Research Article
Austin J Anal Pharm Chem. 2014;1(1): 1005.
Degradation Markers in Nutritional Products a Review
Sochr J, Cinkova K and Svorc L*
Department of Chemical and Food Technology, Slovak University of Technology, Slovak Republic
*Corresponding author: :Svorc L, Institute of Analytical Chemistry, Chemical and Food Technology, Slovak University of Technology in Bratislava, Radlinského 9, 812 37 Bratislava, Slovak republic.
Received: June 29, 2014; Accepted: July 08, 2014; Published: July 08, 2014
Abstract
The main objective of the present review is to develop a brief overview of the current state of the knowledge on the analytical determination of oxidation and/or degradation parameters in nutritional products. Basically, since it is one of the major causes of food quality deterioration, and it has been a challenge for manufacturers and food scientists alike, lipid oxidation has traditionally been the most studied process throughout the history. There are many analytical methods to perform the detection of changes in food quality, focusing on the determination of the formation of oxidation products or on the changes in the standard profile of specific compounds. In addition, sensory analyses to identify oxidative changes in foods are also common. However, it is not the only parameters to measure the oxidation of food. Proteins and carbohydrates are also susceptible to oxidation; and therefore, many undesirable and/or harmful metabolites can also be found in food after this type of degradation that food industry must necessary control. The proposed summarized review will focus on both oxidation processes in order to better understand the evolution throughout the history of food control.
Keywords: Nutritional product; Oxidation; Degradation; Analysis
Introduction
In general, the oxidation of food stuffs is a process that must be avoided. However, the process occurs naturally when food is exposed to air and it is potentiated by heat, light, chemical catalysts or enzymatic processes. The oxidation causes the loss of nutritional value of food and changes the chemical composition. For example, oxidation of fats and oils leads to rancidity and in fruits it can result in the formation of compounds which discolor damaging the product.
The food alterations could deteriorate the quality of the product and also generate undesirable compounds with direct consequences on human health. There are many reports published in the scientific literature about the possible toxicity of certain metabolites generated from lipid oxidation such as cholesterol oxidation products; COPs, commonly known as oxy sterols; OS [1,2]; and phytosterol oxidation products; POPs; which have unhealthy effects at higher concentrations. Cytotoxicity, atherogenesis, mutagenesis, carcinogenesis changes in cellular membrane and inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase, HMG-CoA reductase, activity have been widely described [3-6].
Therefore, it is mandatory to control the oxidation processes using the technology available. Throughout the history, several analytical methods have been proposed to keep under control the oxidation of nutritional products. There are very different techniques that can be applied depending on the final objective and the product under study. The oxidation parameters to measure can be classified in three important groups depending of the sample: lipids, proteins or carbohydrates. Another classification could be done taking into account the technique used to control the oxidation: chemical or sensory methods. Finally, a third classification could be showed comparing two modes: general oxidation or determination of target compounds.
This summary review consists in a globalized approach to know, from an analytical point of view, the evolution in food control in terms of oxidation parameters. In addition, the methods and techniques that are commonly employed today to determine the rate of oxidation of food are presented in more detail.
Lipid Oxidation
Lipids are an important component of food being also used in a large amount of industrial applications. Lipids in food, either naturally occurring or added exogenously are used as nutrient and also provide a heat transfer medium for food processing and render desirable texture and flavour to the products. They are one of the major and essential macronutrients required for growth and maintenance of living organisms. However, overconsumption of lipids, especially certain saturated lipids and trans fats, has been associated with diseases such as obesity, hypertension, cardiovascular disease or cancer [3,7-10], mainly when these lipids are oxidized generating other compounds. Although there are others hypothesis that differ regarding the issue of that fats are the unique responsible of cardiovascular disease [11,12].
Lipid oxidation is an important cause of deterioration in quality of food both during manufacturing and product shelf life, and negatively affects the integrity of biological systems. The oxidative changes cause development of off-flavors, loss of nutrients and bioactives, and even the formation of potentially toxic compounds originating products unsuitable for the human consumption.
The overall mechanism of lipid oxidation consists of three phases: the initiation, with the formation of free radicals; the propagation, with the free-radical chain reactions; and the termination, with the formation of stable non-radical products.
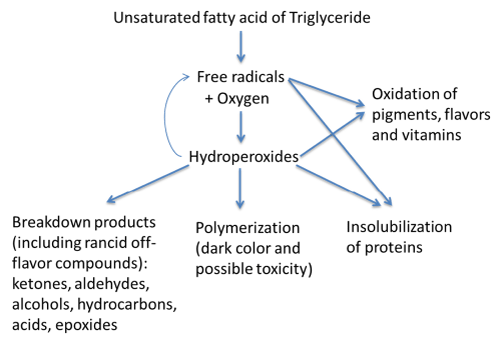
The most important lipids involved in the oxidation process are the unsaturated fatty acid moieties, oleic, linoleic and linolenic. Lipid peroxidation generates a large number of by-products, including breakdown molecules resulting from cleavage of the oxidized fatty acyl chain as is shown in Figure 1. The rate of oxidation of fatty acids increases with the degree of unsaturation. The free radicals reactions are thermodynamically difficult, for this reason the production of the first few radicals mandatory must occur by some catalytic means such as hydro peroxide decomposition, light and heat exposure and metal catalysis. In addition, auto oxidation is the most common process. It is defined as the spontaneous reaction of lipids with atmospheric oxygen through a chain reaction of free radicals (Figure 1).
Figure 1: Lipid oxidation mechanism.
Sensory evaluation
Sensory analysis is a scientific discipline that applies the principles of experimental design and statistical analysis to data obtained from human senses (sight, smell, taste, touch and hear) for the evaluation of consumer products. The discipline requires panels of human assessors, on whom the products are tested, and recording the responses made. By applying statistical techniques to the results it is possible to make inferences and insights about the products under test. Sensory analysis can mainly be classified into three sub-sections: effective testing (dealing with objective facts about products); affective testing (dealing with subjective facts such as preferences); and perception (the biochemical and psychological aspects of sensation).Sensory evaluation is used to study similarities or differences in a wide range of dishes/products; to analyze food samples for improvements; to gauge responses for a dish/product, e.g. acceptable or unacceptable; to explore specific characteristics of an ingredient or dish/food product; to check whether a final dish/food product meets its original specification; and to provide objective and subjective feedback data to enable informed decisions to be made.
Depending on the product, different parameters are studied and specific preparation processes are applied. For example, to measure the quality of solid food, cold cuts parameters such as color intensity, juiciness, hardness, cohesiveness, rancid taste and general acceptability, are studied [13]; in contrast, to evaluate meat it is required to prepare the food with a specific protocol where the temperature or the mode of cooking and serving to the panelists must be indicated. In that case, parameters such as aroma, appearance, color, flavor intensity, saltiness, moistness/juiciness, tenderness, and overall acceptability are evaluated [14,15]. In infant formulas are also evaluated similar parameters such as bitterness, saltiness, savoriness, sourness, sweetness, and pleasantness [16].In all cases an expert panel of at least 6-8 members is selected using a specific point number in the hedonic scale.
Oxygen absorption
In the initial stage of autoxidation a high consumption of oxygen occurs, the fat or oil increases the weight; therefore this event theoretically should reflect its oxidation level. The procedure based on weight gain is simple and cheap equipments are required. An oil sample is heated in a special oven with no air circulation, and periodically testing for weight gain. This method indicates oxygen absorption through mass change. It is one of the oldest methods for evaluating oxidative stability, but nowadays it is still used [17,18]. The method is only useful when highly unsaturated oils, such as marine or vegetable oils containing a high content of polyunsaturated fatty acids, are evaluated.
Oxygen consumption can be also measured directly by monitoring the drop of oxygen pressure. Using a headspace oxygen method, an oil sample is placed in a closed vial that contains certain amount of oxygen at high temperature (100°C). The pressure reduction in the vial, which is due to the oxygen consumption, is monitored and recorded automatically. Then, the oxygen uptake can be calculated [19]. This method is simple and reproducible and could be the best method of analysis to assess the oxidative stability of fats and oils. However, it can be found interferences in products where proteins are present in a substantial ratio since proteins are also oxidized.
Measure compositional changes
Lipid oxidation may also be assessed by measuring the initial quantitative compositional change. In foods that contain fats or oils, unsaturated fatty acids are the main compounds that change during oxidation. Changes in fatty acid composition provide an indirect measure of the extent of lipid oxidation. In the majority of these methods the lipids are extracted, derivatized and measured by gas chromatography with different detectors; GC; [20-23] or GC coupled to mass spectrometry; GC-MS [24,25]. In recent years, ultrahigh performance liquid chromatography; UHPLC; has also widely been used. A recent published method determines the volatile carbonyls in oils by UHPLC coupled to diode array detector; DAD; and GC-MS [26] with dynamic headspace sampling and 2,4-dinitrophenylhydrazine; DNPH; as divartisation agent. An alternative approach to the study of a complete profile is the use of these techniques to determine selective target compounds related with the oxidation of foods. An example is the determination of oxidative markers such as hexanal in vegetable oils by an automated dynamic headspace sampler coupled to GC-MS [27].
Measure of primary compounds of oxidation
The most traditional measurement of lipid oxidation is the Peroxide Value; PV. Peroxides are the main products that appear at the beginning of the autoxidation. They can be measured by techniques based on the generating iodine from potassium iodide, or the oxidation of ferrous ions to ferric ions [18,28]. The content is generally expressed in terms of million equivalents of oxygen per kilogram of fat. Although PV is applicable at the early stages of oxidation, it is highly empirical. The accuracy is questionable, the results vary depending on the procedure used, and the test is very sensitive to changes in the temperature. During the course of the oxidation, PV increases, reaching a maximum, and then decline.
A high number of methods have been developed for determination of PV, among which the iodometric titration, ferric ion complexes measurement spectrophotometry, and IR spectroscopy are most frequently used. Specifically, with Fourier Transform Infrared Spectroscopy; FTIR; the hydro peroxides can be quantitatively determined via measurement of their characteristic O-H stretching absorption band.
Initially, conjugated dienes and trienes were often used to control the level of oxidation. During the formation of hydroperoxides from unsaturated fatty acids conjugated dienes are typically produced, due to the rearrangement of the double bonds. The resulting conjugated dienes exhibit an intense absorption at 234 nm; similarly, trienes absorb at 268 nm. If UV absorption increases then it reflects the formation of primary oxidation products in fats and oils. In addition, there is a good correlation between conjugated dienes and PV.
Measurement of Secondary Compounds of Oxidation
Measurement of malondialdehyde: MDA
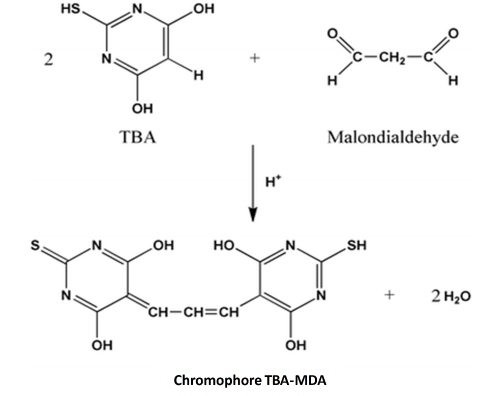
The thiobarbituric acid; TBA; test was proposed over 40 years ago [29,30] and it is one of the most widely used methods to measure the level of lipid peroxidation by determination of MDA in foods containing fats. It is simple and its results are highly correlated with scores obtained in the sensory evaluation [25,31-35]. The basic principle of the method is the reaction of one molecule of MDA and two molecules of TBA to form a red MDA-TBA complex (Figure 2), which can be quantified spectrophotometrically at 530nm (Figure 2).
Figure 2: Reaction of 2-thiobarbituric acid; TBA; and malonaldehyde; MDA.
It is noteworthy that this method has been criticized for several reasons. The method is not specific and selective; and the sensitivity could be further improved, since it has no acceptable limits of detection for measuring MDA [33,36-38]. Other TBA-reactive substances; TBARS, including sugars and other aldehydes, could interfere with the TBA reaction. Abnormally low values may result if MDA reacts with proteins in an oxidizing system [39]. In many cases, the TBA test is recommended for comparing samples of a single material at different states of oxidation. Currently, it is known that TBARS assay is a general method for the detection of lipid peroxidation [40] and not only is measured MDA but also thiobarbituric acid reacts with other compounds to generate different colored species that interfere with the assay. Some studies have identified several compounds using mass spectrometry [41].
An interesting comparison between different methods to measure MDA by TBA derivatisation is presented in a work published in 2001[42]. The authors demonstrated that the quantification of MDA in milk powder samples using three variants of the TBA test, overestimated the MDA content because the high temperature used during the derivatisation step potentiated the oxidation, enhancing the MDA contents or promoting the formation of other by-products that interfere in the spectrophotometric assay. To solve this issue, free MDA has also been determined using direct methods without derivatisation in biological systems [43,44].There are others alternatives to measure MDA by derivatization with 2,4-dinitrophenylhydrazine; DNPH [42,45-47].
p -Anisidine Value; p –AnV
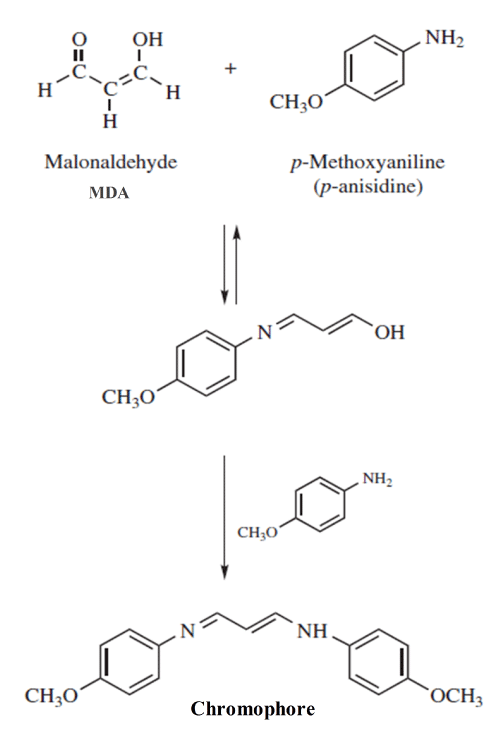
It is a method to measure the content of aldehydes (mainly 2-alkenals and 2,4-alkadienals) generated during the decomposition of hydroperoxides. It is based on the color reaction of p-methoxyaniline and the aldehyde compounds (see Figure 3 as example). The reaction of p-anisidine with aldehydes under acidic conditions provides yellowish products that absorb at 350 nm (Figure 3).
Figure 3: Reaction between p-anisidine and malonaldehyde; MDA.
Measurement of 4-hydroxynonenal; 4-HNE; and 4-hydroxyhexanal; 4-HHE
These compounds are also secondary components of the lipid oxidation. They are both generated in tissue and food from polyunsaturated membrane lipids through a free radical-induced lipid peroxidation process. The biological properties of these aldehydes have been widely studied and some methods have beenproposed with different techniques such as high performance liquid chromatography; HPLC; with ultraviolet detection; UV [48]; TLC densitometry, gas chromatography coupled to mass spectrometry; GC-MS [3,49] or HPLC with mass spectrometry in tandem; MS/MS [10].
Measurement of totox value; TV
This parameter gives a measurement value of the total oxidation, including primary and secondary oxidation products [50-54]. It is a combination of PV and p-AnV: TV = 2PV + p-AnV. This value reflects the oxidation level at early and later stages of oxidation reaction, respectively. With this equation, both hydroperoxides and their breakdown products are estimated and a more adequate result is offered to control the progressive oxidative deterioration of fats and oils. Since two very different values are combined, many authors disagree with this parameter.
Measurement of carbonyls content
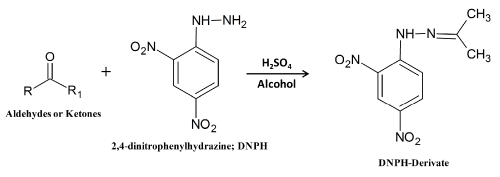
The carbonyl compounds, including ketones and aldehydes, are the secondary oxidation products generated by the degradation of hydroperoxides, and are suggested to be the major responsible for off-flavors associated with the rancidity of food products. The total carbonyl content is measured by a colorimetric method. The carbonyl compounds formed during lipid oxidation are reacted with DNPH followed by the reaction of the resulting hydrazones with alkali (see Figure 4).
Figure 4: Reactions between carbonyls (aldehydes or ketones) and 2,4-dinitrophenylhydrazine; DNPH.
The determination of total content of carbonyls has been frequently used in oxidative stability studies. Improved variants can be found recently [55]. However, this procedure has been criticized because the determination conditions cause degradation of hydroperoxides into carbonyl derivatives, obtaining overestimated results.
Oil stability index; OSI
During lipid oxidation, volatile organic acids such as formic acid and acetic acid are produced at high temperatures together with hydro peroxides. In addition, other secondary products, including alcohols and carbonyl compounds, can be further oxidized to carboxylic acids. The OSI method measures the volatile acids by monitoring the change in electrical conductivity when effluent from oxidizing oils is passed through water. The OSI value is defined as the point of maximal change of the rate of oxidation, attributed to the increase of conductivity by the formation of volatile organic acids. This method shows the important disadvantage of requiring a high level of oxidation, i.e. the method has low sensitivity. There are commercially available equipments such as the Rancimat® (Metrohm Ltd., USA) and the Oxidative Stability Instrument® (Omnion Inc., USA), both are employed for determining the OSI value. Recently, a published article compares the oxidation in oils including OSI, and by several analytical methods above mentioned [56].
Hydrocarbons and fluorescence assay
Formation of saturated hydrocarbons such as ethane, propane and pentane, can be measured for monitoring lipid oxidation. Classically, these compounds have been determined by GC to assess rancidity of fats and oils as well as freeze-dried muscle foods. Usually, significant correlations exist between short-chain saturated hydrocarbons levels and rancid odor scores. Fluorescence assays are also helpful to assess lipid oxidation in muscle foods and biological tissues.
Others techniques
There are others techniques and different variants in the methodologies to measure the lipid oxidation such as Free Radicals, Differential Scanning Calorimetry; DSC, Nuclear Magnetic Resonance Spectroscopy; NMR, etc. In the first case, the free radicals are short-lived intermediates which appear at the initial steps of the oxidation in fats and oils. Those compounds can be measured with different techniques such as Electron Spin Resonance; ESR; that is of great value for the study of the early stages of lipid oxidation and prediction of oxidative stability of fats and oils. In addition, there is a strong linear correlation between ESR and Rancimat® or ESR and oxygen consumption analyses. The second technique, which is based on thermal release of oxidation reactions, has the potential of a nonchemical method for assessing oxidative stability of fats and oils, indicating the onset of advanced oxidation (termination). Finally, NMR is also a very useful technique for this purpose since the results obtained usually correlated adequately with the values obtained with to tox, conjugated diene and TBA assays.
Degradation of Proteins and Carbohydrates
During food processing, the molecular structure of proteins may suffer changes. In addition, chemical reactions such as lipid and protein oxidation, non-enzymatic browning and enzymatic activity are also responsible for color, flavor or odor alterations. As in the case of lipid oxidation, there are a large amount of analytical methods to detect these undesirable processes.
In addition to sensory tests, the protein oxidation is measured in terms of the appearance of carbonyl groups, normally using spectrophotometric techniques. Sodium dodecyl sulfate polyacrylamide gel electrophoresis; SDS-PAGE; and immune blotting are also usually performed [57,58] to study protein degradation (proteolysis) or protein oxidation.
Classically, the food safety procedures for determination of essential amino acids content of mechanically processed products from red meat animals and poultry is based on hydrolysis of a powder prepared by blending samples in acetone-chloroform. Samples are injected into a HPLC using gradient elution on an ion-exchange column for separation and with fluorescence detection. A variant of the elution program allows also the determination of tryptophan. In addition, ß-alanine, 1-methyl-histidine, and 3-methyl-histidine from beef, pork and poultry products are determined to estimate muscle content of products. A colorimetric procedure for assay of hydroxyproline is also used as adjunct method for protein quality estimation [59]. Cysteine as cystic acid and methionine as methionine sulfone are also determined with similar conditions [60]. Currently, new methodologies provided by commercial companies allow determining a complete amino acids profile. The most used are named PicoTag [61] and more recent AcqTag [62] (Waters, Milford MA, USA).
On one hand, the classic methods for determination of protein by determination of nitrogen use Kjeldahl analysis. As example milk casein measurement is based on precipitation of casein at acid pH. Precipitated milk casein is removed by filtration and the nitrogen content of either the precipitate or filtrate is determined by Kjeldahl analysis [63].Furthermore, others methods have been devised to measure protein concentration based on UV-vis spectroscopy and HPLC [64]. Few research groups have used HPLC coupled to mass spectrometer of high resolution such as time of flight; TOF [65].
In the case of carbohydrate, an important number of technologies and methodologies are also found in the literature to control its stability. The Maillard reaction, that generates various compounds with high toxicity, is well known. The study of those compounds is of a great interest. A typical compound to control is acrylamide. It has been demonstrated that acrylamide have neurotoxic and carcinogenic effects. It is widely recognized that acrylamide is mainly formed through the Maillard reaction from free asparagine and reducing sugars. The major sources of dietary acrylamide are potato products, processed cereals and coffee. This compound can be determined by LC-MS/MS after clean-up with solid phase extraction; SPE [66], by GC equipped with a nitrogen-phosphorus detector; NPD; with headspace solid-phase micro extraction; SPME [67] and also by GC-MS/MS [68]. Acrylamide is not the only compound generated during Maillard reaction. In the last years, others carboxylic acid and amides have been found and measured by HPLC-MS, GC-MS, and GC coupled to flame ionization detector; FID; or enzymatically [69].
Additionally, furosine is a marker of the impairment of lysine residues in protein which is generated at the early stage of the Maillard reaction in thermally treated foods. This compound has been determined by HPLC [70]. Highlight gas chromatography-orthogonal acceleration time-of-flight mass spectrometry; GC-oa TOF; is an emerging technique used for quantifying furan ones generated in model Maillard reactions [71]. Finally, some studies based on the use of HPLC-TOF are recently appearing with remarkable results [72- 74].
Conclusion
Food degradation may be controlled in many different ways, from controlling changes in the initial steps with the formation of primary oxidation products, to observe and study the last oxidation stages by measurement of secondary compounds. Sensory analysis, widely used, is able to evaluate the degradation mainly from a subjective point of view. To carry out quantitative evaluations and to control oxidative changes in foods, there are, in the scientific literature, a great amount of more objective methods. Each method shows both advantages and disadvantages, thus it is important to select the most adequate method, depending on the system under investigation and the state of oxidation itself. The use of two or more methods to determine different parameters is highly recommended. The information regarding to evaluate the food degradation in a global way with techniques of total screening using mass spectrometry of high resolution such as TOF is very limited. It is necessary to use these new techniques to expand the global knowledge about those degradation processes.
References
- Guardiola F, Codony R, Addis PB, Rafecas M, Boatella J. Biological effects of oxysterols: current status. Food Chem Toxicol. 1996; 34: 193-211.
- Grootveld M, Silwood CJL, Addis P, Claxson A. Health effects of oxidized heated oils. Foodservice Research International. 2001; 13: 41-55.
- Beckman JK, Howard MJ, Greene HL. Identification of hydroxyalkenals formed from omega-3 fatty acids. Biochem Biophys Res Commun. 1990; 169: 75-80.
- Kanner J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol Nutr Food Res. 2007; 51: 1094-1101.
- Gorelik S, Lapidot T, Shaham I, Granit R, Ligumsky M, Kohen R, et al. Lipid peroxidation and coupled vitamin oxidation in simulated and human gastric fluid inhibited by dietary polyphenols: health implications. J Agric Food Chem. 2005; 53: 3397-3402.
- Naber EC, Allred JB, Winget CJ, Stock AE. Effect of cholesterol oxidation products on cholesterol metabolism in the laying hen. Poult Sci. 1985; 64: 675-680.
- Hurt RT, Kulisek C, Buchanan LA, McClave SA. The obesity epidemic: challenges, health initiatives, and implications for gastroenterologists. Gastroenterol Hepatol (N Y). 2010; 6: 780-792.
- Buttar HS, Li T, Ravi N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol. 2005; 10: 229-249.
- Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L. Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci. 2013; 14: 10497-10538.
- Long EK, Olson DM, Bernlohr DA. High-fat diet induces changes in adipose tissue trans-4-oxo-2-nonenal and trans-4-hydroxy-2-nonenal levels in a depot-specific manner. Free Radic Biol Med. 2013; 63: 390-398.
- Mann J, McLean R, Te Morenga L. Evidence favours an association between saturated fat intake and coronary heart disease. BMJ. 2013; 347: f6851.
- Malhotra A, Shafiq N, Arora A, Singh M, Kumar R, Malhotra S. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst Rev. 2014; 6: CD001918.
- Muguerza E, Ansorena D, Astiasarán I. Improvement of nutritional properties of Chorizo de Pamplona by replacement of pork backfat with soy oil. Meat Sci. 2003; 65: 1361-1367.
- Khiari Z, Omana DA, Pietrasik Z, Betti M. Evaluation of poultry protein isolate as a food ingredient: physicochemical properties and sensory characteristics of marinated chicken breasts. J Food Sci. 2013; 78: S1069-1075.
- Hayes JE, Desmond EM, Troy DJ, Buckley DJ, Mehra R. The effect of enhancement with salt, phosphate and milk proteins on the physical and sensory properties of pork loin. Meat Sci. 2006; 72: 380-386.
- Ventura AK, Beauchamp GK, Mennella JA. Infant regulation of intake: the effect of free glutamate content in infant formulas. Am J Clin Nutr. 2012; 95: 875-881.
- Olcott HS, Einset E. A weighing method for measuring the induction period of marine and other oils. Journal of the American Oil Chemistry Society. 1958; 35: 161-162.
- Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002; 127: 183-198.
- Velasco J, Dobarganes C. Oxidative stability of virgin olive oil. European Journal of Lipid Science and Technology. 2002; 104: 661-676.
- Fruhwirth GO, Wenzl T, El-Toukhy R, Wagner FS, Hermetter A. Fluorescence screening of antioxidant capacity in pumpkin seed oils and other natural oils. European Journal of Lipid Science and Technology. 2003; 105: 266-274.
- Torkamani AE, Juliano P, Ajlouni S, Singh TK. Impact of ultrasound treatment on lipid oxidation of Cheddar cheese whey. Ultrason Sonochem. 2014; 21: 951-957.
- Olivares A, Navarro JL, Flores M. Characterization of volatile compounds responsible for the aroma in naturally fermented sausages by gas chromatography-olfactometry. Food Sci Technol Int. 2013.
- Mubiru E, Shrestha K, Papastergiadis A, De Meulenaer B. Improved gas chromatography-flame ionization detector analytical method for the analysis of epoxy fatty acids. J Chromatogr A. 2013; 1318: 217-225.
- Keshvari M, Asgary S, Jafarian-Dehkordi A, Najafi S, Ghoreyshi-Yazdi SM. Preventive effect of cinnamon essential oil on lipid oxidation of vegetable oil. ARYA Atheroscler. 2013; 9: 280-286.
- Lorenzo JM, Bedia M, Bañón S. Relationship between flavour deterioration and the volatile compound profile of semi-ripened sausage. Meat Sci. 2013; 93: 614-620.
- Zhu H, Li X, Shoemaker CF, Wang SC. Ultrahigh performance liquid chromatography analysis of volatile carbonyl compounds in virgin olive oils. J Agric Food Chem. 2013; 61: 12253-12259.
- Ha J, Seo DW, Chen X, Hwang JB, Shim YS. Determination of hexanal as an oxidative marker in vegetable oils using an automated dynamic headspace sampler coupled to a gas chromatograph/mass spectrometer. Anal Sci. 2011; 27: 873-878.
- Ruíz A, Ayora Cañada MJ, Lendl B. A rapid method for peroxide value determination in edible oils based on flow analysis with Fourier transform infrared spectroscopic detection. Analyst. 2001; 126: 242-246.
- Yamazaki S. [Thiobarbituric acid (TBA) method]. Rinsho Byori. 1967; 15: 616-619.
- Serafini-Cessi F, Cessi C. Thiobarbituric acid test as an index of phospholipid peroxidation. Sperimentale. 1968; 118: 371-378.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351-358.
- Martysiak-Zurowska D, Stolyhwo A. Content of Malondialdehyde (MDA) in infant formulae and follow-on formulae. Pol J Food Nutr Sci. 2006; 15: 323-328.
- Cesa S. Malondialdehyde contents in infant milk formulas. J Agric Food Chem. 2004; 52: 2119-2122.
- Kolakowska A, Deutry J. Some comments on the usefulness of 2-thiobarbituric acid (TBA) test for the evaluation of rancidity in frozen fish. Nahrung. 1983; 27: 513-518.
- Squires EJ, Valdes EV, Wu J, Leeson S. Research note: utility of the thiobarbituric acid test in the determination of the quality of fats and oils in feeds. Poult Sci. 1991; 70: 180-183.
- Papastergiadis A, Mubiru E, Van Langenhove H, De Meulenaer B. Malondialdehyde measurement in oxidized foods: evaluation of the spectrophotometric thiobarbituric acid reactive substances (TBARS) test in various foods. J Agric Food Chem. 2012; 60: 9589-9594.
- Ohya T. Reactivity of alkanals towards malondialdehyde (MDA) and the effect of alkanals on MDA determination with a thiobarbituric acid test. Biol Pharm Bull. 1993; 16: 1078-1082.
- Gutteridge JM, Quinlan GJ. Malondialdehyde formation from lipid peroxides in the thiobarbituric acid test: the role of lipid radicals, iron salts, and metal chelators. J Appl Biochem. 1983; 5: 293-299.
- Jiang ZY, Woollard AC, Wolff SP. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange. Comparison with the TBA assay and an iodometric method. Lipids. 1991; 26: 853-856.
- Lee YJ, Yoon WB. Effects of particle size and heating time on thiobarbituric acid (TBA) test of soybean powder. Food Chem. 2013; 138: 841-850.
- Jardine D, Antolovich M, Prenzler PD, Robards K. Liquid chromatography-mass spectrometry (LC-MS) investigation of the thiobarbituric acid reactive substances (TBARS) reaction. J Agric Food Chem. 2002; 50: 1720-1724.
- Fenaille F, Mottier P, Turesky RJ, Ali S, Guy PA. Comparison of analytical techniques to quantify malondialdehyde in milk powders. J Chromatogr A. 2001; 921: 237-245.
- Bull AW, Marnett LJ. Determination of malondialdehyde by ion-pairing high-performance liquid chromatography. Anal Biochem. 1985; 149: 284-290.
- Csallany AS, Der Guan M, Manwaring JD, Addis PB. Free malonaldehyde determination in tissues by high-performance liquid chromatography. Anal Biochem. 1984; 142: 277-283.
- Sim AS, Salonikas C, Naidoo D, Wilcken DE. Improved method for plasma malondialdehyde measurement by high-performance liquid chromatography using methyl malondialdehyde as an internal standard. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 785: 337-344.
- Czauderna M, Kowalczyk J, Marounek M. The simple and sensitive measurement of malondialdehyde in selected specimens of biological origin and some feed by reversed phase high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879: 2251-2258.
- Faizan M, Esatbeyoglu T, Bayram B, Rimbach G. A fast and validated method for the determination of malondialdehyde in fish liver using high-performance liquid chromatography with a photodiode array detector. J Food Sci. 2014; 79: C484-488.
- Lang J, Celotto C, Esterbauer H. Quantitative determination of the lipid peroxidation product 4-hydroxynonenal by high-performance liquid chromatography. Anal Biochem. 1985; 150: 369-378.
- Michalski MC, Calzada C, Makino A, Michaud S, Guichardant M. Oxidation products of polyunsaturated fatty acids in infant formulas compared to human milk--a preliminary study. Mol Nutr Food Res. 2008; 52: 1478-1485.
- Ali MA, Nouruddeen ZB, Muhamad II, Latip RA, Othman NH. Effect of microwave heating on the quality characteristics of canola oil in presence of palm olein. Acta Sci Pol Technol Aliment. 2013; 12: 241-252.
- Hashemi MB, Niakousari M, Saharkhiz MJ, Eskandari MH. Stabilization of sunflower oil with Carum copticum Benth & Hook essential oil. J Food Sci Technol. 2014; 51: 142-147.
- Poiana MA. Enhancing Oxidative Stability of Sunflower Oil during Convective and Microwave Heating Using Grape Seed Extract. Int J Mol Sci. 2012; 13: 9240-9259.
- Chiavaro E, Rodriguez-Estrada MT, Bendini A, Rinaldi M, Cerretani L. Differential scanning calorimetry thermal properties and oxidative stability indices of microwave heated extra virgin olive oils. J Sci Food Agric. 2011; 91:198-206.
- Poulli KI, Chantzos NV, Mousdis GA, Georgiou CA. Synchronous fluorescence spectroscopy: tool for monitoring thermally stressed edible oils. J Agric Food Chem. 2009; 57: 8194-8201.
- Endo Y, Li C, Tagiri-Endo M, Fujimoto K. A modified method for the estimation of total carbonyl compounds in heated and frying oils using 2-propanol as a solvent. Journal of the American Oil Chemists' Society. 2001; 78: 1021-1024.
- Liu P, Kerr BJ, Chen C, Weber TE, Johnston LJ, Shurson GC. Methods to create thermally oxidized lipids and comparison of analytical procedures to characterize peroxidation. J Anim Sci. 2014; 92: 2950-2959.
- Andersen E, Andersen ML, Baron CP. Characterization of oxidative changes in salted herring (Clupea harengus) during ripening. J Agric Food Chem. 2007; 55: 9545-9553.
- Andersen E, Andersen ML, Baron CP. Characterization of oxidative changes in salted herring (Clupea harengus) during ripening. J Agric Food Chem. 2007; 55: 9545-9553.
- Ashworth RB. Amino acid analysis for meat protein evaluation. J Assoc Off Anal Chem. 1987; 70: 80-85.
- MacDonald JL, Krueger MW, Keller JH. Oxidation and hydrolysis determination of sulfur amino acids in food and feed ingredients: collaborative study. J Assoc Off Anal Chem. 1985; 68: 826-829.
- Bazzichi L, Palego L, Giannaccini G, Rossi A, De Feo F, Giacomelli C, et al. Altered amino acid homeostasis in subjects affected by fibromyalgia. Clin Biochem. 2009; 42: 1064-1070.
- Mayer HK, Fiechter G. Application of UHPLC for the determination of free amino acids in different cheese varieties. Anal Bioanal Chem. 2013; 405: 8053-8061.
- Lynch JM, Barbano DM, Fleming JR. Indirect and direct determination of the casein content of milk by Kjeldahl nitrogen analysis: collaborative study. J AOAC Int. 1998; 81: 763-774.
- Pierri G, Kotoni D, Simone P, Villani C, Pepe G, Campiglia P, et al. Analysis of bovine milk caseins on organic monolithic columns: an integrated capillary liquid chromatography-high resolution mass spectrometry approach for the study of time-dependent casein degradation. J Chromatogr A. 2013; 1313: 259-269.
- Lai P, Okazawa A, Izumi Y, Bamba T, Fukusaki E, Yoshikawa M, et al. Gallic acid oxidizes Met residues in peptides released from bovine β-lactoglobulin by in vitro digestion. J Biosci Bioeng. 2012; 114: 297-305.
- Gielecinska I, Mojska H. Optimisation and validation of the analytical procedure for the determination of acrylamide in coffee by LC-MS/MS with SPE clean up. Rocz Panstw Zakl Hig. 2013; 64: 85-90.
- El-Ghorab AH, Fujioka K, Shibamoto T. Determination of acrylamide formed in asparagine/D-glucose maillard model systems by using gas chromatography with headspace solid-phase microextraction. J AOAC Int. 2006; 89: 149-153.
- Mojska H, Gielecinska I, Stos K. Determination of acrylamide level in commercial baby foods and an assessment of infant dietary exposure. Food Chem Toxicol. 2012; 50: 2722-2728.
- Smuda M, Voigt M, Glomb MA. Degradation of 1-deoxy-D-erythro-hexo-2,3-diulose in the presence of lysine leads to formation of carboxylic acid amides. J Agric Food Chem. 2010; 58: 6458-6464.
- Gökmen V, Serpen A, Morales FJ. Determination of furosine in thermally processed foods by hydrophilic interaction liquid chromatography. J AOAC Int. 2009; 92: 1460-1463.
- Fay LB, Newton A, Simian H, Robert F, Douce D, Hancock P, et al. Potential of gas chromatography-orthogonal acceleration time-of-flight mass spectrometry (GC-oaTOFMS) in flavor research. J Agric Food Chem. 2003; 51: 2708-2713.
- Kunert C, Walker A, Hofmann T. Taste modulating N-(1-methyl-4-oxoimidazolidin-2-ylidene) α-amino acids formed from creatinine and reducing carbohydrates. J Agric Food Chem. 2011; 59: 8366-8374.
- Shrestha K, De Meulenaer B. Antioxidant activity of Maillard type reaction products between phosphatidylethanolamine and glucose. Food Chem. 2014; 161: 8-15.
- Jiang D, Peterson DG. Identification of bitter compounds in whole wheat bread. Food Chem. 2013; 141: 1345-1353.