Review Article
Austin J Anal Pharm Chem. 2014;1(3): 1011.
Development of Hemosomal Drug Delivery System
Nagi F Idris1, Yallappamaharaj R Hundekar2*, Ramchandra N Chilkawar3, Basavaraj K Nanjwade4, Meghana S Kamble5 and Teerapol Srichana6
1Department of Pharmacology, Omer Al-Mukhtar University, Libya
2Department of Quality Assurance, Elegant Drugs Pvt. Ltd., India
3Department of Pharmaceutics, KLE College of Pharmacy, India
4Department of Pharmaceutics, Omer Al-Mukhtar University, Libya
5Department of Pharmaceutics, P.E. Society’s Modern College of Pharmacy, India
6Drug Delivery System Excellence Centre, Prince of Songkla University, Thailand
*Corresponding author: :Yallappamaharaj R Hundekar, Department of Quality Assurance, Elegant Drugs Pvt. Ltd., Chalmatti-581204, Karnataka, India
Received: July 23, 2014; Accepted: August 11, 2014; Published: August 23, 2014
Abstract
Haem is found in all living cells as well tissues in the form of proteins such as cytochromes, hemoglobin’s and peroxides. This is act as catalyst for the formation of oxygen radicals. Encapsulation of haem or hemoglobin with in lipid vesicles or liposome’s is called “Hemosomes” or it is also called Liposome-encapsulated hemoglobin (LEH). Which are 0.1 to 10 microns in dimension, and it is a technology of preparing artificial red blood substitutes. The synthetic cells are stable being somewhat smaller and stronger than normal red blood cells and are having same electrophoretic movements. Hemoglobin (Hb) encapsulation within a liposome is one of the strategies in the development of artificial oxygen carriers. The review article includes preparation techniques, characterization and stability of Hemosomal drug delivery system.
Keywords: Hemosomes; Hemoglobin encapsulation; Red blood cells; Oxygen carriers; LEH
Introduction
Hemosomes is a part of vesicular drug delivery system. Haem means hemoglobin and some means cell-like, Hemoglobin, also spelled as hemoglobin, iron-containing protein in the red blood cells of many animals. As early as 1957, the author Chang TMS prepared encapsulated hemoglobin to form artificial red blood cells [1]. In the 1950s, the first form of encapsulated hemoglobin was developed but limited technical possibility. Liposome-Encapsulated Hemoglobin (LEH) has been found to be an effective oxygen carrier without any adverse effects of vasoconstriction. The liposome encapsulation appears to increase plasma retention time; however, adverse immune interactions occur with the liposome [2].
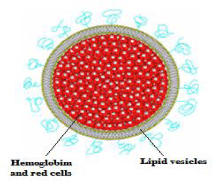
The blood loss occurs in the body due to operational surgical or any other injury. The mostly problems occurs with blood transfusion replacement therapy such as allergic reactions, hemolytic transfusion reactions, embolism, circulatory overload, coagulation disturbances and the blood borne diseases transmission such as AIDS and hepatitis. Actual blood has a short shelf life of 21 days outside the body [3], in emergency situations there is a need of universally transfusable (nonallergenic), oxygen-carrying blood replacement fluid to provide temporary life support until availability of an enough blood supply [4]. In such a situation “Hemosomes” (artificial red blood cells), the vesicular delivery system is useful. This maintains the temporary life till enough blood available to the body (Figure 1).
Figure 1: Hemoglobin Vesicles (Hemoglobin encapsulated, embedded and coated vesicles)
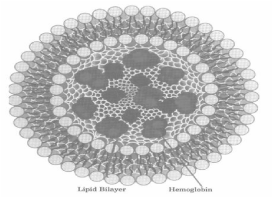
Hemoglobin encapsulated in lipid vesicles has been developed. Due to this the vesicles permeable to glucose and other molecules which are needed to reduce met hemoglobin. That is a type of hemoglobin and is useless for carrying oxygen and delivering it tissues. The earliest types of encapsulated hemoglobin had a short circulation half-life and formed met hemoglobin. The circulation half-life was improved by surface changes using negative surface charges like polyethylene-glycol (PEG). These modifications improved the half-life to over 24 hours. Met hemoglobin formation was reduced by reducing enzymes such as met hemoglobin reductase system. In animal studies, results were mostly successful, but complement activation occurred in rats and pig [5] (Figure 2).
Figure 2: Hemoglobin encapsulated within liposomes.
Hemoglobin vesicles or liposome–encapsulated hemoglobin (LEH) mimics membrane enclosed cellular structure of red blood cells. Compared to free modified hemoglobin preparations, LEH is characterized by spatial isolation of hemoglobin by an oxygen permeable lipid layer that eliminates the free modified toxicity. With that, co–encapsulation of reductants antioxidative enzymes and oxygen–affinity modifiers inside liposome’s is possible to enhance resuscitative capacity of LEH [6]. The lipid bilayer of encapsulated hemoglobin prevents renal clearance, extra vacation and direct contact of hemoglobin with vascular smooth muscle, thus prevent vascular constriction, increasing the in vivo half–life of LEH [7]. Also this membrane reduces interactions between hemoglobin and nitric oxide, adverse effects such as hypertension and histological damage in myocardial lesions, are not induced, as are found for a cellular–type HBOCs (hemoglobin–based oxygen carriers) [8].
The Success and progress in the generation of encapsulated hemoglobin can be improved by following considerations:
- Increasing storage stability and then infusion.
- Effects of lipid decreasing on the reticuloendothelial systems.
- Avoiding peroxidation of lipid.
- Solving the problem of met hemoglobin formation.
- Inclusion of most of functioning red blood cell enzymes [9].
In this work we report synthesis of a simple phospholipid to enhance hemoglobin encapsulation inside liposome’s.
A part of optimization of LEH formulation in this article we present synthesis of novel lipid and LEH formulation development as an oxygen carrier.
The overall goal of our work is the development of safe, efficacious and economically viable oxygen carrying red blood cell substitute composed of hemoglobin solution encapsulated in a liposome.
Hemosomes or LEH have many important advantages
Compared to un encapsulated hemoglobin LEH has many advantages which include the following:
- Minimised nephrotoxicity.
- Metabolism by RES (reticuloendothelial system) of the liver and spleen same as red cells [10].
- Potential to Co–encapsulate Allosteric Modifiers and Antioxidants with Hemoglobin. During manufacture of LEH Allosteric modifiers can be co–encapsulated with the hemoglobin in order to control the oxygen affinity (P50) [11]. Hemoglobin protectants can also be encapsulated in the liposome in order to retain the hemoglobin in the oxy–hemoglobin state [12].
- Decreased Vasoactivity, because physical properties of LEH have closer to red cells, it produces less of a hypertensive response than that observed with cell-free hemoglobin [13]. Recent studies demonstrate that the vasoconstrictor activity of LEH is 60 times less than that of un encapsulated Hb [14].
- Diffusive Properties Closer to Red Cells. The rate of oxygen release from LEH is slower than from cell-free hemoglobin i.e. closer to the rate of release from intact red cells [15]. Rapid oxygen release from un encapsulated Hb may cause hypertension secondary to auto regulation at the level of the arterioles [16].
- Decreased Likelihood of Neurotoxicity, because of the protective lipid encapsulation of the hemoglobin with LEH [17].
The hemosomes or LEH formulation produced by following characteristics
- It is a homogeneous LEH formulation that is approximately 0.25 microns in diameter, unlike the originally described LEH formulation which contained large particles of > 1 micron (~ 30% of the population).
- Due to the addition of albumin to the new formulation and the use of a PEG (poly ethylene glycol) coating now the LEH is a volume expander. Recent research demonstrates that intravascular volume expansion is a very important additional aspect of a resuscitative fluid (i.e. no oxygen transport is possible without adequate intravascular volume) [18].
- By addition of PEG to the LEH formulation has increased the circulation half-life of LEH from 18 hours up to 65 hours [19].
- Prior LEH and other liposome formulations have been reported to cause an acute thrombocytopenic [20]. Coating the surface of LEH with PEG as well as greatly reducing the negative lipid component from 10% to 2% of the formulation has also greatly reduced the thrombocytopenic response in small animals as studied.
Progress has been limited by three major obstacles
We have made considerable progress in all three following fronts.
- Selecting an acceptable microencapsulation material;
- Developing a microencapsulation process that yields the desired size range, but avoids denaturation of Hb; and,
- Encapsulating sufficient Hb while maintaining an acceptable final viscosity [21].
Materials used for LEH preparation
Egg Phosphatidylcholine (EPC), Dimyristoyl Phosphatidylcholine (DMPC) and bovine Brain Phosphatidylserine (BPS; horse heart cytochrome C, the sodium salt of diphosphoglyceric acid and tetra-methylene phenylenediamine; cholesterol and Dicetyl Phosphate (DCP), Inc. Hemoglobin derived from outdated human blood at - 150 mg/ml Cs. All other chemicals were reagent grade and used without additional purification. Polycarbonate membranes were obtained from Nucleopore [22,23].
Techniques for Hemosomes or LEH preparation
Aseptic techniques are followed in the preparation of hemosomes; the widely used techniques are included such as,
- Film hydration followed by sonication,
- Membrane extrusion,
- French press extrusion,
- Micro fluidization or homogenization,
- Detergent dialysis,
- Reverse-phase evaporation, reverse- micelle: and other mechanisms.
- Most recently approach is multiple emulsions [24].
Among above all mainly three methods are used for producing LEH:
- Film hydration,
- Reverse evaporation and
- Double emulsion.
Film hydration
This method used to form multilamellar vesicles, which are then reduced in size by a Micro-fluidizer TM M1 10 (Micro fluidics. Newton, Ma). Most recently modified this process (prior to Micro fluidization) by rotary evaporation (operating under vacuum at room temperature) of as much water as possible from the formed LEH in Hb solution. This results in a somewhat dry Hb/LEH film that deposit on the walls of a round bottom flask. Hb solution is added to rehydrate and re suspend the LEH. Micro fluidization processing at temperatures of about 5°C follows. The LEH so formed are washed at least three times in isotonic phosphate buffered saline(PBS) and centrifuged at 20,000 X g for 20mins to remove all un encapsulated Hb solution and any remaining organic solvent (if any). The washed liposome’s are then re suspended in isotonic PBS containing 7.5 g% egg albumin [25,26].
Reverse-phase evaporation
In this technique, sonication is not used as it damages the hemoglobin. The approach used here in involves formation of an emulsion of aqueous concentrated Hb solution in an organic solvent mixture of diethyl ether and trichlorotrifluoroethane, which contains in dissolved form the formulated phospholipids/ lipids. Organic solvents are slowly evaporated at room temperature in a rotary evaporator operating under partial vacuum, as the organic solvents are removed. LEH spontaneously form in the excess lipid system. The evaporation procedure is continued until dryness to maximize removal of all organic solvent and water so that the Hb concentration within the LEH is as high as possible. This results in the deposition of an apparently dry Hb/LEH film on the walls of the round bottom flask. Concentrated Hb solution is then added under agitation to rehydrate and re suspend the LEH. Micro fluidization, washing and re suspension procedures used for LEH are the same as described above for film hydration [27,28].
Double emulsion
In this method the formation of a water-in-oil-in- water type multiple emulsion with removal of organic solvent and then size reduction in a Micro fluidizer to form LEH . First a primary emulsion is formed of Hb solution droplets dispersed in an organic solvent blend containing in dissolved form the formulated lipids/ phospholipids. Components and compositions are exactly the same as used above for reverse evaporation. The primary emulsion is then well-dispersed into precursor Hb solution to form the double emulsion. The remaining steps in the process to form LEH that involve organic solvent removal, micro fluidization and washing are similar to those reported above for the reverse evaporation method [29,30].
Method of preparation of LEH
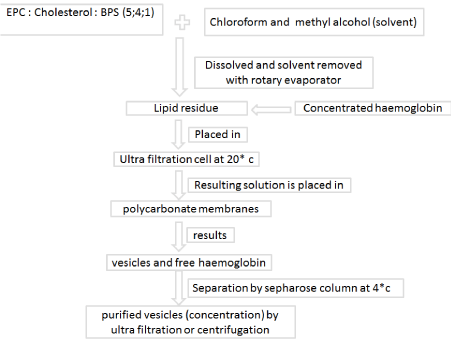
The solvent (chloroform and methyl alcohol) is subjected to remove with a rotary evaporator from 75 pmol of a mixture of EPC, cholesterol and BPS 5: 4: 1 or of DMPC, cholesterol and DCP 5: 4: 1. Concentrated hemoglobin (3 ml) in buffer (100mM NaCl, 20mM HEPES, 1mM EDTA, pH 7.4) is added to the lipid, which is dispersed by overtaxing. The mixture is then placed at 20°C in an ultra-filtration cell (Amicon Model 12) and forced by argon pressure through polycarbonate membranes twice each through membranes with 6000 A, 4000 A and 2000 A pores. The resulting mixture of vesicles and free hemoglobin is separated at 4°C on a column of Sepharose CL-6B (Pharmacia). The purified vesicles may then be concentrated on an, & 100000 cut-off ultra filter (Nucleopore), or by centrifugation at 5000 rev/min for 20 min [31] (Figure 3).
Figure 3: Manufacturing technique of Liposome-Encapsulated Hemoglobin (LEH) preparation.
Evaluation parameters of LEH or Hemosomes
After preparation of hemosomes or LEH was tested for following parameters
- Liposome size,
- Phospholipid concentration,
- Total hemoglobin,
- Percent methemoglobin,
- Oxygen-binding capacity and
- Sterility [32].
Stability studies
Wet and dry storage stability: Wet storage stability will be evaluated for hemosomes or LEH formulations that determined to be effective and safe. Samples of LEH suspended in isotonic/isooncotic PBS-albumin (7.5g %) solution stored at 4°C and -20’C are tested for oxygen-carrying capacity, liposome-encapsulated Hb concentration and functionality (i.e. % oxy-, reduced, and met-). LEH size and Hb leakage (i.e., extra vesicular Hb), using suitable methods [33].
Applications
- Liposome Encapsulated Hemoglobin (LEH) or hemosomes, may be effective in acute brain ischemia, because its size (230 nm) may not only prevent extravasations but also allow oxygen delivery beyond the obstruction with plasma to areas where Red Blood Cells (RBCs) seldom reach, thereby reducing the oxygen diffusion distance [34].
- LEH with a high oxygen affinity accelerates Gastric wound healing in rats [35].
- LEH used as an oxygen carrier for fetal and adult rat liver cell culture [36].
- LEH enhances chemotherapy to suppress metastasis in mice [37].
LEH inhibits tumor necrosis factor release from rabbit alveolar macrophages by a posttranscriptional mechanism [38] (Table 1).
Title of patent
Inventors
Innovation
Patent no.
Reference
Lipid encapsulated hemoglobin cells
Irving F. Miller et al.
Synthetic erythrocytes, Lipid encapsulated hemoglobin cells preferably cholesterol one or more phospholipids are included
US4133874
36
Hemoglobin containing liposomes and process for preparing the same
Suzuki, Kazuhiko et al.
Hemoglobin containing liposomes and process for preparing the same by thin film process comprising liposome forming lipid material in organic solvent
EP 0170247 B1
37
Processes for reconstituting lyophilized erythrocytes and/or hemosomes
Goodrich, Raymond Paul et al.
Processes for the reconstitution of lyophilized red blood cells and/or hemosomes comprising the use of solutions including monosaccharide hexoses and pentoses, and biocomparible amphipathic...
EP0392813 A2
38
Liposomes on which adsorption of proteins is inhibited
Terumo Kabushiki Kaisha et al.
Liposomes on which adsorption of proteins is inhibited which are agglutination-free
0 354 855 B1
39
Storage-stable lipid vesicles and method of preparation
Murray Weiner et al. [44]
Storage-stable lipid vesicles and method of preparationcontaining inositol hexaphosphate
4397846
40
Liposome encapsulated hemoglobin
Kazuhiko Suzuki et al. [45]
The invention relates to hemoglobin-containing liposomes in which an aqueous solution of hemoglobin is incorporated comprising liposome membrane mainly composed of hydrogenated phospholipids
5049391
41
Scaled-up production of liposome-encapsulated hemoglobin
Martha C. Farmer et al.
A method for production of liposome-encapsulated haemoglobin comprising chloroform, HSPC, cholesterol, DMPG, alpha-tocopherol
US4776991
42
Hemoglobin-encapsulated liposome
Ogata, et al.
preparing a hemoglobin-containing solution comprising a concentrated stroma-free hemoglobin solution
5674528
43
Table 1: Patent Technologies in Hemosomes.
Future Perspective
Hemosomes or Liposome Encapsulated Hemoglobin (LEH) have wide range of applications in pharmaceuticals, the problems of blood loss in the body have been occurred due to operational surgery or any other injury, menstrual cycle in women’s, blood transfusion replacement therapy etc. In such cases these hemosomes or LEH maintains the temporary life till enough blood available to the body, also these are useful in acute brain ischemia. LEH with a high oxygen affinity accelerates gastric wound healing in rats. Such problems are common so next in few years this type of Hemosomal drug delivery systems have very valuable role, so the research also going on for developing such delivery system with various drug components, peptides, proteins etc.
Conclusion
The forgoing review shows the aspects related with the vesicular system i.e. hemosomes or LEH for delivery of oxygen accurately that are safe for use as a red blood cell substitute without any adverse effects of vasoconstriction by addition of PEG to the LEH formulation has increased the circulation half-life of LEH by slowly releasing of hemoglobin. The vesicular delivery systems still play an important role in the selective targeting and controlled delivery of various drugs. Researcher are implementing their efforts in improving the design of vesicular system by making them steady in nature, in order to prevent leaching of contents, oxidation and their uptake by natural defense mechanism. As their flexibility in design possess a wide range of potential, its application must be explored throughout the world by encouraging the participation of researcher in the field of vesicular drug delivery system.
References
- Chang TMS. Hemoglobin Corpuscles. J Biomaterials Artificial Cells Artificial Organs. 1988; 16: 1–9.
- Chang TM. Future generations of red blood cell substitutes. J Intern Med. 2003; 253: 527-535.
- HuestisW, Bove JR, Busch S. Practical Blood Transfusion. 2nd edn. Little Brown, Boston. 1976.
- Gaber BP, Yager P, Sheridan JP, Chang EL. Encapsulation of hemoglobin in phospholipid vesicles. FEBS Lett. 1983; 153: 285-288.
- Pierre LaFolie. “If this really works all the way, then mankind will have taken a big step forward. This is like landing on the moon."
- Agashe H, Lagisetty P, Awasthi S, Awasthi V. Improved formulation of liposome-encapsulated hemoglobin with an anionic non-phospholipid. Colloids Surf B Biointerfaces. 2010; 75: 573-583.
- Kawaguchi AT, Haida M, Yamano M, Fukumoto D, Ogata Y, Tsukada H. Liposome-Encapsulated Hemoglobin Ameliorates Ischemic Stroke in Nonhuman Primates: An Acute Study. The J. of pharmacology and experimental therapeutics. 2010; 332: 429–436.
- Taguchi K, Iwao Y, Watanabe H, Kadowaki D, Sakai H, Kobayashi K, et al. Repeated injection of high doses of hemoglobin-encapsulated liposomes (hemoglobin vesicles) induces accelerated blood clearance in a hemorrhagic shock rat model. Drug Metab Dispos. 2011; 39: 484–489.
- Chang TM. Future generations of red blood cell substitutes. J Intern Med. 2003; 253: 527-535.
- Rudolph AS, Spielberg H, Spargo BJ, Kossovsky N. Histopathologic study following administration of liposome-encapsulated hemoglobin in the normovolemic rat. J Biomed Mater Res. 1995; 29: 189-196.
- Farmer MC, Johnson SA, Beissinger RL, Gossage JL, Lynn AB, Carter KA, et al. Liposome-encapsulated hemoglobin: a synthetic red cell. Adv Exp Med Biol. 1988; 238: 161-170.
- Sakai H, Sato A, Masuda K, Takeoka S, Tsuchida E. Encapsulation of concentrated hemoglobin solution in phospholipid vesicles retards the reaction with NO, but not CO, by intracellular diffusion barrier. The J. of biological chemistry. 2008; 283: 1508–1517.
- Rudolph AS, Cliff R, Kwasiborski V, Neville L, Abdullah F, Rabinovici R. Liposome-encapsulated hemoglobin modulates lipopolysaccharide-induced tumor necrosis factor-alpha production in mice. Crit Care Med. 1997; 25: 460-468.
- Rudolph AS, Sulpizio A, Hieble P, MacDonald V, Chavez M, Feuerstein G, et al. Liposome encapsulation attenuates hemoglobin-induced vasoconstriction in rabbit arterial segments. J Appl Physiol. 1997; 82: 1826-1835.
- Sakai H, Masada Y, Horinouchi H, Ikeda E, Sou K, Takeoka S, et al. Physiological capacity of the reticuloendothelial system for the degradation of hemoglobin vesicles (artificial oxygen carriers) after massive intravenous doses by daily repeated infusions for 14 days. J Pharmacol Exp Ther. 2004; 311: 874–884.
- Winslow RM. Current status of blood substitute research: towards a new paradigm. J Intern Med. 2003; 253: 508-517.
- Rogers B, Yakopson V, Teng ZP, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Radic Biol Med. 2003; 35: 872-881.
- Taguchia K, Miyasatoa M, Ujihiraa H, Sakaic H, Otagiri M. Hepatically-metabolized and -excreted artificial oxygen carrier, hemoglobin vesicles, can be safely used under conditions of hepatic impairment. Toxicology and Applied Pharmacology. 2010; 248: 234–241.
- Phillips WT, Klipper RW, Awasthi VD, Rudolpha S, Cliff R, Kwasiborski V, et al. Polyethylene glycol-modified liposome-encapsulated hemoglobin: A long circulating red cell substitute. The J. of pharmacology and experimental therapeutics. 1999; 288: 665–670.
- Awasthi VD, Garcia D, Klipper R, Goins BA, Phillips WT. Neutral and anionic liposome-encapsulated hemoglobin: effect of postinsertedpoly (ethylene glycol)-distearoylphosphatidylethanolamine on distribution and circulation kinetics. The J. of pharmacology and experimental therapeutics. 2004; 309: 241–248.
- Hunt CA, Burnette RR. Lipid Microencapsulation of Hemoglobin. Springer link. 1985; 147-149.
- Bartlett. Phosphorus Assay in Column Chromatography. Biol. Chem. 1959; 234: 466-468.
- Van Assendelft OW. Spectrophotometry of Hemoglobin Derivatives. Royal Vangorum Ltd. The Netherlands. 1970.
- Beissinger RL. Grossweiner L. Development of synthetic blood substitute utilizing hemoglobin vesicles. 1984.
- Beissinger RL, Farmer MC, Gossage JL. Liposome-encapsulated hemoglobin as a red cell surrogate. Preparation scale-up. Trans. Am. Soc. Art. Int. Organs. 1986; 32: 58-63.
- Vidal-Naquet A, Gossage JL, Sullivan TP, Haynes JW, Gilruth BH, Beissinger RL, et al. Liposome-encapsulated hemoglobin as an artificial red blood cell: characterization and scale-up. Biomater Artif Cells Artif Organs. 1989; 17: 531-552.
- Hunt CA, Burnette RR, MacGregor RD, Strubbe AE, Lau DT, Taylor N, et al. Synthesis and evaluation of a prototypal artificial red cell. Science. 1985; 230: 1165-1168.
- Szoka F Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978; 75: 4194-4198.
- Matsumoto SY, Kita, Yonezawa DJ. Colloid Interface Sci. 1976; 57: 353.
- Zheng S, Zheng Y, Beissinger RL, Fresco R. Liposome-encapsulated hemoglobin processing methods. Biomater Artif Cells Immobilization Biotechnol. 1992; 20: 355-364.
- Gaber BP, Yager P, Sheridan JP, Chang EL. Encapsulation of hemoglobin in phospholipid vesicles. FEBS Lett. 1983; 153: 285-288.
- Cliff RO, Kwasiborski V, Rudolph AS. A comparative study of the accurate measurement of endotoxin in liposome-encapsulated hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 1995; 23: 331-336.
- Okamoto Y, Kawaguchi A, Kise Y, Tanaka M, Ogoshi K, Makuuchi H, et al. Liposome-encapsulated hemoglobin accelerates gastric wound healing in the rat. Tokai J Exp Clin Med. 2009; 34: 99-105.
- Chang TM. Future generations of red blood cell substitutes. J Intern Med. 2003; 253: 527-535.
- Kawaguchi AT, Fukumoto D, Haida M, Ogata Y, Yamano M, Tsukada H, et al. Liposome-encapsulated hemoglobin reduces the size of cerebral infarction in the rat: evaluation with photochemically induced thrombosis of the middle cerebral artery. Stroke. 2007; 38: 1626-1632.
- Montagne K, Huang H, Ohara K, Matsumoto K, Mizuno A, Ohta K, et al. Use of liposome encapsulated hemoglobin as an oxygen carrier for fetal and adult rat liver cell culture. J Biosci Bioeng. 2011; 112: 485-490.
- Murayama C, Kawaguchi AT, Kamijo A, Naito K, Iwao K, Tsukamoto H, et al. Liposome-Encapsulated Hemoglobin Enhances Chemotherapy to Suppress Metastasis in Mice. Artif Organs. 2014.
- Langdale LA, Maier RV, Wilson L, Pohlman TH, Williams JG, Rice CL, et al. Liposome-encapsulated hemoglobin inhibits tumor necrosis factor release from rabbit alveolar macrophages by a posttranscriptional mechanism. J Leukoc Biol. 1992; 52: 679-686.
- Beissinger RL, Grossweiner L. Developmant of synthetic blood substitute utilizing hemoglobin vesicles. 1984.
- Miller IF. Lipid encapsulated hemoglobin cells. 1979.
- Szebeni J. Encapsulation of Hemoglobin in Phospholipid Liposomes: Characterization and Stability. Biochemistry J. 1984; 220: 685-692.
- Goodrich RP. Processes for reconstituting lyophilized erythrocytes and/or hemosomes. 1990.
- Yoshoda H, Terumo KK. Liposomes on which adsorption of proteins is inhibited. 1994.
- Weiner M. Storage-stable lipid vesicles and method of preparation. 1983.
- Suzuki K, Sakaguchi K. Liposome encapsulated hemoglobin. 1991.