Research Article
Austin J Anal Pharm Chem. 2014;1(4): 1020.
Development of an In Vitro Dissolution Method for Novel Formulation: A Systematic and Scientific Approach
Harshal A Pawar1* and K G Lalitha2
1Research Scholar, Ultra College of Pharmacy, Madurai-625020, India
2Professor and Head, Department of Pharmaceutical Chemistry, Ultra College of Pharmacy, Madurai- 625020, India
*Corresponding author: :Harshal A Pawar, Research Scholar, Ultra College of Pharmacy, 4/235, College Road,Thasildar Nagar, Madurai-625020, Tamil Nadu, India.
Received: October 21, 2014; Accepted: October 30, 2014; Published: November 01, 2014
Abstract
The development of a discriminatory dissolution method for novel drug formulations has been a challenge to the pharmaceutical industry. Most of the times official dissolution method is not available for newly developed dosage forms. Parameters such as saturation solubility in different pH medium, dissolution behavior of formulations, influence of sink conditions, stability, and discriminatory effect of dissolution testing need to be studied for the selection of a proper dissolution medium for novel formulations. The present paper suggests a simple, scientific and systematic approach for development of an in vitro dissolution method for such novel formulations. In-house developed Metoprolol tartrate matrix tablet and Captopril sustained release beads were used as a model formulation. The developed methods were successfully applied for in vitro dissolution study of in-house developed novel formulations.
Keywords: Novel drug; Dissolution method; Captopril; Metoprolol tartrate; Quality Control
Introduction
Dissolution study is very important test for various pharmaceutical dosage forms especially for those administered orally. The incorporation of adjuvant (e.g., diluents, lubricants, and surfactants) into the formulation of a solid oral dosage form can cause significant effects on the dissolution rate of drugs, especially those that are hydrophobic and poorly soluble [1]. Dissolution is an official test routinely used in Quality Control (QC) and Research and Development (R and D) Laboratories for the evaluation of pharmaceutical products. The purpose of in-vitro dissolution studies in QC is to check batch to batch consistency and detection of manufacturing deviation while in R and D the focus is to provide some predictive estimate of the drug release in respect to the in vivo performance of a drug product [2]. In the case of Class 2 drugs in the Biopharmaceutics Classification System (BCS), dissolution may be the rate-limiting step for drug absorption, so suitable dissolution tests can be used to predict differences in bioavailability among different formulations [3]. The choice of dosage form is often of critical importance in establishing a successful product for oral administration of this class of drugs [4].
As a regulatory test, dissolution is used to approve minor changes in formulation, changes in the site of manufacturing and also to assess the scale-up of the bio-batch to the production batch.
The present paper suggests a scientific and systematic approach for development of an in vitro dissolution method for such novel formulations. In-house developed Metoprolol tartrate matrix tablet and Captopril sustained release beads were used as a model formulation.
Materials and Methods
Materials
Metoprolol tartrate and Captopril were obtained as a gift sample from Rubicon Research, Mumbai and Kwality Pharmaceuticals, Amritsar, India, respectively. Metolar-50 (Cipla) tablets were purchased from local market. All the chemicals and reagents used were of analytical grade.
Instrumentation
Dissolution test was performed in an Electro lab dissolution test system (TDT-08L), in accordance to USP Pharmacopoeia general method. A double-beam UV-Vis spectrophotometer (Shimadzu 1800, Japan) with 1.0 cm quartz cells was used for all absorbance measurements.
Formulation used for study
Two different formulations were used to conduct the study. The formulations were prepared in laboratory. The details of the formulation are as mentioned below:
- Metoprolol tartrate matrix tablet: Labeled to contain 50 mg of the Metoprolol tartrate and prepared by wet granulation method using natural polymers. Three batches (Coded as F1, F2 and F3) were prepared with low, medium and high concentration of polymer.
- Captopril sustained release beads: Labeled to contain 50 mg of the Captopril. The beads were prepared by ionic gelation technique using sodium alginate and filled in hard gelatin capsule. Three batches (Coded as F1, F2 and F3) were prepared with low, medium and high concentration of polymer.
Determination of analytical wavelength
- Metoprolol tartrate
- Captopril
A standard stock solution of drug was prepared by dissolving 100 mg of Metoprolol tartrate in pH6.8 phosphate buffer to obtain a solution of 1000μg/mL. From standard stock solution, 1 mL solution was pipette in 100 mL volumetric flask and the volume was made up mL) was scanned between 200 to 400 nm to determine the λ max against pH6.8 phosphate buffer as blank in UV spectrophotometer (Shimadzu 1800).
A standard stock solution of drug was prepared by dissolving 100 mg of Captopril in 0.01N HCl to obtain a solution of 1000μg/ mL. From standard stock solution, 1 mL solution was pipette in 100 mL volumetric flask and the volume was made up to the mark with the same medium. The resulting solution (10μg/mL) was scanned between 200 to 400 nm to determine the λ max against 0.01 N HCl as blank in UV spectrophotometer (Shimadzu 1800).
Preparation of standard calibration curve
- Metoprolol tartrate
- Captopril
Appropriate aliquots were withdrawn from the standard stock solution prepared above into different volumetric flasks and diluted with pH6.8 phosphate buffer to obtain solution of 1, 5, 10, 15, 20, 25 and 30μg/mL. The absorbances of these solutions were taken at 275 nm using pH 6.8 phosphate buffers as blank.
Appropriate aliquots were withdrawn from the standard stock solution prepared above into different volumetric flasks and diluted with 0.01 N HCl to obtain solution of 5, 10, 15, 20,25,50,75 and 100 μg/mL. The absorbance's of these solutions were taken at 205 nm using 0.01NHCl as blank.
Dissolution method development
- For Metoprolol Tartrate Sustained Release Tablets
- For Captopril Sustained Release Beads
In vitro drug release testing of Metoprolol tartrate marketed immediate release tablets (Metolar-50,Cipla) was performed in distilled water and different buffers of pH 1.2(0.1N HCl), 4.5, 6.8, 7.4 as dissolution mediums (500mL) using USP apparatus 2 (paddle) at 50 rpm for 60minutes. The selected dissolution parameters for the study were based on official dissolution method of Metoprolol Extended release tablets USP and the method reported in the literature [5].
In vitro drug release testing of Captopril capsules (50mg,compounded in laboratory) was performed in distilled water and different buffers of pH 2.1 (0.01N HCl), pH 4.5, 6.8, 7.4 as dissolution mediums (900mL) using USP apparatus I (Basket) at 50 rpm for 60minutes. The selected dissolution parameters for the study were based on official dissolution media reported in USP for Captopril Tablets and the method reported in the literature [6].
The dissolution study was performed on in-house developed formulation (F1-F3) using above optimized conditions to test the discriminatory power of the method.
Results and Discussion
Determination of analytical wavelength
- Metoprolol tartrate
- Captopril
- Standard curve of Metoprolol tartrate in pH 6.8 phosphate buffer
- Standard curve of Captopril in 0.01 N HCl
- Dissolution method development for Metoprolol tartrate sustained release tablets
- Dissolution method development for Captopril sustained release beads
- Aulton, M. E. Pharmaceutics: The Science of Dosage Form Design, 2nd ed.; Churchill Livingstone: New York, 2001.
- Azarmi S, Roa W, Löbenberg R . Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007; 328: 12-21.
- Dissolution Testing of Immediate Release Solid Oral Dosage Forms; Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), U.S. Government Printing Office: Washington, DC, 1997.
- Pouton CW . Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur J Pharm Sci. 2006; 29: 278-287.
- Nellore RV, Singh RG, Hussain AS, Tillman LG, Augsburger LL. Development of metoprolol tartrate extended-release matrix tablet formulations for regulatory policy consideration. Journal of controlled release 1998; 50, 247-256.
- Azevedo RDCP, Ribeiro GP, and Araújo MBD. Development and validation of dissolution test for captopril in capsules by HPLC. Revista Brasileira de Ciências Farmacêuticas 2008; 44: 261-269.
- Lunch JR, Metoprolol tartrate. In K. Florey(ed) Analytical profiles of drug substances, Volume 12, Academic Press, New York, USA;1983:326-354.
- Gollapudi R, Harika JRR Tadikonda, Vanaja A. Formulation and in vitro evaluation of sustained release matrix tablets of Losartan potassium. Int J Ad Pharm Sci 2011: 2: 31-36.
- Singh S, Narandra M, Shaikh A. Preformulation studies of Captopril for Novel oral drug system, IJAPR, 3, 2012; 1096-1099.
- US Pharmacopeial Forum, Pharmacopoeial Previews, Vol. 30, 2004, 351-363.
- US Pharmacopoeia NF-25 <1092> The Dissolution Procedure: Development and Validation (32nd ed.) US Pharmacopoeial Convention, Rockville, MD, 2009.
- C.K. Brown, H.P. Chokshi, B. Nickerson, R.A. Reed, B.R. Rohrs, P.A. Shah, Acceptable analytical practices for dissolution testing of poorly soluble compounds Pharm. Technol. 28 (2004) 56-65.
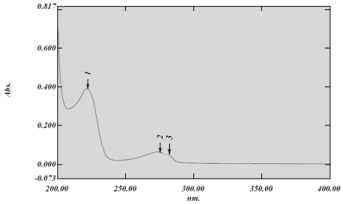
The UV- spectrum of Metoprolol tartrate in 0.01 N HCl is represented in Figure 1. The λ maximal's were found to be at about 222 nm, 275nm and 281nm which were similar to the values reported in literature [7, 8].
Figure 1: UV-Spectrum of Metoprolol tartrate.
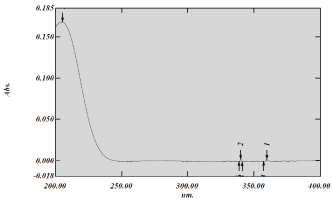
The UV- spectrum of Captopril in 0.01 N HCl is represented in Figure 2. The λ max was found to be about 205 nm which was similar to the value reported in literature [9].
Figure 2: UV-Spectrum of Captopril.
Standard calibration curve
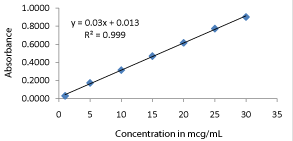
The concentration range of 1-30 μg/ml of Metoprolol tartrate was selected for development of standard curve in pH 6.8 phosphate buffer. The standard curve is constructed by plotting absorbance against concentration (Figure 3). The value of R2 was found to be 0.9991 indicating linear relationship of drug concentration and absorbance in the selected range. The regression equation obtained for the straight line was y = 0.03x + 0.0137; where 'y' is the absorbance and 'x' is concentration.
Figure 3: Calibration curve of MT in pH 6.8 buffer.
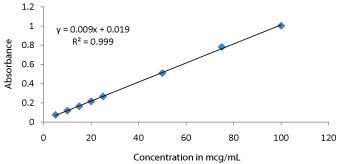
The concentration range of 5-100 μg/ml of Captopril was selected for development of standard curve in 0.01 N HCl. The standard curve is constructed by plotting absorbance against concentration (Figure 4). The value of R2 was found to be 0.9994 indicating linear relationship of drug concentration and absorbance in the selected range. The regression equation obtained for the straight line was y = 0.0099x + 0.0191; where 'y' is the absorbance and 'x' is concentration.
Figure 4: Calibration curve of Captopril in 0.01 N HCl.
In-Vitro dissolution method development
The results of the dissolution studies of the 50 mg Metoprolol tartrate marketed immediate release tablets with water and different buffers are given in Tables 1. The results indicated that less than 80 % of the drug released in 60minutes in the pH 1.2 and 4.5 buffer solution, whereas in buffer of pH 6.8 and water the dissolution is more than 80%. A less significant increase was observed between pH 6.8 and water. The dissolution is more than 90 % in pH 6.8 buffer and water. The dissolution is, however, optimal in pH 6.8 buffer at rpm 50 and this pH was, therefore, selected for further studies.
S. No.
Medium
Parameters
Time (min)
% Dissolved*
1
Water
50rpm, Paddle
60
95.66±0.42
2
pH 1.2 buffer
50rpm, Paddle
60
73.89±0.61
3
pH 4.5 buffer
50rpm, Paddle
60
78.72±0.29
4
pH 6.8 buffer
50rpm, Paddle
60
97.94±0.52
5
pH 7.4 buffer
50rpm, Paddle
60
93.14±0.43
Table 1: Dissolution results for conventional Metoprolol tartrate tablet (Metolar-50, Cipla, India) - Single time point.
The results of the dissolution studies of the 50 mg Captopril Compounded capsule with water and different buffers are given in Tables 2. The results indicated that less than 90 % of the drug released in 60 minutes in the water, pH 4.5, 6.8 and 7.4 buffer solutions. The dissolution is, however, maximum in 0.01N HCl at rpm 50 and this pH was, therefore, selected for further studies.
S. No.
Medium
Parameters
Time (min)
% Dissolved*
1
Water
50rpm, Basket
60
88.65±0.68
2
pH 2.1 buffer(0.01 N HCl)
50rpm, Basket
60
97.98±0.46
3
pH 4.5 buffer
50rpm, Basket
60
77.56±0.25
4
pH 6.8 buffer
50rpm, Basket
60
69.77±0.61
5
pH 7.4 buffer
50rpm, Basket
60
67.31±0.33
Table 2: Dissolution results for Captopril capsules (Compounded capsules) - Single time point.
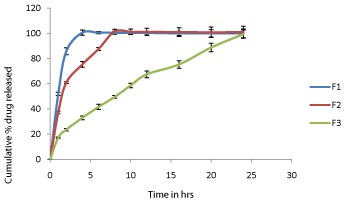
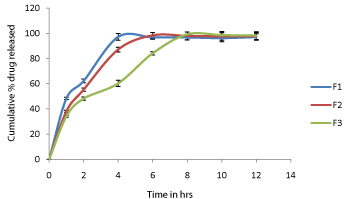
The discriminating power of the dissolution method is the method's ability to detect changes in the drug product [10-12]. The % cumulative drug release of formulation F1 to F3 of Metoprolol tartrate sustained release tablets and Captopril beads are depicted in Figure 5 and Figure 6 respectively. The results are expressed as mean of six observations. Standard error calculated for six observations are represented in Figure 5 and Figure 6 as error bars. Formulation F1 contains low amount of retardant (Polymer), F2 contains medium amount of retardant and F3 contains high amount of retardant. The drug release was decreased from formulation F1 to F3 with increase in concentration of retardant. This indicated that the developed method is discriminatory dissolution method which can able to detect changes in the drug product.
Figure 5: In vitro release of Metoprolol from the matrix tablets (F1-F3).
Figure 6: In vitro drug release profile of Captopril beads (F1 to F3).
Conclusion
This present study explains how to proceed for dissolution study of novel formulations if the dissolution test is not available in official compendium. The procedure consists of several steps like; Use of standard dissolution testing equipment such as the paddle (For tablets) / basket method (For beads) as per general USP guidelines, preliminary method development for the marketed product or compounded immediate release tablets by single point dissolution for 60min., use of the distilled water and different buffers of pH 1.2, 4.5, 6.8, 7.4 as dissolution mediums, collection of samples and their analysis by suitable analytical method to obtain product dissolution profiles, analysis of data to determine the influence of the media on the dissolution (and solubility) of the drug and to ascertain the medium in which greater than 85 % dissolution in a reasonable amount of time (60 minutes) is possible, and dissolution of in house developed tablets using above optimized dissolution conditions. This is simplest scientific and systematic approach for development of an in vitro dissolution method for novel formulation.
References