1Department of Industrial Chemistry, Jnana Sahyadri, Kuvempu University, India
2Department of Chemistry, Vijayanagara Sri Krishnadevaraya University, India
3Department of Chemistry, Karnataka University, India
*Corresponding author: Lokesh K S, Department of Chemistry, Vijayanagara Sri Krishnadevaraya University, India.
Received: December 06, 2014; Accepted: December 23, 2014; Published: December 24, 2014
Citation: Mallikarjuna H, Lokesh KS, Shivaprasad KH and Venugopala Reddy KR. Sensitive Spectrophotometric Method for the Determination of Permetrexed Disodium in Pure and Pharmaceutical Formulations. Austin J Anal Pharm Chem. 2014;1(6): 1029.
Two simple, sensitive, rapid and accurate spectrophotometric methods have been proposed for the assay of disodium N-[p-[2-[(2-amino-4,7 dihydro- 4-oxo-1H-pyrrolo[2,3- d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamate (permetrexed disodium) in pure and pharmaceutical formulations. These methods were based on the measurement of absorbance of tris (bipyridyl) iron (II) [Method A] and the diazocoupling of permetrexed disodium with diazotized p-nitro aniline (DPNA) in basic medium [Method B] at 522 and 508 nm, respectively. Reaction conditions have been optimized to obtain colored complexes of higher sensitivity and longer stability. The absorbance increased linearly with increase in concentrations of permetrexed disodium, which were corroborated by correlation coefficient values (0.9948 and 0.9928 in methods A and B, respectively). The complexes obeyed Beer’s law over the concentration range of 0.2 - 12 μgmL-1 and 0.5 - 10 μgmL-1 in method A and method B, respectively. The common excipients and additives did not interfere in their determination. The developed methods have been successfully applied for the assay of PRX in bulk samples and pharmaceutical formulations.
Keywords: Permetrexed disodium; Spectrophotometry; Assay; Diazotization; Formulations
Drugs play a prominent role in the progress of human civilization. It is very much essential to monitor the quality of the synthesized bulk drug as well as the formulations accurately. Analytical chemistry helps in the quality assurance and control of bulk drug and their dose forms [1, 2]. The pharmaceutical industries have employed several analytical techniques to penetrate chemical estimation not only of the active ingredient (s) but also the quantification of related compounds or impurities in incoming chemicals, drug materials and formulations. The assay of drug samples in bulk and formulations may be determined by means of physical, chemical, physico-chemical and biological methods [1-8]. Physical and physico-chemical methods are more commonly employed. The existing methods of analysis of drugs often need improvement to suit laboratory requirements and facilities available in a particular laboratory set up. Even though the modern methods of analysis (HPLC, GLC, NMR and Mass) offer good speed, precision and accuracy, they involve sophisticated equipments which are costly and pose problems of maintenance [1, 3]. Hence, they may not be in the reach of most of laboratories and small scale industries, which produce bulk drugs and pharmaceutical formulations. Among various techniques, spectrophotometry still plays a significant role in the determination of compounds at micro or nanogram levels [2]. It is simple, economically viable and easy to carry-out. The importance of a spectrophotometric method lies in the chemical reaction(s) upon which the procedures are based, rather than upon the sophistication of the instrument. Many reactions which yield colored species for a particular drug are found to be selective or can be rendered selective through the introduction of masking agent, control of pH, use of solvent extraction techniques, by prior removal of interfering substance and so on. Hence, spectrophotometry is generally preferred in small scale industries and most of the laboratories for routine quality assurance [9].
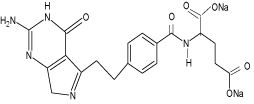
Permetrexed disodium (PRX) chemically designated as N-[4-(2- (2-amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-D]pyrimidin-5-yl)- ethyl)-benzoyl]-L-glutamic acid is similar to folic acid and it belongs to the class of chemotherapeutic drugs called folate antimetabolites with significant antitumor activity in its polyglutamyl forms. The chemical structure of permetrexed disodium is given in Scheme 1. It works by inhibiting three enzymes used in purine and pyrimidine synthesis-thymidylate synthase (TS), dihydrofolated reductase (DHFR) and glycinamide ribonucleotide formyl transferase (GARFT). By inhibiting the formation of precursor purine and pyrimidine nucleotides, pemetrexed prevents the formation of DNA and RNA, which are required for the growth and survival of both normal and cancer cells. It has shown activity against colon cancer, mesothelioma and non small-cell lung cancer [4-5].
Critical literature survey reveals that there are only few HPLC [10-12] methods available for the determination of an antiomeric purity of PRX. Fewer attempts have been made so far, for the development of spectrophotometric method [13-14] for the assay of PRX. This prompted us to develop simple, sensitive and accurate spectrophotometric methods for the determination of PRX in its pure and pharmaceutical formulations.
2, 2’-bipyridyl (BPL), ferric ammonium sulphate dodecahydrate (FAS), NaNO2, NaOH, HCl and methanol were obtained from s.d.fine chemicals Ltd., Mumbai and Para nitro aniline (PNA) was obtained from Merck. Permetrexed disodium, (PRX) was a gift sample obtained from Eli Lilly and Company Ltd, and used as such for the assay. All the solutions were prepared and diluted with distilled water unless it is mentioned.
Standard drug solution: A stock solution of standard PRX (100 μgmL-1) was prepared in distilled water and diluted up to the mark in a 100 mL volumetric flask. This solution was stored in a refrigerator. The solution was diluted as and when required.
Preparation of Fibrillarin (FBL )reagent: FBL was prepared [15] by mixing 0.16 g of 2, 2’-bipyridyl (BPL) in 2 mL of 1 M HCl with 0.16 g FAS diluted with distilled water up to the mark in a 100 mL volumetric flask.
Preparation of Para nitro aniline (PNA): 0.2% (w/v) of PNA was prepared by dissolving 200 mg in 1:9 HCl-H2O mixtures and solution was diluted with distilled water to 100mL. UV-Visible double beam spectrophotometer provided by Shimadzu, Japan was used for the spectrophotometric measurements.
The effect of various parameters viz., the amount of FBL, time for development of colored species and stability of colored species were studied at different temperature (for Method A) or effects of PNA, suitable acids (with their concentration), NaNO2, NaOH, time development for diazotization, maximum color development and the stability of colored species were studied at room temperature (for Method B). After detailed investigations of the various parameters involved in the formation of colored products, a systematic study for the determination of PRX was carried out. The following procedures were recommended for the assay of PRX in pure and pharmaceutical formulations.
Analysis of bulk sample
Method A: Aliquots of standard PRX drug solution containing 0.2-12 μgmL-1 were placed in to a series of 10 mL calibration flasks. To these, 4mL of FBL solution was added. The contents were heated to 60 0C on water bath for 20 min. The solutions were cooled to room temperature and diluted up to the mark with distilled water and the contents were mixed well. The absorbance’s of red colored complex were measured at 522 nm against the reagent blank. Calibration graph was plotted and used to determine the amount of drug in an unknown sample.
Method B: To a series of 10 mL graduated flasks, 1.8 mL of PNA and 1 mL of 0.5 % (w/v) NaNO2 were added and kept aside for 5 min after mixing thoroughly. 2 mL of methanol was added and the contents were allowed to stand for 5 min. Then, aliquots of standard PRX drug solution containing 0.5-10 μgmL-1 were transferred to series of flasks, followed by 1 mL of 1M NaOH solution. The contents were diluted to the mark with distilled water. The absorbances were recorded after 5 min at 508 nm against reagent blank. The amount of PRX was computed from its Beer’s law plot.
Analysis of pharmaceutical preparations: Tablets: Twenty tablets of PRX were weighed and finely powdered. An amount equivalent to 10 mg of the drug was weighed accurately and transferred into a 100 mL beaker. Using the mechanical stirrer, the powder was completely disintegrated in distilled water. The solution was filtered through a Whatman filter paper number 40. The residue was washed with distilled water and the solution was transferred into 100 mL volumetric flask and diluted to the mark with distilled water. The solution was analyzed using both method A and B.
The detection limit (LOD) and Quantification limit (LOQ) for the proposed methods were calculated as per the recommendations of Analytical Method Committee [16].
The effects of common additives and excipients like talc, glucose, starch, lactose, dextrose and magnesium stearate associated with the PRX in its formulations were investigated using the developed methods.
For recovery experiments, known quantities of pure PRX solutions were mixed separately with definite amounts of pre-analyzed formulations and the mixtures were analyzed using the developed methods.
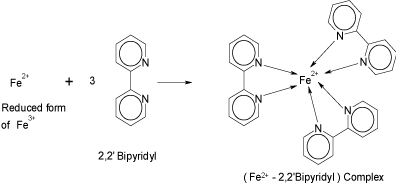
Ferric salts play a prominent role in spectrophotometric determination of many pharmaceutical formulations [17-22]. Acting as an oxidant, a ferric salt gets reduced to ferrous salt, which corresponds to the drug concentration. The amount of Fe (II) could be determined using the reagent, BPL. This property was exploited in the present investigation to develop spectrophotometric method for determination of PRX. PRX underwent oxidation by Fe (III) present in FBL. The Fe (II) so formed readily combined with the BPL of FBL to form a red-colored complex [15], [Fe (bipy) 3]2+ (Scheme 2).
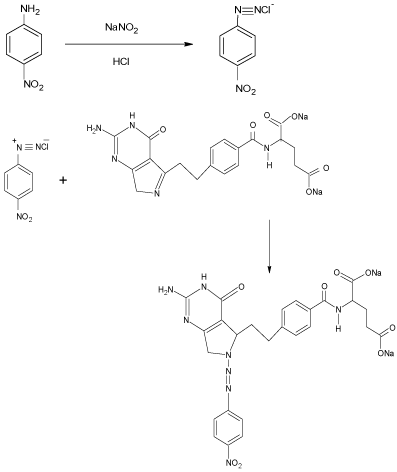
The method B involved the coupling reaction of diazotized p-nitro aniline (DPNA) with the hydrolyzed product of PRX in alkali medium to give a pink colored product which shows maximum absorption at 508 nm. The spectrophotometric properties of colored product as well as the different experimental parameters affecting the color development, sensitivity and its stability were studied carefully and optimized.
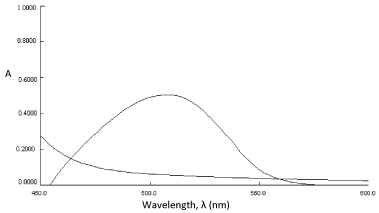
In order to examine the wavelength of maximum absorption (λmax) of colored species, known amount of drug was taken and the colored species was developed separately by following procedures given earlier. The colored products exhibited absorption maxima at 522 nm and 508 nm, respectively for method A and B against the corresponding reagent blank (Figure 1 and 2). The reagent blank showed a negligible absorbance at the corresponding λmax (for both methods). The probable reaction mechanism for the formation of diazo-coupling product is shown in Scheme 3.
In order to establish optimum reaction conditions necessary for the formation of colored product of maximum stability and sensitivity, the absorbance of a series of solutions were measured by varying the one parameter at a time and keeping others fixed [23].
For method A:
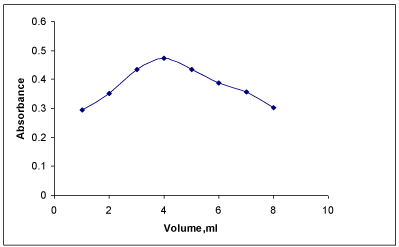
Effect of FBL concentration: The concentration of PRX was fixed and varied amounts of FBL were added and colored species were developed and absorbances were recorded. The experimental results indicated that low absorbances were observed with the volume of the reagent which was less than or more than 4 mL (Figure 3). Hence, a volume of 4 mL was used in this study.
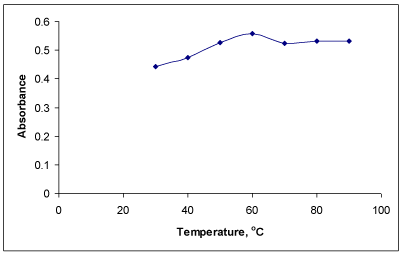
Effect of temperature on colored product: The formation of colored complex was slow at room temperature (25 °C) and required longer time for completion. Hence, reaction was carried out at higher temperatures. It was observed that the maximum absorbance was obtained after heating the reaction mixture at 60 °C (Figure 4) for 20 min as sufficient time is required for the complete development of color. The absorbance related to reaction time is not shown here. The absorbance of the complex remained constant at room temperature for more than 1.5 h.
For method B:
Sequence of addition of reagents: It was found that the sequence as mentioned in the procedure was found to be more accurate and precise for obtaining the colored product of maximum absorbance and stability.
Reaction sequence: The diazo-coupling reaction took place in mild acidic medium in two steps. In the first step, nitrite reacts with PNA in the presence of HCl to form diazonium salt (DPNA). In second step, the DPNA coupled with PRX in NaOH medium to give the diazotized product. Coupling of a diazonium salt form an aromatic primary amine (p-nitro aniline [2]) take place in mild acidic condition. Usually substitution occurs at para position of the active group or at ortho position, if para position is substituted. The probable reaction mechanism is shown in Scheme 3.
Effect of PNA on the sensitivity of the colored product: The use of hydrochloric acid (1:9) as reaction medium was found to give more satisfactory results for the diazotization coupling reaction compared to other acids. A volume of 1.8 mL of 0.2% PNA (prepared in water- HCl) was found to be necessary to yield maximum intensity and high stability colored species. With volume below or above 1.8 mL of PNA, less intense colored product was noticed (Figure 5).
Optimization of NaNO2 concentration: 1 mL of 0.5% (w/v) of NaNO2 was observed to be necessary for diazotization coupling reaction that yielded colored species of maximum stability and intensity. Low absorbance values were noticed with a volume of below or above 1 mL NaNO2. Moreover, the absorbances were unstable. Hence, 1 mL of NaNO2 was used throughout the experiment (Figure 5).
Optimization of MeOH concentration: It was noticed that 2 mL MeOH was necessary to achieve maximum colored product. The colored species were noticed to be unstable or exhibited low absorbances with volume of below or above 2 mL. Hence, 2 mL MeOH was selected for diazotization (Figure 5).
Effect of NaOH: Experiment showed that that the diazotized coupled product was formed only in alkali medium. 1M NaOH was found to be the most suitable as it improved the sensitivity of the reaction and gave reproducible results. The absorbance values increased up to 1 mL of NaOH and decreased upon the addition of more than1 mL. Hence, 1 mL of NaOH was selected for diazotization (Figure 6).
Precision and accuracy of the proposed method: The precision and accuracy of the proposed methods were determined by carrying out the actual determination of five replicates of a fixed amount of PRX. The low values of percentage relative standard deviation and percentage errors indicated good precision and accuracy of the proposed method (Table I).
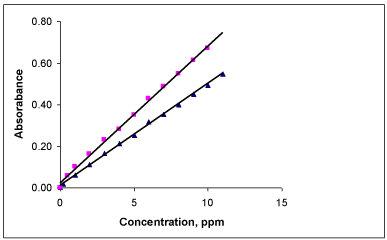
Optical characteristics of the colored product: In order to examine whether the colored product formed in the proposed methods adhere to Beer’s law or not, the absorbance of a series of solutions containing increased amounts of PRX were measured against the corresponding reagent blank at respective λmax values. A linear relationship was found between the absorbances at λmax and concentration of colored species in the range of 0.2 to 12μgmL-1 for method A and 0.5 to 10μgmL- 1 for method B (Figure 7). Regression analyses of Beer’s law plot at their respective λmax values revealed a good correlation. The molar absorptivity and Sandell’s sensitivity values have been evaluated and are summarized in Table 1.
Limit of detection and quantification: The LOD values were found to be 0.0488μgmL-1 and 0.1448μgmL-1, while the LOQ values were 0.1625 μgmL-1and 0.4821 μgmL-1 for method A and B, respectively.
Stability of the colored species: Under the optimum reaction conditions, the colored complex formed in method A was stable for 1.5 h. It was observed that the maximum absorbance was obtained after heating the reaction mixture at 60 0C for 20 min. However, low absorbances were observed at temperatures higher than 60 0C indicating the decomposition of the product.
In method B, when DPNA was treated with PRX in presence of sodium hydroxide, a pink colored product was formed within 5 min. The stability of colored product was studied at different temperatures. It was found that the absorbance values remained constant in the temperature range of 5-40 °C. The resulting final colored product was noticed to be stable for 24-30 h.
Studies on the interference of excipients: It was observed that the common additives and excipients like talc, glucose, starch, lactose, dextrose and magnesium stearate did not interfere in the determination at the levels found in dose forms. The corresponding results are indicated in Table 2.
Recovery studies: Recovery experiments were performed in order to determine the accuracy and reliability of the proposed methods. The total amount of the drug was determined using the proposed methods and the amount of the added drug was calculated by difference. The results were found to be satisfactory (Table 2 and 3).
The proposed methods were applied to the determination of PRX in commercial tablets. The results of analysis of five replicates for each concentration were noticed to be in good agreement with the labeled values. The results of analysis of the PRX in tablets are given in Table III. The developed methods showed a better recovery and no interference of excipients. This indicates that the developed methods are found to be highly accurate and precise. These simple spectrophotometric methods can be employed in pharmaceutical industry to analyze the commercial as well as bulk samples.
The proposed spectrophotometric methods for the permetrexed disodium are simple and accurate. The statistical parameters and recovery study data clearly indicate the reproducibility and accuracy of the proposed methods. The methods are found to be free from interference by common additives and excipients. The wide applicability of the new procedures for routine quality control is well established by the assay of PRX in pure form and tablets. Hence, the proposed methods can be utilized in the pharmaceutical laboratories and industries.
Authors thank VGST, Karnataka Govt. for the financial assistance through VGST-SMYSR grant to Dr. K. S. Lokesh and CESST to Prof. K. R. Venugopala Reddy.
Optical characteristics, precision and accuracy data.
Parameters |
Values for |
|
FBL |
PNA |
|
λmax (nm) |
522 |
508 |
Beer’s law limits (µgmL-1) |
0.2-12 |
0.5-10 |
Molar Absorptivity (l/ mol/ cm)×104 |
2.681 |
1.420 |
Sandell’s sensitivity (ng cm-2) |
17.59 |
13.79 |
Correlation co-efficient (r) |
0.9948 |
0.9928 |
Regression equation (Y) a |
------- |
------- |
Slope (b) |
0.1638 |
0.0696 |
Intercept (a) |
0.0618 |
0.0156 |
Relative standard deviation (%) d |
0.86 |
0.91 |
% Error d |
1.31 |
1.08 |
Limit of detection (µgmL-1 ) |
0.0488 |
0.1448 |
Limit of quantification (µgmL-1 ) |
0.1625 |
0.4821 |
aY= a+bX , where X is the concentration of the drug in μgmL-1.
dAverage of five determinations.
Interference of excipients in the determination of PRX.
Excipients added |
Amount (mg) |
% Recovery* ± %RSD |
Glucose |
30 |
99.58±0.62 |
Talc |
30 |
96.82±0.48 |
Lactose |
30 |
97.78±0.62 |
Starch |
30 |
97.35±0.88 |
Magnesium stearate |
30 |
99.51±0.69 |
Dextrose |
30 |
97.22±0.36 |
*Average of five determinations.
Assay of PRX in pharmaceutical formulations.
Drug (Tablet) |
Labeled amount (mg) |
%Recovery* ± %RSD |
ALIMTAa |
100 |
99.62±0.29 |
500 |
99.87±0.78 |
|
Pure drug added to 100 mg tablet |
200 |
99.74±0.65 |
*Average of five determinations.
aEli Lilly and Company Ltd.
Chemical structure of permetrexed disodium (PRX).
Formation of coloured complex of iron (II).
Probable reaction mechanism for the formation of coupling product of PRX with PNA.
Absorbance spectra of (a) reagent blank FBL and (b) oxidation complex of PRX (3μgmL-1).
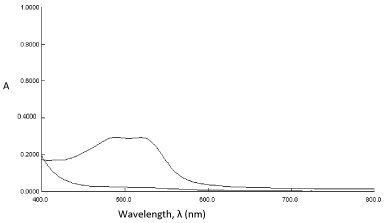
Absorbance spectra of (a) reagent blank, PNA and (b) diazocoupling product of PRX (8μgmL-1).
Effect of volume of FBL on absorbance of colored product of PRX.
Effect of temperatures on absorbance of colored product of PRX.
Effect of volume of reagents on absorbance of coloured product of PRX.
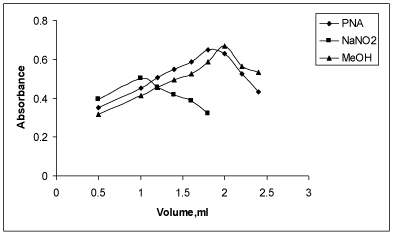
Effect of volume of NaOH on absorbance of colored product of PRX.
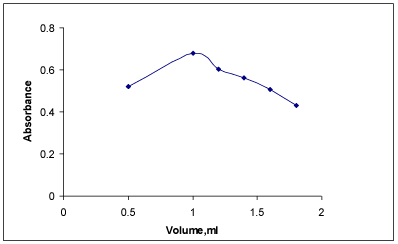
Beer’s law plot of PRX for FBL (▲) and PNA (■).