Research Article
Austin J Anal Pharm Chem. 2014;1(5): 1023.
Hypericum Perforatum L. Protective Effects on Fatand Water-soluble Vitamins after Administration of 7, 12-Dimethylbenz(a)anthracene in Rats
Abdulkadir Levent1*, Gökhan Oto2, Suat Ekin3
1Batman University, Health Services Vocational College, Turkey
2Yüzüncü Yil University, Medical Faculty, Department of Pharmacology, Turkey
3Yüzüncü Yil University, Faculty of Sciences, Department of Biochemistry, Turkey
*Corresponding author: :Abdulkadir Levent, Batman University, Health Services Vocational College, Turkey.
Received: October 22, 2014; Accepted: November 06, 2014; Published: November 11, 2014
Abstract
In this study, we have determinated levels of retinol, retinyl palmitate, β-carotene, a-tocopherol and vitamin C in rat tissues treated with Hypericum Perforatum L. (HP) and 7,12- dimethylbenz[a]anthracene (DMBA) with high-performance liquid chromatography. The present study was designed to evaluate the effect of HP extract on retinol, retinyl palmitate, β-carotene, a-tocopherol and vitamin C in rat tissues administrated DMBA. Results of this study demonstrated that, on the 60th day, there was a significant decrease in levels of vitamins in DMBA-treated group compared to the control group levels. A significant increase in the level of vitamins was observed in the DMBA+HP. -treated group compared to the DMBA treated group.
Keywords: Vitamin; HPLC; Hypericum Perforatum L. 7; 12 dimethylbenz[a] anthracene; Tissue
Introduction
Most tumors either begin the agent production or convert them metabolically into the electrophilic reactants that bind covalently to cellular DNA. For several polycyclic aromatic hydrocarbons (PAHs) such as 7, 12 -dimethylbenz[a] anthracene (DMBA), the ultimate carcinogen is called dihydrodiol epoxide of the bay region that is produced during cellcular metabolism. Thus, free radicals and modified DNA bases have been implied strongly in the carcinogenesis in general [1]. DMBA is one of the PAHs used extensively in experimental studies as a presumptive prototype mutagen, a signal transduction modulator and a known breast and skin carcinogen [2]. In our previous study on serum samplings, the results showed that levels of vitamins decreased in DMBA-treated rat group, but levels of vitamins increased in HP-treated rat group [3].
Hypericum perforatum L. (HP), commonly named as St. John's Wort in Western Europe, is a herbaceous perennial plant of Hypericaceae family widely spread in Europe, Asia, and North Africa; it is also naturalized in North America [4]. Over the last three decades, aggressive analysis of this plant has revealed that it possesses several biological properties, including antidepressant, antiviral and antiproliferative activities [5, 6]. Although HP is the most commonly used plant as an alternative to standard tricyclic antidepressants, other biological properties possessed by this plant have also been utilized for the treatment of cancer and viral infections [7-9]. HP has been used in this study because of its pharmacological properties.
Free radicals are highly reactive molecules that react and damage cells throughout the body. They are suspected of causing cardiovascular diseases, cancers, neurological disorders, cataracts, arthritis, aging and other conditions such as muscle damage and fatigue that could inhibit performance [10]. Antioxidants are molecules, which can safely interact with free radicals, and terminate the chain reaction before vital molecules are damaged. There are several enzyme systems within the body that scavenge free radicals, whereby the principal micronutrient (vitamin) antioxidants are vitamin C, vitamin E and β-carotene [11, 12]. After recording on the cleavage of carotenoids, retinol or vitamin A is the first step of a complex process metabolite. In the diet, they are taken mainly as retinoid carotenoids that are exclusively synthesized by photosynthetic microorganisms and plants. The forms of storage of retinol in liver and adipose tissue are lipophilic esters of retinol, consisting mainly of retinol palmitate [13, 14]. Recently, carotenoids having antioxidant properties and immunity functions have attracted attention of the public and researchers in food and nutrition [15, 16]. Similarly, retinoids with anticarcinogenic and morphologic actions are of major interest. Therefore, it is important to determine, as accurately and simultaneously as possible, the amounts of vitamins absorbed and metabolized in body organs [17].
Our aim is detected the levels of vitamins with HPLC method, and to focus attention to the importance in chemotherapy and its effects on the vitamins in the rats treated with HP and DMBA.
Experimental
Chemicals and reagents
Retinol, retinyl palmitate, β-carotene, α-tocopherol and vitamin C, DMBA, and butylated hydroxyltoluene (BHT) were purchased as standard substances from sigma (St Louis, MO, USA). HPLC-grade methanol, acetonitril, ethanol, chloroform, n-hexane and tetrahydrofuran (THF) solutions were purchased from Merck (Darmstadt, Germany).
Preparation of standard solutions and calibration
Stock solution of retinol, retinyl palmitate, β-carotene and α-tocopherol were prepared at 10 mg mL-1 in chloroform. Stock solution of vitamin C was prepared at 10 mg mL-1 in water. For calibration, solutions of stock were diluted with mobile phase volumetrick flasks in the concentration ranges: 0.02-3 μg mL-1 for retinol, retinyl palmitate, α-tocopherol; 0.03-3 μg mL-1 for β-carotene; and 0.09-4 μg mL-1 for vitamin C. The calibration was accomplished at seven concentrations levels. Calibration curves were calculated by linear regression analysis of the area of the peak versus the concentration in the standard solutions. The slopes, intercepts, correlation coefficient, and related validation parameters such as limit of detection (LOD), limit of quantification (LOQ), slope and intercept were calculated for each vitamin. All solutions were protected from light and manipulated using amber glass vials, and stored at -20 °C.
Instrument and chromatographic conditions
The chromatographic systems consist of an HP Agilent 1100 series with an Agilent series G-1328 DAD detector and an Agilent 1200 series G-1329 ALS auto sampler. The data were handled with Agilent Technologies HP 1100 software. Separation was carried out with a 5μm Inertsil ODS3 reversed phase column (250x4.6 mm ID). Ultra pure water purified by a Milli-Q system from Millipore was used to prepare the solutions. Gradient elution was used at a flow rate 1.5 mL min-1; mobile phase with methanol-THF (70:30, v/v) for 0-8 min and methanol-water (80:20, v/v) for 8-14 min. The chromatographic analysis was carried out at 25 °C. The total time of analysis was 16.0 min. The injection volume was 100 μL. The DAD detection of vitamin C, α-tocopherol, (retinol and retinyl palmitate) and β-carotene were carried out at 270, 290, 325 and 450 nm, respectively. The mobile phase was prepared daily and degassed by ultrasonication before use.
Extraction of Hypericum Perforatum L.
HP was collected from Van, Turkey and dried at room temperature. Dried material (300 mg) was infused in 30 ml of boiled distilled water for 30 min. After decantation and filtration, the filtrate was again dried in an incubator at a temperature of 50 °C. The aqueous extract was then prepared in isotonic physiological solution [18, 19].
Animals' treatment
Animal procedures were approved by the care of the institutional Animals and Use Committee. Sprague-Dawley female rats were placed individually in the standard cages in temperature controlled rooms, 12 h light/dark cycles and maintained with free access to water and a standard laboratory diet.
This study was performed on 36 Sprague-Dawley female rats. Rats were divided into equal three groups. Group 1 was control group (n=12), given olive oil orally (intragastrically). Group 2 (n=12), rats treated with a single dose of DMBA (50mg/kg) in olive oil given orally (intragastrically). Group 3 (n=12), treated with a single dose of DMBA (50mg/kg) in olive oil followed by aqueous extract of HP 100 mg/kg day given orally (intragastrically) for 60 days.
Tissue samples preparation
The rats were anesthetized by inhalation of diethyl ether; and then sacrificed. Tissues were dissected, and put in Petri dishes. Samples were kept frozen at -78 °C until analysis. Tissues were homogenized as gently as possible by hand while tubes were cooled in ice. Tissues were homogenized in Tris-HCl buffer pH 8.0 for 5 min using a homogenizer (Ultra-Turrax T8 IKA-WERKE Gmb&Co. KG Germany) and then centrifuged at 8000 rpm for 15 min. All process was carried out at 4 °C.
Extraction procedure
Samples were thawed at room temperature under plastic sleeve-covered fluorescent lights to minimize sample degradation from exposure to UV light. Retinol, retinyl palmitate, β-carotene and α-tocopherol in tissues were extracted as follows: tissue (100 μL) was deproteinized by adding ethanol (100 μL) (containing 0.025% BHT) and the sample was vortex mixed for 1 min. Since carotenoids and antioxidants are easily oxidized, it is useful to add antioxidants such as BHT to the extraction solvent [20]. The sample was extracted twice with n-hexane (600 μL). The sample was vortex mixed and centrifuged at 12000 rev. min-1 for 10 min. Part (500 μL) of the hexane layer was extracted and evaporated to dry under a stream of nitrogen at 40 °C. The residue was dissolved in THF (25μL) and added methanol (75μL). Vitamin C in tissues was extracted as follows: tissue (100 μL) was precipitated with 60% methanol and 1mM EDTA. The sample was mixed with 400μL tissue of 60% methanol/EDTA, incubated for 10 min at 4 °C before centrifuging at 12000 rev. min-1 [12]. The clear phase was transferred to another tube and evaporated to dry under nitrogen. The dried extracts were dissolved in 100 μL of methanol. Samples of both vitamins were vortex mixed for 1 min. 100 μL was injected via the auto sampler using amber glass vials.
Assay validation
The method was validated according to USP 24 requirements for validation of analytical procedures [21]. Assay validation was involved in the determination of linearity, accuracy, precision, LOD and LOQ. Intra-day and inter-day precision values were estimated at three different concentrations of vitamins three times on the same day to obtain the relative standard deviation (RSD %). Accuracy was determined with recovery study. In this study, the LOD and LOQ were calculated by using following equations [21, 22]: LOD = 3.3s/m; LOQ = 10s/m where s is the standard deviation of the response and m is the slope of the related calibration equation.
Data were presented as means (x̄) ± SEM (standard error of mean). Differences in biochemical parameters were statistically evaluated using one-way analysis of variance (ANOVA) followed by Tukey multiple comparison test.
Results and Discussion
Chromatography method
In the current study, we applied a gradient HPLC method for simultaneous determination of retinol, retinyl palmitate, β-carotene, α-tocopherol and vitamin C in rat tissues [3, 23]. The best mobile phase for the separation of those vitamins were found to be methanol-THF-water: gradient elution was used at a flow rate 1.5 mL min-1; mobile phase with methanol-THF (70:30, v/v) for 0-8 min, methanol-water (80:20, v/v) for 8-14 min. and methanol-THF (70:30, v/v) for 14-16 min. The chromatogram of vitamins in mobile phase and extracted from rat tissue are illustrated in Figure 1, 2, 3 and 4.
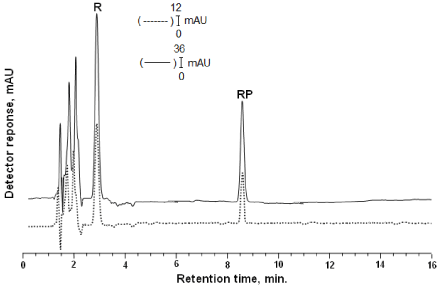
Figure 1: Gradient elution (0-8 min) was performed with methanoltetrahydrofuran (70:30, v/v) as mobile phase at a flow rate of 1.5 mL min-1. Inertsil ODS3 reversed phase column (250x4.6 mm 5 μm) was employed. DAD was adjusted at 325 nm. Chromatogram of vitamins: Solid lines (-) shows that standard of retinol (R) (tR:2.99) and retinyl palmitate(RP) (tR:8.56) in the mobile phase; dashed line (---) shows that retinol and retinyl palmitate the extracted from rat tissue.
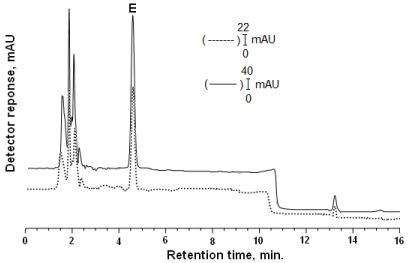
Figure 2: DAD was adjusted at 290 nm. Chromatogram of α-tocopherol (E) (tR:4.63): Solid lines (-) shows that standard of α-tocopherol in the mobile phase; dashed line(---) shows that α-tocopherol the extracted from rat tissue. The gradient elution parameters as indicated in Figure 1.
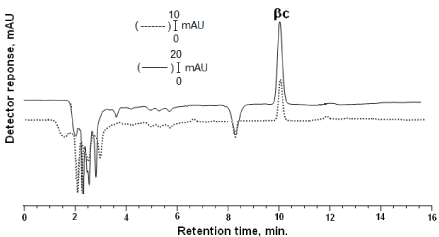
Figure 3: DAD was adjusted at 430 nm. Chromatogram of β-carotene (β-C) (tR:10.03): Solid lines (-) shows that standard of β-carotene: in the mobile phase; dashed line (---) shows that β-carotene the extracted from rat tissue. The gradient elution parameters as indicated in Figure 1.
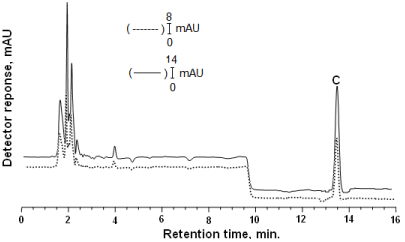
Figure 4: Gradient elution (8-14 min) was performed with methanol-water (80:20, v/v) as mobile phase at a flow rate of 1.5 mL min-1. Inertsil ODS3 reversed phase column (250x4.6 mm 5 μm) was employed. DAD was adjusted at 270 nm. Chromatogram of vitamin C (C) (tR: 13.51): Solid lines (-) shows that standard of vitamin C: in the mobile phase; dashed line (---) shows that vitamin C the extracted from rat tissue.
Linearity of method
Resolution was always good in the linearity range studied. Linearity was checked for each vitamin using seven standard solutions with concentrations ranging from (0.02-3 μg mL-1 for retinol, retinyl palmitate, α-tocopherol; 0.03-3 μg mL-1 for β-carotene; and 0.09- 4 μg mL-1 for vitamin C) the theoretical amounts of vitamins in the studied liquid mixture. Peak areas and analyte concentrations were found to be linearly related to this range for all vitamins (Table 1). Linear regression was used to determine the slope and intercept. The correlation coefficients of the calibration curves were tested for all vitamins.
Vitamins
Retinol
Retinyl palmitate
β-carotene
α-tocopherol
Vitamin C
Days
1
2
3
1
2
3
1
2
3
1
2
3
1
2
3
Slope
198.11
213.81
193.08
193.25
229.67
226.66
64.619
66.066
73.662
42.1
37.891
40.186
153.3
163.03
171.52
Intercept
-0.588
-979
-3.337
-5.243
-13.62
-15.28
-2.959
-1.762
-2.964
-2.548
-1.237
-2.961
-15.90
-12.21
-27.27
Corr. coeff.(r)
0.9992
0.9969
0.9954
0.9922
0.9971
0.9936
0.9815
0.9862
0.9974
0.9974
0.9969
0.9991
0.9981
0.9988
0.9966
Linearity range (μg mL-1)
0.02-3
0.02-3
0.03-3
0.02-3
0.09-4
Mean of slope
201.67
216.53
68.116
40.059
162.62
Mean of intercept
-4.435
-11.38
-2.5615
-2.249
-18.46
Mean of r
0.9985
0.9952
0.9852
0.9958
0.9983
LOD(ng mL-1)
3.96
5.04
7.56
5.93
20.25
LOQ(ng mL-1)
11.99
15.29
22.90
17.97
61.37
Table 1: Statistical data for the calibration graphs of vitamin standards in mobile phase.
Precision of method
The developed method was validated according to the standard procedures. The precision of the method was evaluated by replicate determination of inter-day and intra-day reproducibility. Intra-day repeatability was determined by replicate injection of standard solutions of vitamins (medium concentration) 9 times on the same day. Inter-day reproducibility was determined by analysis of standard solutions on three different days. RSDpercentage was calculated to check the precision of the method. Intra- and inter-day precision data for standard solutions of vitamins were summarized in Table 2. These results indicate that the method developed achievments of a high degree of precision, and reproducibility.
Retinol
(μg mL-1)
Retinyl palmitate (μg mL-1)
β-carotene (μg mL-1)
α-tocopherol (μg mL-1)
Vitamin C
(μg mL-1)
Intra-day
RSD% (n=9)
2.81
2.95
3.04
2.54
3.55
Inter-day
RSD% (n=9)
3.25
3.97
4.23
3.37
5.23
Table 2: Precision of analysis of vitamin standards in mobile phase.
Accuracy of method
The accuracy of the method was tested by measurement of recovery. The known amounts of the vitamins were added to tissue samples and the mixtures were analyzed according to the proposed extraction method. The signal of tissue extracts to which increasing amounts of each vitamin was added, and compared to the signal of the corresponding standards injected directly into the HPLC system. The recovery of each vitamin was determined at medium concentration level and for one sample from each group. The recovery results were calculated using calibration equations. The results were presented in Table 3. Recovery (%) data confirm the accuracy of the method.
Compound
Amount added
(μg mL-1)
Control
Recovery (%)
DMBA
Recovery (%)
DMBA+HP
Recovery (%)
Retinol
0.75
96
93
103
Retinyl palmitate
0.75
95
92
97
β-carotene
1.00
93
90
94
α-tocopherol
0.75
97
94
97
Vitamin C
1.50
104
109
106
Table 3: Recovery of analysis of vitamin in the rat tissue extracted.
Level of vitamins
Average of vitamins in rat tissues of the control DMBA and DMBA+HP groups are shown in Table 4. In statistical analysis, in the liver samples, the levels of retinol (μg mL-1) between control (3.43 0.07) and DMBA (2.29 ± 0.05), DMBA+HP (2.74 ± 0.08) groups, a significant decrease (p<0.001) was observed, but there is a significant increase (p<0.01) between DMBA (2.29 ± 0.05) and DMBA+HP (2.74 ± 0.08) groups. However, in the kidney samples, a significant decrease (p<0.05) was observed in the levels of retinol (g mL-1) between control (1.75 0.06) and DMBA (1.09 ± 0.01) groups, but no significant difference (p>0.05) was found between DMBA (1.09 ± 0.01) and DMBA+HP (1.12 ± 0.01) groups. In our previous study, in the serum samples, differences among groups (control, DMBA and DMBA+HP) in the levels of retinol were found as statistically (p<0.001) [3]. [24] Yilmaz et al., 2010 reported that the differences were significant at P <0.001 for vitamins, in the plasma vitamin A and vitamin E levels were significantly lower in the gastroesophageal cancer group than in the control group. [25] Ahlersova et al., 2000 reported that between control DMBA and retinyl acetate+DMBA groups average tumour volume was found to be significantly (p<0.01) decreasing in rat mammary carcinogenesis. [26] Bukhari et al., 1998 reported that the results indicate that vitamin A deficiency enhances the effect of chemical carcinogenesis as DMBA. In this study, a significant decrease (p<0.001) was observed in the levels of retinol between control and DMBA, DMBA+HP groups. These results indicate that relative doses of vitamin A significantly may inhibit the development of DMBA-induced rat tumors.
Compound
Liver, x̄ ± SEM (mg mL-1)
Kidney, x̄ ± SEM (μg mL-1)
Control
DMBA
DMBA+HP
Control
DMBA
DMBA+HP
Retinol
3.43 ± 0.07a
2.29 ± 0.05a,b
2.74 ± 0.08a,b
1.75 ± 0.06c
1.09 ± 0.01c
1.12 ± 0.01
Retinyl palmitate
2.04 ± 0.04a
1.19 ± 0.03a,b
1.36 ± 0.02a,b
1.07 ± 0.03a
0.57 ± 0.03a
0.63 ± 0.02
β-carotene
1.38 ± 0.02a
0.75 ± 0.02a,a1
0.95 ± 0.03a,a1
0.29 ± 0.01a
0.09 ± 0.04a,a1
0.19 ± 0.02a,a1
α-tocopherol
3.85 ± 0.04a
2.19 ± 0.02a,a1
2.65 ± 0.03a,a1
2.01 ± 0.04a
1.08 ± 0.03a,
1.67 ± 0.03a,
Vitamin C
0.68 ± 0.05a
0.39 ± 0.03a,a1
0.75 ± 0.03a1
0.54 ± 0.05c
0.28 ± 0.02c
0.35 ± 0.02
Table 4: Vitamin levels at 60th day in control, DMBA and DMBA+HP groups in the rat tissues.
In the liver samples, a significant decrease (p<0.001) was observed in the levels of retinyl palmitate (μg mL-1) between control (2.04 ± 0.04) and DMBA (1.19 ± 0.03), DMBA+HP (1.36 ± 0.02) groups, but there is a significant increase (p<0.01) between DMBA (1.19 ± 0.03) and DMBA+HP (1.36 ± 0.02) groups. However, in the kidney samples, the levels of retinyl palmitate (μg mL-1) between control (1.07 ± 0.03) and DMBA (0.57 0.03) groups, a significant decrease was observed (p<0.001), but between other groups, no significant difference was found (p>0.05). In our previous study, in the serum samples, differences among groups (control, DMBA and DMBA+HP) were found statistically (p<0.01) in the levels of retinyl palmitate [3]. [27] Shklar et al., 1993 reported that tumor mass decreased in the groups of retinyl palmitate and β-carotene in vitro changes of normal human keratinocytes derived from the oral mucosa after treatment with the DMBA, retinyl palmitate and β-carotene.
In the liver samples, the levels of β-carotene (μg mL-1) between control (1.38 ± 0.02) and DMBA (0.75 ± 0.02), DMBA+HP (0.95 ± 0.03) were found to have a significant decrease (p<0.001), but there is a significant increase (p<0.001) DMBA (0.75 ± 0.02) and DMBA+HP (0.95 ± 0.03). In the kidney samples, the levels of β-carotene (μg mL-1) between control (0.29 0.01) and DMBA (0.09 ± 0.04), DMBA+HP (0.19 ± 0.02) were found to have a significant decrease (p<0.001), there is a significant increase (p<0.001) and DMBA (0.09 ± 0.04) and DMBA+HP (0.19 ± 0.02). Researchers reported that β-carotene, vitamin C and α-tocopherol could act synergistically to inhibit the growth of experimentally induced oral cancer by DMBA [27-29].
In the liver samples, a significant decrease (p<0.001) was observed in the levels of α-tocopherol (μg mL-1) between control (3.85 ± 0.04) and DMBA (2.19 0.02), DMBA+HP (2.65 ± 0.03) groups, but there is a significant increase (p<0.001) between DMBA (2.19 0.02) and DMBA+HP (2.65 ± 0.03) groups. However, in the kidney samples, the levels of α-tocopherol (μg mL-1) between control (2.01 0.04) and DMBA (1.08 ± 0.03), DMBA+HP (1.67 ± 0.03) groups, a significant decrease was observed (p<0.001), but between DMBA (1.08 ± 0.03) and DMBA+HP (1.67 ± 0.03) groups a significant difference was not found (p>0.05). [30] Yamata et al., 2008 reported the protective effects of α-tocopherol on skin damage in hairless mice by DMBA [15].
In the liver samples, the levels of vitamin C (μg mL-1) between control (0.68 ± 0.05) and DMBA (0.39 0.03) groups, a significant decrease was observed (p<0.001), but there is a significant increase (p<0.001) between DMBA (0.39 ± 0.03) and DMBA+HP (0.75 ± 0.03) groups. However, in the kidney samples, the levels of vitamin C (μg mL-1) between control (0.54 ± 0.05) and DMBA (0.28 0.02) groups, a significant decrease was observed (p<0.05) but between other groups, a significant difference (p>0.05) was not found. In our previous study, in the levels of vitamin C, between control and DMBA groups a significant decrease was observed (p<0.05) [3]. [31, 32] Aralkumaran et al., 2006-2007 reported that Vitamin C was found to be significantly (p<0.05) decreasing in mammary carcinoma bearing rats. In this study, in the levels of vitamin C, between control and DMBA, DMBA+HP groups, a significant decrease was observed (p<0.001), (p<0.05), respectively. Vitamin C protects the organism against the genotoxic effects of several radical-generating mutagens, scavenging the free radicals and alteration in metabolic activation of carcinogens, enzymatic superoxide-generating system (hypoxanthine/xanthine oxidase) DMBA [33-35]. The observed decreases in the level of vitamin in DMBA and DMBA+HP groups may be due to the excessive utilization of these antioxidants for quenching enormous free radicals generated in cancer condition related with increased free radical production that is due to DMBA. [36] Yavuz et al., 2010 reported the interaction of DMBA with fish sperm double-stranded DNA based on decreasing of the oxidation signal of adenine base.
Conclusion
The results obtained from this study demonstrated that the vitamin levels decreased in DMBA-treated group, but increased in HP-treated group. Hence, considering the antioxidant property of HP, the bioactive compounds derived from this plant may be supplemented with anticancer medicines. As a result, the observed beneficial effects treated with HP on antioxidant status in rats subjected to DMBA indicate that the vitamin levels changed as chemopreventive agent. We suggest that decreased vitamin levels be related with increased free radical production that is due to DMBA, and the potent chemopreventive efficacy of HP might be due to its antioxidant effects.
References
- Muqbil I, Banu N . Enhancement of pro-oxidant effect of 7,12-dimethylbenz (a) anthracene (DMBA) in rats by pre-exposure to restraint stress. Cancer Lett. 2006; 240: 213-220.
- De Flora, S.; Scarfi, S.; Izzotti, A.; D'agostini, F.; Chang, C.C.; Bagnasco, M; et al, Induction by 7,12-dimethylbenz(a)anthracene of molecular and biochemical alterations in transformed human mammary epithelial stem cells, and protection by N-acetylcysteine. J. E. Int. J Oncol. 2006, 29, 521-529.
- Levent, A.; Ekin, S.; Oto, G. Simultaneous determination of retinol, retinyl palmitate and β-carotene in rat serum treated with 7,12 dimethylbenz[a]anthracene and Hypericum Perforatum L. by high-performance liquid chromatography with diode-array detection. Central european journal of chemistry. Cent. Eur. J. Chem., 2010, 8, 108-115.
- Di Carlo G, Borrelli F, Ernst E, Izzo AA . St John's wort: Prozac from the plant kingdom. Trends Pharmacol Sci. 2001; 22: 292-297.
- Lenard J1, Rabson A, Vanderoef R . Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and rose bengal: inhibition of fusion and syncytia formation. Proc Natl Acad Sci U S A. 1993; 90: 158-162.
- Butterweck V . Mechanism of action of St John's wort in depression : what is known? CNS Drugs. 2003; 17: 539-562.
- Gulick RM, McAuliffe V, Holden-Wiltse J, Crumpacker C, Liebes L, Stein DS, et al . Phase I studies of hypericin, the active compound in St. John's Wort, as an antiretroviral agent in HIV-infected adults. AIDS Clinical Trials Group Protocols 150 and 258. Ann Intern Med. 1999; 130: 510-514.
- Jacobson JM, Feinman L, Liebes L, Ostrow N, Koslowski V, Tobia A, et al . Pharmacokinetics, safety, and antiviral effects of hypericin, a derivative of St. John's wort plant, in patients with chronic hepatitis C virus infection. Antimicrob Agents Chemother. 2001; 45: 517-524.
- Schempp, C.M.; Winghofer, B.; Muller, K.; Schulte-Monting, J.; Mannel, M.; Schopf, E.; et al.; Effect of oral administration of Hypericum perforatum extract (St. John's Wort) on skin erythema and pigmentation induced by UVB, UVA, visible light and solar simulated radiation. Phytother. Res. 2003, 17, 141-146.
- Fang YZ, Yang S, Wu G . Free radicals, antioxidants, and nutrition. Nutrition. 2002; 18: 872-879.
- Moss, R.W. Antioxidants against cancer, Equinox press, Inc. New York, 2000.
- Zhao B, Tham SY, Lu J, Lai MH, Lee LK, Moochhala SM . Simultaneous determination of vitamins C, E and beta-carotene in human plasma by high-performance liquid chromatography with photodiode-array detection. J Pharm Pharm Sci. 2004; 7: 200-204.
- Norum KR, Blomhoff R . McCollum Award Lecture, 1992: vitamin A absorption, transport, cellular uptake, and storage. Am J Clin Nutr. 1992; 56: 735-744.
- Van Merris, V.; Meyer, E.; De Wasch, K.; Burvenich, C. Simple quantification of endogenous retinoids in bovine serum by high-performance liquid chromatography-Diode-array detection. Anal, Chim, Acta., 2002, 468, 237-244.
- Someya K, Totsuka Y, Murakoshi M, Kitano H, Miyazawa T . The effect of natural carotenoid (palm fruit carotene) intake on skin lipid peroxidation in hairless mice. J Nutr Sci Vitaminol (Tokyo). 1994; 40: 303-314.
- Terry P, Jain M, Miller AB, Howe GR, Rohan TE . Dietary carotenoids and risk of breast cancer. Am J Clin Nutr. 2002; 76: 883-888.
- Hosotani K, Kitagawa M . Improved simultaneous determination method of beta-carotene and retinol with saponification in human serum and rat liver. J Chromatogr B Analyt Technol Biomed Life Sci. 2003; 791: 305-313.
- Tahri A, Yamani S, Legssyer A, Aziz M, Mekhfi H, Bnouham M, et al . Acute diuretic, natriuretic and hypotensive effects of a continuous perfusion of aqueous extract of Urtica dioica in the rat. J Ethnopharmacol. 2000; 73: 95-100.
- Bnouham M, Merhfour FZ, Ziyyat A, Mekhfi H, Aziz M, Legssyer A . Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia. 2003; 74: 677-681.
- Su Q, Rowley KG, Balazs ND . Carotenoids: separation methods applicable to biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2002; 781: 393-418.
- Riley, C.M.; Rosanske, T.W. Development and Validation of Analytical Methods, Elsevier, New York, 1996.
- Swartz, M.E.; Krull, I.S. Analytical Method Development and Validation, Marcel Dekker, New York, 1997.
- Levent, A.; Oto, G.; Ekin, S.; Berber, i. Method Validation and Simultaneous Determination of Retinol, Retinyl Palmitate, β-Carotene, a-Tocopherol and Vitamin C in Rat Serum Treated with 7,12 Dimethylbenz[a]Anthracene and Plantago major L. by High-Performance Liquid Chromatography Using Diode-Array Detection. Combinatorial Chemistry & High Throughput Screening, 2013, 16, 142-149
- Yilmaz, Ö.; Dursun, H.; Keles, S.; Yilmaz, A.; Okçu, N. Plasma vitamin A and E evels in patients with gastroesophageal cancer in Eastern Anatolia. Turk. J. Med. Sci., 2010, 40, 115-119.
- Ahlersová E, Ahlers I, Kubatka P, Bojková B, Môciková K, Gajdosová S, et al . Melatonin and retinyl acetate as chemopreventives in DMBA-induced mammary carcinogenesis in female Sprague-Dawley rats. Folia Biol (Praha). 2000; 46: 69-72.
- Bukhari, S.M.H.; Bajwa, R.; Khan, S.A.; Chaudry, N.A.; Tayyab, M. Vitamin A deficiency enhances the effects of 7-12 dimethylbenz (a) anthracene (DMBA) induced skin carcinogenesis in albino rats. J. Coll. Physicians Surg. Pak., 1998, 8, 101-105.
- Shklar G, Schwartz J, Trickler D, Cheverie SR . The effectiveness of a mixture of beta-carotene, alpha-tocopherol, glutathione, and ascorbic acid for cancer prevention. Nutr Cancer. 1993; 20: 145-151.
- Chien, H.; Huang, S.Y.; Lin, Y.H.; Shieh, Y.H.; Shieh, M.J.; Shieh, M.J. et al, The preventive effect of carotenoids on MBA induced oral carcinoma in male hamsters. 2000, 25, 199-206.
- Chien, H.; Huang, S.Y.; Lin, Y.H.; Shieh, Y.H.; Shieh, M.J.; Wang, H.F.; et al. Nutr. Sci. J. 2001, 26, 98-108.
- Yamada Y, Obayashi M, Ishikawa T, Kiso Y, Ono Y, Yamashita K . Dietary tocotrienol reduces UVB-induced skin damage and sesamin enhances tocotrienol effects in hairless mice. J Nutr Sci Vitaminol (Tokyo). 2008; 54: 117-123.
- Arulkumaran, S.; Ramprasath, V.R.; Shanthi, P.; Sachdanandam, P. Restorative effect of Kalpaamruthaa, an indigenous preparation, on oxidative damage in mammary gland mitochondrial fraction in experimental mammary carcinoma. Mol. Cel. Biochem. 2006, 291, 77-82.
- Arulkumaran, S.; Ramprasath, V.R.; Shanthi, P.; Sachdanandam, P. New simplified procedures for the extraction and simultaneous high-performance liquid chromatographic analysis of retinol, tocopherols and carotenoids in human serum. Chem.-Biol. Interact., 2007, 167, 99-106.
- Van Poppel, G.; Vanden Berg, H. Chemopreventive efficacy and anti-lipid peroxidative potential of Jasminum grandiflorum Linn on 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis. Cancer. Letter. 1997, 114, 195-202.
- Khaidakov M, Bishop ME, Manjanatha MG, Lyn-Cook LE, Desai VG, Chen JJ, et al . Influence of dietary antioxidants on the mutagenicity of 7,12-dimethylbenz[a]anthracene and bleomycin in female rats. Mutat Res. 2001; 480-481: 163-70.
- Hertz N, Lister RE . Improved survival in patients with end-stage cancer treated with coenzyme Q(10) and other antioxidants: a pilot study. J Int Med Res. 2009; 37: 1961-1971.
- Yardim, Y. Keskin, E. Levent, A. Özsöz, M. Sentürk, Z. Voltammetric studies on the potent carcinogen, 7,12-dimethylbenz[a]anthracene: Adsorptive stripping voltammetric determination in bulk aqueous forms and human urine samples and detection of DNA interaction on pencil graphite electrode. Talanta, 2010, 80, 1347-1355.