2
drugs
[37]
4PA
BDDE
DPV
AA
n/a
n/a
n/a
[45]
Au
BDDE
SWV
AA
n/a
0.1
n/a
[27]
AuNPs/PANIox
BDDE
SWV
AA
0.15 – 500
0.03
n/a
[28]
(Au/PAH)4/(PSS/PAH)4/PS
BDDE
CV
AA
5 – 100
0.8
n/a
[36]
n/a
BDDE
CA
NADH
n/a
0.05
n/a
[25]
n/a
BDDE
DPV
SWV
n/a
0.3 – 100
0.7 – 100
0.09
0.04
human urine, tap and river water
This work
Table 3: Comparison of basic characteristics of proposed method with the previsously reported electroanalytical methods for determination of DA.
Conclusion
In this study, an unmodified and cathodically pretreated BDD electrode was applied as a sensitive electrochemical sensor for the direct DA determination. CV, DPV and SWV were applied for the characterization of the electrochemical behaviour and quantification of DA. Proposed analytical method is simple and rapid in comparison with commonly used chromatographic methods. The low LOD (0.04μM) was obtained as a consequence of very high S/N ratio using SWV technique. The method was also sufficiently selective because most of the species in human urine did not interfere in high excess. The practical analytical utility of method was successfully demonstrated on the analysis of model human urine, tap and river water samples (recoveries varied from 96.8 to 98.7 %). Based on obtained facts the presented method with BDD electrode could offer rapid and sensitive analytical alternative in diagnostics and clinical practice.
Acknowledgment
This work was supported by the Grant Agency of the Slovak Republic (grant No. 1/0051/13) and the Slovak Research and Development Agency under the Contract Nos. APVV-0797-11 and APVV-0122-12.
References
- Arias-Carrión ó, Pöppel E. Dopamine, learning, and reward-seeking behavior. Act. Neuro Exp. 2007; 67: 481 – 488
- Liu L, Du J, Li B, Yuan B, Han B, Jing M, et al. Amplified voltammetric detection of dopamine using ferrocene-capped gold nanoparticle/streptavidin conjugates. Biosensors and Bioelectronics 2013; 41: 730 – 735
- Kollins S H, Adcock R A. ADHD, altered dopamine neurotransmission, and disrupted reinforcement processes: Implications for smoking and nicotine dependence. Progress in Neuro-Psychopharmacology & Biological Psychiatry 2014; 52: 70 – 78
- Muzzi C, Bertocci E, Terzuoli L, Porcelli B, Ciari I, Pagani R, et al. Simultaneous determination of serum concentrations of levodopa, dopamine, 3-O-methyldopa and a-methyldopa by HPLC. Biomedicine and Pharmacotherapy 2008; 62: 253 – 258
- Naccarato A, Gionfriddo E, Sindona G, Tagareli A. Development of a simple and rapid solid phase microextraction- gas chromatography-triple quadrupole mass spectrometry method for the analysis of dopamine, serotonin and norepinephrine in human urine. Analytica Chimica Acta 2014; 810: 17 – 24
- Khuhawar MY, Zardari LA, Majidano SA, Majidano AA. Capillary gas chromatographic determination of dopamine by pre-column derivatization method using trifluoroacetylacetone as derivatizing reagent. J. Chem. Soc. Pak. 2010; 32(6): 781 – 785
- Vuorensola V, Sirén H, Karjalainen U. Determination of dopamine and methoxycatecholamines in patient urine by liquid chromatography with electrochemical detection and by capillary electrophoresis coupled with spectrophotometry and mass spectrometry. J. Chromatography B 2003; 768(2): 277 – 289
- Zhao H-X, Mu H, Bai Y-H, Yu H, Hu Y-M. A rapid method for the determination of dopamine in porcine muscle by pre-column derivatization and HPLC with fluorescence detection. J. Pharm. Analysis 2011; 1(3): 208 – 212
- Guo L, Zhang Y, Li Q. Spectrophotometric determination of dopamine hydrochloride in pharmaceutical, banana, urine and serum samples by potassium ferricyanide-Fe(III). The Japan Society for Analytical Chemistry 2009; 25: 1451 – 1455
- Zhu M, Huang X, Li J, Shen H. Peroxidase-based spectrophotometric methods for the determination of ascorbic acid, norepinephrine, epinephrine, dopamine and levodopa. Analytica Chimica Acta 1997; 357: 261 – 267
- Van de Merbel NC, Hendriks G, Imbos R, Tuunainen J, Rouru J, Nikkanen H. Quantitative determination of free and total dopamine in human plasma by LC-MS/MS: the importance of sample preparation. Bioanalysis 2011; 3(17): 1949 – 1961
- Ke N J, Lu S-S, Cheng S-H. A strategy for the determination of dopamine at a bare glassy carbon electrode: p-Phenylenediamine as a nucleophile. Electrochem.Communications 2006; 8: 1514 – 1520
- Huang D-Q, Chen Ch, Wu Y-M, Zhang H, Sheng L-Q, Xu H-J, et al. The determination of dopamine using glassy carbon electrode pretreated by a simple electrochemical method. Int. J. Electrochem. Sci. 2012; 7: 5510 – 5520
- Mahanthesha KR, Swamy BEK. Pretreated/carbon paste electrode based voltammetric sensors for the detection of dopamine in presence of ascorbic acid and uric acid. J. Electroanal. Chem. 2013; 703: 1 – 8
- Shankar SS, Swamy BEK, Chandrashekar BN, Gururaj KJ. Sodium do-decyl benzen sulfate modified carbon paste electrode as an electrochemical sensor for the simultaneous analysis of dopamine, ascorbic and uric anid: A voltammetry study. J. Molecular Liquids 2013; 177: 32 – 39
- Ensafi AA, Arashpour B, Rezaei B, Allafchian AR. Voltammetric behavior of dopamine at a glassy carbon electrode modified with NiFe2O4 magnetic nanoparticles decorated with multiwall carbon nanotubes. Materials Science and Engineering C 2014; 39: 78 – 85
- Raj CR, Okajima T, Ohsaka T. Gold nanoparticle arrays for the voltammetric sensing of dopamine. J. Electroanal. Chem. 2003; 543: 127 – 133
- Yosypchuk O, Barek J, Pecková K. Voltametrické stanovení 1-nitropyrenu a 1-aminopyrenu na borem dopované diamantove filmové elektrode. Chem. Listy 2010; 104: 186 – 190
- Musilová J, Barek J, Pecková K. Použití diamantových filmových elektrod dopovaných borem pro stanoveníí organických látek. Chem. Listy 2009; 103: 469 – 478
- Uslu B, Topal BD, Ozkan SA. Electroanalytical investigation and determination of pefloxacin in pharmaceuticals and serum at boron-doped diamond and glassy carbon electrodes. Talanta 2008; 74(5): 1191 – 1200
- švorc L, Sochr J, Tomcík P, Rievaj M, Bustin D. Simultaneous determination of paracetamol and penicillin V by square-wave voltammetry at a bare boron-doped diamond electrode. Electrochimica Acta 2012; 68: 227 – 234
- Bandžuchová L, Švorc L, Sochr J, Svítková J, Chýlkováá J. Voltammetric method for sensitive determination of herbicide picloram in environmental and biological samples using boron-doped diamond film electrode. Electrochimica Acta 2013; 111: 242 – 249
- Kapalka A, Fóti G, Comninellis C. The importance of electrode material in environmental electrochemistry: Formation and reactivity of free hydroxyl radicals on boron-doped diamond electrodes. Electrochimica Acta 2009; 54(7): 2018 – 2023
- Švorc L, Rievaj M, Bustin D. Green electrochemical sensor for environmental monitoring of pesticides: Determination of atrazine in river waters using a boron-doped diamond electrode. Sensors and Actuators B: Chemical 2013; 181: 294 – 300
- Fujishima A, Rao TN, Popa E, Sarada BV, Yagi I, Tryk DA. Electroanalysis of dopamine and NADH at conductive diamond electrodes. J. Electroanalytical Chemistry 1999; 473: 179 – 185
- Wei B M, Sun L-G, Xie Z-Y, Zhii J-F, Fujishima A, Einaga Y, et al. Selective determination of dopamine on a boron-doped diamond electrode modified with gold nanoparticle/polyelectrolyte-coated polystyren colloids. Adv. Funct. Mater. 2008; 18: 1414 – 1421
- Weng B J, Xue J, Wang J, Ye J-S, Cui H, Sheu F-S, et al. Gold-cluster sensors formet electrochemically at boron-doped-diamond electrodes: Detection of dopamine in the presence of ascorbic acid and thiols. Adv. Funct. Mater. 2005; 15: 639 – 647
- Song M-J, Lee S-K, Kim J-H, Lim D-S. Dopamine sensor based on a boron-doped diamond electrode modified with a polyaniline/Au nanocomposites in the presence of ascorbic acid. Analytical Sciences 2012; 28: 583 – 587
- Muñoz P, Huenchuguala S, Paris I, Segura-Aguilar J. Dopamine oxidation and autophagy. Parkinson’s Disease 2012; ID 920953
- Shahrokhian S, Zare-Mehrjardi HR. Application of thionine-nafion supported on multi-walled carbon nanotube for preparation of a modified electrode in simultaneous voltammetric detection of dopamine and ascorbic acid.Electrochimica Acta 2007; 52: 6310 – 6317
- Yang S, Yin Y, Li G, Yang R, Li J, Qu L. Immobilization of gold nanoparticles on multi-wall carbon nanotubes as an enhanced material for selective voltammetric determination of dopamine. Sensors and Actuators B 2013; 178: 217 – 221
- Chandra U, Swamy BEK, Gilbert O, Sherigara BS.Voltammetric resolution of dopamine in the presence of ascorbic acid and uric acit at poly (calmagite) film coated carbon paste electrode. Electrochimica Acta 2010; 55: 7166 – 7174
- Ensafi AA, Taei M, Khayamian T. Simultaneous determination of ascorbic acid, dopamine, and uric acid by differential pulse voltammetry using tiron modified glassy carbon electrode. Int. J. Electrochem. Sci. 2010; 5: 116 – 130
- Li K-C, Yin C-Y, Chen S-M. Simultaneous determination of AA, DA, and UA based on bipolymers by electropolymerization of luminol and 3,4-ethylenedioxythiophene monomers. Int. J. Electrochem. Sci. 2011; 6: 3951 – 3965
- Reddaiah K, Reddy MM, Raghu P, Reddy TM. An electrochemical sensor based on poly (solochrome dark blue) film coated electrode for the determination of dopamine and simultanous separation in the presence of uric acid and ascorbic acid: A voltammetric method.Colloids and Surfaces B: Biointerfaces 2013; 106: 145 – 150
- Ensafi AA, Taei M, Khayamian T. A differential pulse voltammetric method for simultanous determination of ascorbic acid, dopamine, and uric acid using poly (3-(5-chloro-2-hydroxyphenylazo)-4,5-dihydroxynaphtalen-2,7-disulfonic acid) film modified glassy carbon electrode. J. Electroanalytical Chemistry 2009; 633: 212 – 220
- Ribeiro FAS, Tarley CRT, Borges KB, Pereira AC. Development of a square wave voltammetric method for dopamine determination using a biosensor based on multiwall carbon nanotubes paste and crude extract of Curcubita pepo L.Sensors and Actuators B 2013; 185: 743 – 754
- Shankar S S, Swamy B E K, Chandrashekar B N. Electrochemical selective determination of dopamine at TX-100 modified carbon paste electrode: A voltammetric study. J. Molecular Liquids 2012; 168: 80 – 86
- Goyal RN, Singh SP. Simultaneous voltammetric determination of dopamine and adenosine using a single walled carbon nanotube – modified glassy carbon electrode. Carbon 2008; 46: 1556 – 1562
- Beitollahi H, Mohadesi A, Mohammadi S, Pahlavan A, Karimi-Maleh H, Akbari A. New voltammetric strategy for determination of dopamine in the presence of high concentrations of acetaminophen, folic acid and N-acetylcysteine. J. Molecular Liquids 2012; 169: 130 – 135
- Sathisha TV, Swamy BEK, Reddy S, Chandrashekar BN, Eswarappa B. Clay modified carbon paste electrode for the voltammetric detection of dopamine in presence of ascorbic acid. J. Molecular Liquids 2012; 172: 53 – 58
- Shahrokhian S, Zare-Mehrjardi H R. Cobalt salophen-modified carbon-paste electrode incorporating a cationic surfactant for simultaneous voltammetric detecion of ascorbic acid and dopamine. Sensors and Actuators B 2007; 121: 530 – 537
- Thomas T, Mascarenhas RJ, Nethravathi C, Rajamathi M, Swamy BEK. Graphite oxide bulk modified carbon paste electrode for the selective detection of dopamine: A voltammetric study. J. Electroanal. Chem. 2011; 659: 113 – 119
- Nancy TEM, Anithakumary V, Swamy BEK. Solar graphene modified glassy carbon electrode for the voltammetric resolution and detection of dopamine, ascorbic and uric acid. J. Electroanalytical Chemistry 2014; 720 – 721: 107 – 114.
- Kondo T, Niwano Y, Tamura A, Imai J, Honda K, Einaga Y, et al. Enhanced electrochemical response in oxidative differential pulse voltammetry of dopamine in the presence of ascorbic acid at carboxyl-terminated boron-doped diamond electrodes. Electrochimica Acta 2009; 54: 2312 – 2319