
Research Article
Austin J Anal Pharm Chem 2023; 10(1): 1153.
Synthesis and Biological Evaluation of Curcumin-NSAIDs Conjugates
Gaurav Chaudhary1, Sarika Chaudhary2*, Lovy Sharma3, Janhavi Singh4, Sweeti Bana5 and Kamakshi Verma6
*Corresponding author: Sarika ChaudharyDepartment of Pharmacology, I. T. S College of Pharmacy, Muradnagar, Ghaziabad, India.
Received: November 22, 2022; Accepted: January 11, 2023; Published: January 17, 2023
Abstract
The present research work involves the synthesis of a total of four Curcumin-NSAIDs conjugates and their subsequent biological screening for Rheumatoid Arthritis, Anti-inflammatory, and Anti-ulcer activities. Curcumin is a phytochemical having versatile therapeutic potential. The idea of conjugating curcumin with various NSAIDs is somewhat novel in the sense that it helps in obtaining the target of incorporating certain desired properties in the curcumin like enhanced bioavailability, reduction in the side effects and also giving good therapeutic potential in terms of determination of rheumatoid arthritis, anti-inflammatory and anti-ulcer activities of synthesized conjugates using appropriate animal models. Additionally, studies have been done on the in vivo bioavailability of curcumin-NSAIDs conjugates and curcumin in Sprague-Dawley rats and on the antiarthritic efficacy of curcumin-NSAIDs conjugates and curcumin in a modified streptococcal cell wall-induced arthritis model in Balb/c mice to resemble rheumatoid arthritis in human. Curcumin-NSAID conjugates have shown improved stability in all of the studies mentioned when compared to curcumin and curcumin-NSAID conjugates; they also increased curcumin’s bioavailability by more than five folds and significantly reduced arthritis symptoms in a streptococcal cell wall-induced arthritis model when compared to other conjugates and curcumin. The structural features of all the synthesized compounds have been confirmed by appropriate spectral analysis. Other supporting techniques employed for structural and purity aspects include Melting point determination, Thin Layer Chromatography analysis, and Recrystallization. Therefore, conjugating curcumin with medicinal molecules proved to be a creative way to increase its bioavailability and enhance its wide range of pharmacological effects.
Keywords: Bioavailability; Anti-Inflammatory; Anti-Ulcer; Rheumatoid Arthritis etc.
Introduction
Curcumin is a yellow polyphenolic chemical compound isolated from the rhizomes of turmeric. Its chemical name is diferuloyl methane (Curcuma longa) [1-3]. Its medicinal efficacy has been attributed to its strong antioxidant, and anti-inflammatory properties [4,5]. Curcumin has yet to be authorized as a medicinal drug, despite its numerous pharmacological characteristics. This is due to its limited oral bioavailability (1 % or lower) [6]. Curcumin’s low bioavailability has been linked to its poor solubility, permeability, and water stability, as well as its fast pre-systemic metabolism [7].
Curcumin is a crystalline powder that is orange-yellow in color and nearly insoluble in water. Curcumin has lately been studied for its potential to treat high cholesterol, inflammation, scabies, AIDS, viral infections, HIV, and chronic anterior uveitis, a vision issue [8,9]. Curcuma longa is a medication from the old Indian system of medicine that resembles a wizard. It is used in Ayurveda to treat high “Kapha” and “Pitta” conditions [10].
Curcuma longa, C. zanthrorrhiza, C. zedoaria, C. Angustifolia, C. aromatica, and C. caesia, are among the 70 species in the Curcuma genus, of which several are medicinally significant [11]. Tubers, rhizomes, and oil of Curcuma longa are the most commonly utilized medicinally. The rhizomes are rough brown on the outside and bright orange on the inside. Rhizomes are rectangular, ovate, or colored, with a unique odor and a strong, bitter taste [12]. Curcuma longa is a tropical crop highly cultivated in India, Bangladesh, China, Malaysia, Indonesia, Thailand, Cambodia, and the Philippines. The major turmeric-growing states in India include Tamil Nadu, Orissa, Karnataka, Andhra Pradesh, Maharashtra, and Kerala. More than 90% of the world’s entire output is produced in India [13,14].
Chemistry of Curcumin
Curcumin, also known as diferuloylmethane, is a symmetric chemical moiety having the chemical formula C21H20O6 and a molecular weight of 368.38. Its IUPAC name is (1E, 6E)-1, 7-bis (4-hydroxy-3-methoxyphenyl) -3,5-dione -1,6-heptadiene. Two aromatic ring systems with o-methoxy phenolic groups are connected by a seven-carbon linker made up of an,-unsaturated -diketone moiety [15-19]. Keto-enol tautomerism exists in the diketo group, which can exist in many types of conformers depending on the environment [20]. Curcumin, which accounts for 2-5% of turmeric’s active ingredients, is the spice’s most active component. The curcuminoids, which were originally identified by Vogel in 1842, are responsible for turmeric’s distinctive yellow. Curcumin (Cur; diferuloylmethane, 10 %), demethoxycurcumin (Dem; 15 %), and bisdemethoxycurcumin (Bis; 5 %) are the three types of curcuminoids (Curs) found naturally in Curcuma longa. Curcumin (C21H20O6) was initially reported in 1910 by Lampe and Milobedeska, who discovered that its structure is diferuloylmethane [21].
Poor Bioavailability of Curcumin
Reduced bioavailability of any medication within the body is caused by poor absorption, a high rate of metabolism, and/or rapid excretion from the body. Curcumin has been shown in studies to have high intrinsic activity and, as a result, effectiveness as a therapeutic agent for a variety of diseases. However, Research on curcumin absorption, distribution, metabolism, and excretion over the last three decades has indicated poor absorption and fast metabolism of curcumin, severely limiting its bioavailability [22].
Curcumin has poor water solubility, chemical instability, and a poor pharmacokinetic profile, which is well known. Despite its effectiveness and safety, curcumin's medicinal potential is still debatable, owing to its low absorption in humans, even when given at large dosages [23]. Curcumin has limited oral bioavailability due to a low absorption rate in the small intestine, significant reductive and conjugative metabolism in the liver, and excretion through the gall bladder. Curcumin binding to enterocyte proteins, which can alter its structure, adds to the low bioavailability [24].
Several techniques have been tried to combat poor curcumin absorption and fast removal from the body, including adjuvants that prevent curcumin metabolism as well as new solid and liquid oral administration vehicles [25]. These new drug delivery methods have the potential to solve the pharmaceutical problem of curcumin administration by increasing solubility, prolonging plasma residence time, and optimizing pharmacokinetic profile and cellular absorption [26].
Non-Steroidal Anti-Inflammatory Drugs
NSAIDs are one of the most often recommended therapies in the world. They are classified into the following groups based on their chemical structure and selectivity: propionic acids, acetic acids, acetylated salicylates, non-acetylated salicylates, enolic acids, anthranilic, and selective COX-2 inhibitors [27].
The major therapeutic action of NSAIDs is their ability to prevent the production of particular prostaglandins (PGs) by inhibiting the cyclooxygenase enzymes (COX-1 and COX-2). As a result, drugs should alleviate joint pain and inflammation while causing less GI damage than non-selective NSAIDs. On the other hand, they are widely known for causing stomach and Duodenal Ulcers (DU). Furthermore, NSAIDs are linked to a high prevalence of upper gastrointestinal (GI) problems, which leads 5-15 % of patients to stop taking them [28].
The reason behind NSAID-induced GI side effects is that these medicines block prostaglandin production, lowering the protective GI mucosal barrier and increasing the risk of bleeding [28].
Because of the built-in gastro-protective properties of curcumin, Curcumin-NSAIDs conjugates have the potential to lessen or alleviate the negative effects of long-term non-steroidal anti-inflammatory medication (NSAID) use [28].
The severity and frequency of GI bleeding and ulceration induced by NSAID use are well-documented to increase with age. [29] In the elderly, using NSAIDs four times increases the risk of GI bleeding. The reason behind NSAID-induced GI side effects is that these medicines block prostaglandin production, lowering the protective GI mucosal barrier and increasing the risk of bleeding [30].
Misoprostol, H2-receptor antagonists (H2RA), and proton pump inhibitors are GI-protective medications that can help prevent NSAID-induced gastroduodenal ulcers (PPI) [31]. This method is used by about 20% of older people who are on chronic NSAIDs [32]. Substituting COX-2 selective NSAIDs for nonselective NSAIDs is another way to reduce GI side effects. COX-2 inhibitors have been shown in several trials to produce less harm to the GI mucosa than non-selective NSAIDs [33-35]. Rhame et al. investigated older individuals on low-dose aspirin, they corroborated similar findings. They discovered that celecoxib had a better GI safety profile than non-selective NSAIDs [36].
On the other hand, COX-2 inhibitors raise the risk of cardiovascular adverse effects [37]. As a result, finding the optimal GI protection options for those using chronic NSAIDs necessitates tailoring a patient's GI risk factors to their cardiovascular risk factors [31].
Conjugation as an important strategy has been adopted resulting in a large number of drug molecules with enhanced solubility, bioavailability, and in vivo performance of small and large molecules. In our study, the drug-drug conjugate was adopted as a novel approach to synthesize different Curcumin NSAIDs Conjugates to increase the bioavailability of curcumin, enhance the therapeutic benefit of NSAIDs, and in addition, the inherent beneficial effects of curcumin in the GIT has the potential to alleviate the side effects and severity of arthritis treatment. The enhanced bioavailability of curcumin in Curcumin NSAIDs Conjugates was by over many folds can be attributed to the improved stability of Conjugates. Conjugates also exhibited better anti-inflammatory activity in arthritis in Balb/c mice. Thus, the conjugation of Curcumin with NSAIDs molecules could be a novel strategy for improvement of its bioavailability and for potentiating its numerous pharmacological activities.
Pharmaceutical aspects of Curcumin NSAID conjugates
Since a long time, Curcumin had a drawback of poor bioavailability, which has been overcome by many fold through Esterification of hydroxyl group of curcumin and Carboxyl group of different NSAIDs. NSAIDs have been used to cure inflammation which is caused by arthritis and it is proved if used for prolong period as in case of arthritis causes peptic ulcers. Conjugating Curcumin with NSAIDs leads to higher efficiency for anti-inflammatory effect when compared to NSAIDs individually. Formed conjugates also decreases chances of ulceration. Several pharmaceutical compounds with improved solubility, stability, permeability, bioavailability, organoleptic characteristics, and in vivo performance have been produced as a result of the essential method of conjugation. Drug-drug conjugate was used in our study as a novel approach to synthesize different curcumin NSAIDs conjugates in order to increase the bioavailability of curcumin, improve the therapeutic benefit of curcumin and NSAIDs, and in addition, the inherent beneficial effects of curcumin in the GIT has the potential to lessen the side effects and severity of arthritis treatment. Therefore, conjugating Curcumin with NSAIDs molecules could be a creative way to increase its bioavailability and enhance its wide range of pharmacological effects. These studies required further investigation.
Materials and Methods
Isolation and Purification of Curcumin
In a soxhlet assembly, turmeric rhizome powder was extracted with ethyl acetate until all the coloring matter was removed. Then condensed the crude extract into a semisolid dark-tinted substance. One gram of crude extract was mixed with 25 mL of Hexane for 12 hours. After 12 hours, the solution containing a lump of crude extract was agitated for 3 hours with a magnetic stirrer at 600 rpm. The bulk splits into little fragments during the churning and was eventually reduced to powder. The suspended lump powder was separated by centrifugation and dried in a 40°C oven. The total curcumin content of the resulting crude powder was determined. The open capillary technique was used to measure the melting point of curcuminoid powder. Recrystallization was used to separate pure curcumin from crude curcumin.
TLC separation of curcuminoids using various solvent systems:
Three curcuminoids were detected in acetone extract using TLC. The TLC pre-coated silica gel plates (Merk-60 F254, 0.25mm thick) were developed in a Camag twin trough glass tank that had been impregnated with the mobile phase for 1 hour and10 cm height was developed on each plate. Various mobile solvents of variable polarity were used to optimize the mobile phase composition. After the development plates had been removed and dried, UV light was used to examine the spots.
Silica gel column chromatography
The acetone extract was chromatographed on a silica gel (60-120 mesh) glass column. Curcuminoids (5gm) were mixed with silica gel(8 gm) and put onto a 462 cm column, which was then eluted with chloroform, chloroform: methanol, and chloroform: methanol with an increase in polarity. All of the obtained fragments were run on a 60 F254 silica gel TLC plate with a developing solvent system of chloroform: methanol (95:5) and identified as yellow spots. The organic solvent was extracted using a rotary evaporator and comparing fractions with Rf values were pooled. UV spectrophotometry at 420 nm was used to determine the total curcuminoid concentration of each curcuminoid sample.
Purification of each curcuminoid
Each Curcuminoid was extracted using column chromatography and in methanol, it is dissolved before being heated. After full dissolution, add chloroform to make a 5:2 ratio of methanol to chloroform. Store at 5°C overnight. Filtration was used to separate the crystals. Petroleum ether was used to precipitate the crystals. Individual crystal purity was determined using HPLC.
Synthesis of Curcumin-NSAIDs Conjugate
An equimolar quantity of 4-dimethyl aminopyridine (DMAP) was added to the aqueous solution of curcumin (5 g, 13.6 mmol) in dichloromethane: ethyl ether (70:30, 500 ml) and the mixture was agitated for 30 minutes. Different NSAIDs and 1-Ethyl-3-(3-dimethyl aminopropyl) carbodiimide (EDAC) (20.4 mmol) were added to the above-mentioned mixture and agitated for another 24 hours. Thin layer chromatography (TLC) was used to track the reaction's progress, with the hexane: ethyl acetate (55:45) combination as the eluent. The prepared reaction mixture, was rinsed with 5% Hydrochloric acid and concentrated after the reaction was completed. Using column chromatography the crude product was purified with a stationary phase of silica gel (60-120 mesh) and a mobile phase of hexane: ethyl acetate (55:45). The different monoconjugated fractions were collected, concentrated, and dried to produce Curcumin-NSAIDs Conjugates.
Bioavailability
The bioavailability of curcumin and its NSAIDs conjugates was estimated in Sprague-Dawley rats. The animals were divided into five groups of six each, according to random distribution. By oral gavage, curcumin (650 mg/kg) and various curcumin-NSAID conjugates (150 mg/kg) solutions in 0.5 percent CMC were given. Under a light ether anesthetic, blood samples (~0.5 ml) were taken from the retro-orbital plexus and placed at intervals into microcentrifuge tubes that had been heparinized (containing 50 l of 1000 U of heparin). To separate the plasma, the blood samples were centrifuged at 10,000 rpm for 7 minutes at 15°. As previously mentioned, plasma was used to extract curcumin. Briefly, 180 μl of plasma was mixed with 50 μl of internal standard (600 ng/ml rhodamine G) and the mixture was vortexed for 60 seconds. The sample was vortexed for a further 2 minutes after the addition of ACN (220 μl) and then centrifuged for 5 minutes at 10,000 rpm. By using HPLC, the quantity of curcumin in the supernatant was measured [38].
Anti-Inflammatory Activity
The research was carried out on healthy adult rats (male and female) weighing 120-160 g. They were given regular pellet feed as well as water to eat. The rats were split into six groups, each with six rats. One set of animals was given the placebo, while the other was given the usual medication (Naproxen). The remaining groups were assigned to the test chemicals. Naproxen and sample compound were given orally by preparing 1% CMC suspension to groups (standard, control, and test compound) respectively. After 30 minutes, 0.1 mL of freshly produced carrageenan suspension in 0.9 percent NaCl solution was injected subcutaneously into the paw and the quantity was calculated. At 1 hour and 2 hours, the foot volume was measured again and the mean increase in paw volume in each group was calculated. A water plethysmometer was used to determine the paw volume. The difference in volume was used to calculate the amount of edema that had been produced. The following calculation is used to calculate the percentage of inhibition value:
[1- Dt/Dc]*100
Dt =Paw volumes of edema in the test
Dc==Paw edema volumes in control.
Anti-Bacterial Activity
The agar-diffusion technique was used to determine preliminary antibacterial activity. A nutritive agar well diffusion test was carried out with 15 lbs (121°C) pressure and immediately after removal from the autoclave, it was chilled to 50-55°C in the water, as described by Magaliet al. (2004). The cooling medium was poured onto sterile Petri plates with a constant 4mm depth, equal to about 40mL, and kept for solidification on a 90mm plate. After the medium had solidified, the culture was injected in a laminar airflow on the media. Within 15 minutes of changing the density of the inoculum, a sterile cotton swab was dipped into the normal bacterial suspension or injected with 1mL of organism solution. The sterile swab was used to ensure that the inoculums were evenly distributed throughout the surface of the nutrient agar medium. To ensure adequate moisture absorption, the plates were left intact for 3 to 5 minutes. The 7mm cork borer was sterilized in the preparation of the agar wells, and the diluted test compound solution concentrations of 25, 50, 75, 100, and 200 g/ml in each well were put, with ciprofloxacin controlled at 100%. For bacterial growth, 37°C and 20-25°C incubation plates were incubated in an incubator. Inhibition zones have been discovered in the vicinity of the agar wells. The diameters of the zones were measured in millimeters.
The inhibition % can be determined using the formula:
Percentage inhibition = (I) diameter of inhibition zone in mm/ (90) diameter of Petri plate in mm
Anti-Ulcer Activity
Wistar albino male rats weighing 200-250 g were split into Six groups (n=6) and given free access to water for two days. The animals were dehydrated for another 24 hours on the third day before undergoing surgery under general anesthesia with Ketamine/diazepam. Group one animals were used as a typical ulcerative control group, with their pylorus ligated and just saline. The same model was used to ligate the pylorus of the rats in the remaining five groups. The animals in groups were administered 20 mg/kg (dissolved in saline) curcumin, CDC, CAC, CNC, and CIC via oral gavage and were decapitated after 19 hours. The excised stomach was then filled with 15 mL of 4 percent formalin after the gastric juice was collected. The fixed stomach was opened along its larger curvature and the ulcer spots were measured, ulcer lesion index and percentage inhibitions were calculated [39-41].
Rheumatoid Arthritis
Balb/c mice with body weights between 15 and 20 g were chosen to create an animal model of antigen-induced arthritis. By injecting modified Streptococcus mutant cell wall extract (SCW) that had already been prepared with proteolytic enzymes intravenously, arthritis was generated. The animals were divided into seven groups, each with six animals, to test the antirheumatic activity of curcumin and curcumin NSAID conjugates. Group 1: normal control; Group 2: disease control; Group 3 oral curcumin 62.50 mg/kg/day; Group 4: oral CAC 107.1 mg/kg/day; Group 5: oral CNC 107.1 mg/kg/day; Group 6: oral CDC 107.1 mg/kg/day; Group 7: oral CIC 107.1 mg/kg/day. Seven days before the induction of arthritis, medication administration began and it continued throughout the trial. The study lasted for fourteen days. On the eighth day of the trial, 10 mg/kg of a modified SCW extract was intravenously injected to cause arthritis. The test samples were still being given to the animals up to the fourteenth day, during which time they were being watched for changes in joint thickness, tail thickness, body weight, and mobility [42-45].
Result and Discussion
Bioavailability
The plasma concentration vs. time profiles in rats, of curcumin (650mg/kg) following single oral administration of CDC, CIC, CNC, and CAC (150 mg/kg), are shown in Figure 3. The pharmacokinetic parameters were estimated by one compartmental analysis of the experimental data. The peak plasma concentration (Cmax) of curcumin obtained was 80 ± 2.41 ng/ml. The mean values of AUC0-8 of curcumin were 3.765 ± 0.205 mg/ml/h. CDC exhibited Cmax of 58.93±1.49 ng/ml, mean values of AUC0-8 of CDC were 3.840 ± 0.262 mg/ml/h, CIC exhibited Cmax of 57.93 ± 1.79 ng/ml, mean values of AUC0-8 of CIC were 4.264±0.224 mg/ml/h, CNC exhibited Cmax of 49.93 ± 1.99 ng/ml, mean values of AUC0-8 of CNC were 4.031 ± 0.203 mg/ml/h, CAC exhibited Cmax of 47.93±2.09 ng/ml, mean values of AUC0-8 of CAC were 4.044±0.182 mg/ml/h. On comparing the AUC0-8 values of curcumin and with other conjugates and it is found that enhancement in the bioavailability of curcumin was observed in another conjugate.
Figure 1: Disease target of curcumin.
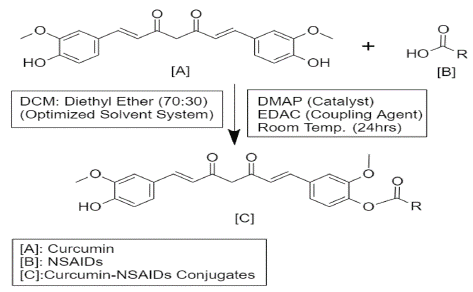
Figure 2: Synthesis of Curcumin-NSAIDs Conjugates.
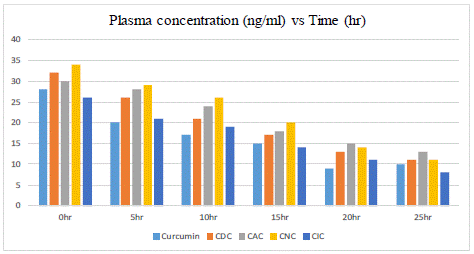
Figure 3: Stability studies of the curcumin-diclofenac conjugate in different media.
Anti-Inflammatory Activity
By assessing the % inhibition of edema with a 1 and 2-hour interval, anti-inflammatory action was discovered. After one hour of delivery, naproxen (48.3%), CDC (53.6%), CAC (64.28%), CNC (67.85%), and CIC were shown to have the highest levels of inhibition (51.78%). After two hours of treatment, it was discovered that naproxen (54.23%), CDC (59.32%), CAC (71.18%), CNC (76.27%), and CIC had the highest levels of inhibition (57.62%). The outcomes demonstrate the respective benefits of curcumin conjugates.
Anti-Bacterial Activity
The antibacterial properties of synthesized compounds (CDC, CAC, CNC, and CIC) were assessed in vitro against pathogens such as gram-positive (Bacillus pumilus, Staphylococcus aureus) and gram-negative (Escherichia coli, Klebsiella pneumoniae). At doses of 1000 g/mL, the bactericidal activity of all four synthesized compounds was assessed. In comparison to the standard (ciprofloxacin), which had a value of 51.94%, the mean percentage of zone inhibition for the CDC was determined to be 54.99%, while it was 58.33% for CAC, 60.83% for CNC, and 45.27% for CIC. The aforementioned information demonstrates the curative properties of curcumin conjugates.
Anti-Ulcer Activity
The mean ± SE value of gastric ulcer lesion index has been shown as 59.66±3.21 of normal saline, 7.52 ± 1.24 of curcumin, 10.21±1.77 of CDC, 11.28 ± 2.32 of CAC, 14.06±1.88 of CNC and 22.37±2.06 of CIC. The percentage inhibition of ulcerative lesions has been shown in fig7. The above data show significant results. The mean ± SE value of the stomach ulcer lesion index was determined to be 59.66±3.21 for normal saline, 7.52 ± 1.24 for curcumin, 10.21 ± 1.77 for the CDC, 11.28 ± 2.32 for the CAC, 14.06±1.88 for the CNC and 22.37±2.06 for the CIC. Figure 7 displays the % inhibition of the ulcerative lesion. The figures up top indicate a substantial outcome.
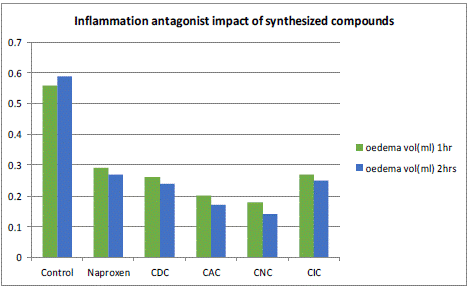
Figure 4: Anti-inflammatory effect of synthesized compounds.
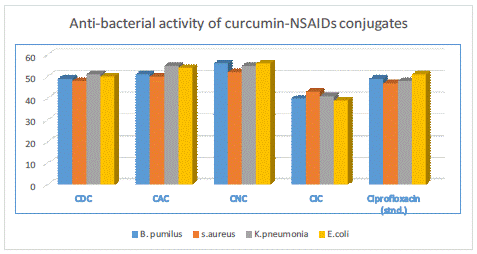
Figure 5: Anti-bacterial effect of synthesized compounds.
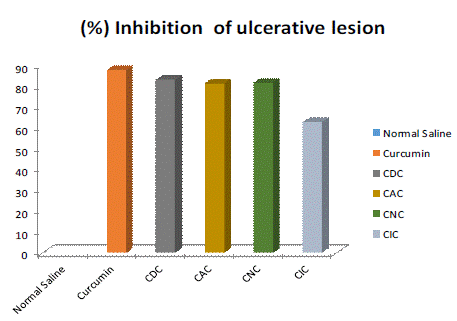
Figure 6: The percentage inhibition of ulcerative lesion.
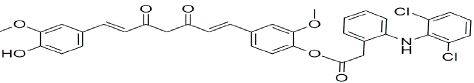
Figure 7: CDC-Curcumin-Diclofenac Conjugate
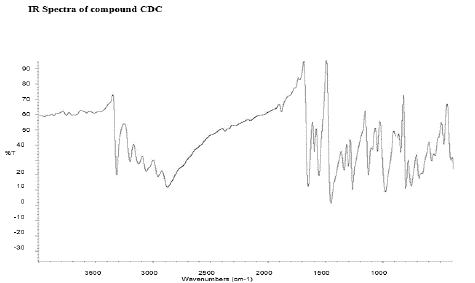
Figure 8: FT-IR peaks of CDC-Curcumin-Diclofenac Conjugate.
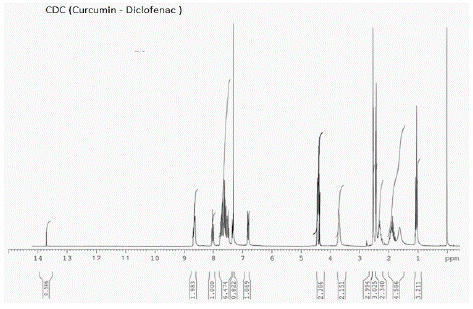
Figure 9: ¹H NMR peaks of CDC-Curcumin-Diclofenac Conjugate.
Figure 10: CAC- Curcumin-Aceclofenac Conjugate.
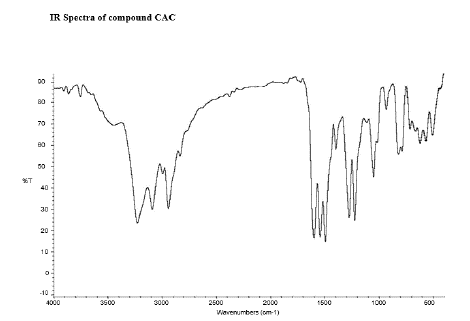
Figure 11: FT-IR peaks of CDC-Curcumin-Diclofenac Conjugate.
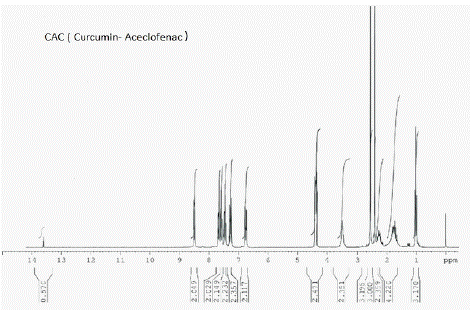
Figure 12: 1H NMR peaks of CAC-Curcumin-Aceclofenac Conjugate.
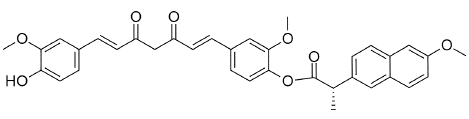
Figure 13: CNC-Curcumin-Naproxen Conjugate.
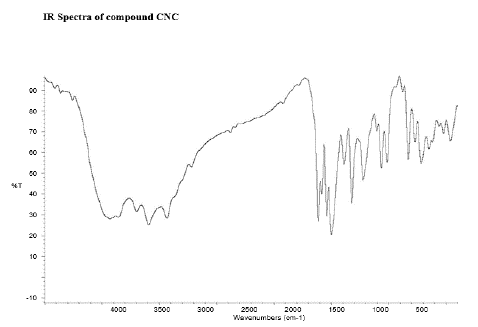
Figure 14: FT-IR peaks of CNC-Curcumin-Naproxen Conjugate
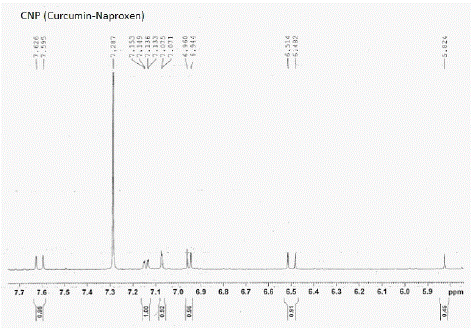
Figure 15: ¹H NMR peaks of CNC-Curcumin- Naproxen Conjugate.
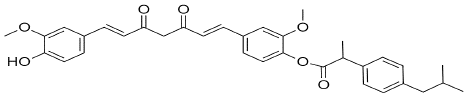
Figure 16: CIC-Curcumin-Ibuprofen Conjugate
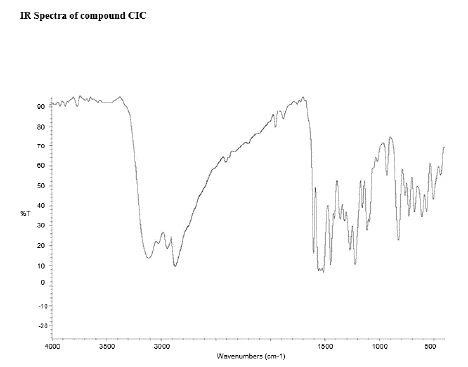
Figure 17: FT-IR peaks of CIC-Curcumin- Ibuprofen Conjugate
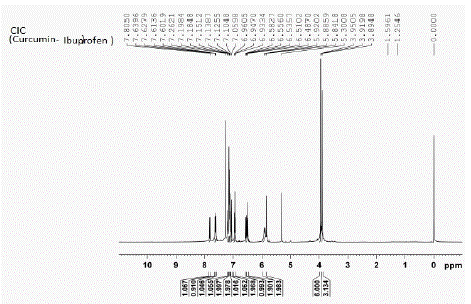
Figure 18: ¹H NMR peaks of CIC-Curcumin- Ibuprofen Conjugate.
Rheumatoid Arthritis
Each animal received a score between 5 and 20 points to represent the degree of arthritis. As a result, the statistics demonstrated how severe arthritis was among various populations (Table 1).
Group
Treatment
Total Score (mean±SD)
Diff. in weight (mean±SD)
Mobility
Appetite
1
Normal control
6.00 ± 0.85
0.02 ± 0.62
Good
Normal
2
Disease control
19.71 ± 0.62
4.54 ± 0.45
Very poor
Poor
3
Curcumin
18.49 ± 1.70
0.32 ± 0.21
Very poor
Poor
4
CAC
7.05 ± 2.10
0.017 ± 0.19
Good
Normal
5
CDC
7.02 ± 0.86
0.021 ± 0.24
Good
Normal
6
CNC
8.00 ± 2.80
0.010 ± 0.61
Good
Normal
7
CIC
12.80 ± 1.42
0.26 ± 0.85
Moderate
Normal
Table 1: Evaluation of the antiarthritic activity of curcumin, and synthesized compounds in balb/c mice
Confirmation of Structural Features of Synthesized Compounds
CDC-Curcumin-Diclofenac Conjugate
FT-IR Peaks
There were specific IR peaks due to Curcumin at 3508 cm-1 1628 cm-1 1603 cm-1 1510 cm-1 1427 cm-1 and 1278 cm-1 The spectrum of Curcumin-Diclofenac Conjugate showed an infrared band at around 1602.85 cm-1 (C=C); 3387.00 cm-1 (N-H amine) peaks; stretching vibrations due to phenolic hydroxyl groups at 3200–3500 cm-1; stretching vibration at 1490 cm-1 associated with the aromatic C=C bond and a bending vibration at 1246 cm-1 attributed to the phenolic C-O group; a stretching band at 1735 cm-1 due to the C=O ester group.
¹H NMR of compounds
¹H NMR (CDCl3): d 3.75 (s, 3H), 3.94 (s, 3H), 4.09 (s, 2H), 5.87 (d, 2H), 6.47 (d, 2H, J=16.4 Hz), 6.58 (d, 1H, J=8.04 Hz), 6.73 (s, 1H), 6.98 (m, 3H), 7.06 (m, 3H), 7.15 (m, 3H), 7.34 (m, 3H), 7.57 (d, 1H, J=7.2 Hz), 7.61 9d, 1H, J=6.92 Hz).
FT-IR Peaks
There were specific IR peaks due to Curcumin at 3508 cm-1 1628 cm-1 1603 cm-1 1510 cm-1 1427 cm-1 and 1278 cm-1. The spectrum of Curcumin-Aceclofenac Conjugate showed the infrared band at around 3320 cm -1 (N-H stretching); 3287 cm -1 (O-H stretching); 1715 cm -1 (C=O stretching); 1284 cm-1 (C-N aromatic amine); 1344.38 cm -1 (O-H in-plane bending; 3000 cm -1 (C-H Stretching); 1455 cm -1 (Aromatic ring stretch); 1659 cm -1 (C=C stretch); stretching vibrations due to phenolic hydroxyl groups at 3200–3500 cm-1
¹H NMR of compound
¹H NMR (CDCl3): d 3.66 (s, 3H), 3.90 (s, 3H), 4.02 (s, 2H), 5.82 (d, 2H), 6.471(d, 2H, J=16.4 Hz), 6.53 (d, 1H, J=8.04 Hz), 6.68 (s, 1H), 6.88 (m, 3H), 7.08 (m, 3H), 7.17 (m, 3H), 7.36 (m, 3H), 7.59 (d, 1H, J=7.2 Hz)
FT-IR Peaks
There were specific IR peaks due to Curcumin at 3508 cm-1 1628 cm-1 1603 cm-1 1510 cm-1 1427 cm-1 and 1278 cm-1. The spectrum of Curcumin-Naproxen Conjugate showed an intense, well-defined peak, infrared band at around 1252 cm-1 due to C–O stretching; 1583 cm-1 due to COO– stretching; C–C aromatic stretching at 1631 cm-1; C–H aliphatic stretch at 2840 cm-1; stretching vibrations due to phenolic hydroxyl groups at 3200–3500 cm-1; stretching vibration at 1490 cm-1 associated with the aromatic C=C bond and a bending vibration at 1246 cm-1 attributed to the phenolic C–O group; a stretching band at 1735 cm–1 due to the C=O ester group.
¹H NMR of compounds
¹H NMR (CDCl3): d 3.70 (s, 3H), 3.92 (s, 3H), 4.11 (s, 2H), 5.83 (d, 2H), 6.40 (d, 2H, J=16.4 Hz), 6.51 (d, 1H, J=8.04 Hz), 6.70 (s, 1H), 6.94 (m, 3H), 7.04 (m, 3H), 7.10 (m, 3H), 7.31 (m, 3H), 7.56 (d, 1H, J=7.2 Hz)
CIC- Curcumin-Ibuprofen Conjugate
FT-IR Peaks
There were specific IR peaks due to Curcumin at 3515 cm-1 1632 cm-1 1598 cm-1 1510 cm-1 1427 cm-1 and 1278 cm-1. The spectrum of Curcumin-Naproxen Conjugate showed an infrared band at 1252 cm-1 due to C–O stretching; 1580 cm-1 due to COO– stretching; C–C aromatic stretching at 1634 cm-1; C–H aliphatic stretch at 2840 cm-1; stretching vibrations due to phenolic hydroxyl groups at 3210–3500 cm-1; and a bending vibration at 1246 cm-1 attributed to the phenolic C-O group; a stretching band at 1735 cm-1 due to the C=O ester group.
¹H NMR of compounds
¹H NMR (CDCl3): d 3.69 (s, 3H), 3.88 (s, 3H), 3.99 (s, 2H), 5.80 (d, 2H), 6.02 (d, 2H, J=16.4 Hz), 6.58 (d, 1H, J=8.04 Hz), 6.71 (s, 1H), 6.91 (m, 3H), 7.06 (m, 3H), 7.08 (m, 3H), 7.31 (m, 3H), 7.50 (d, 1H, J=7.2 Hz)
Drug-drug conjugates were used in our study as a novel approach to synthesize CDC, CIC, CNC, and CAC to increase the bioavailability of curcumin, improve the therapeutic benefit of diclofenac, and in addition, the inherent beneficial effects of curcumin in the GIT have the potential to reduce the side effects and severity of arthritis treatment. The improved stability of CDC, CIC, CNC, and CAC as well as their affinity for the enzymes attached to the gut can be related to the increased bioavailability of curcumin in other conjugates. Furthermore, they demonstrated superior anti-inflammatory efficacy in mice used in a modified SCW model of arthritis. Additionally, they demonstrated strong antibacterial and antiulcer action. Therefore, conjugating curcumin with medicinal molecules may be a creative way to increase its bioavailability and enhance its wide range of pharmacological effects.
Acknowledgement
The authors are grateful to the Director of Dr. KNMIPER, Modinagar, Ghaziabad, for providing us with the facility to execute this work.
Conflict of Interest
Authors declare no conflict of interest.
References
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curcumin”: From kitchen to clinic. Biochem Pharmacol. 2008; 75: 787-809.
- Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005; 223: 181-190.
- Jayaprakasha G, Jaganmohan Rao L, Sakariah K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006; 98: 720-724.
- Kohli K, Ali J, Ansari M, Raheman Z. Curcumin: A natural anti-inflammatory agent. Indian J Pharmacol. 2005; 37: 141.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises. Mol Pharmaceut. 2007; 4: 807-818.
- Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002; 11: 105-111.
- Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008; 17: 1411-1417.
- Agrawal DK, Mishra PK. Curcumin and its analogues: potential anticancer agents. Med Res Rev. 2010; 30: 818-860.
- Itokawa H, Shi Q, Akiyama T, Morris-Natschke SL, Lee KH. Recent advances in the investigation of curcuminoids. Chin Med. 2008; 17: 3-11.
- Dastur JF. Medicinal Plants of India and Pakistan. Treasure House of Books. Bombay. 1970.
- Haddad M, Sauvain M, Deharo E. Curcuma as a parasiticidal agent: a review. Planta Med. 2011; 77: 672-678.
- Zhu ZY. Flora Sichuanica. Sichuan Nationalities Publishing House, 1992.
- Ravindran PN, Ravindran K, Nirmal B, Sivaraman K. Turmeric: the genus Curcuma; Medicinal and aromatic plants industrial profiles. CRC Press. 2007.
- Khanna NM. Turmeric is nature’s precious gift. Current Sci. 1999; 76: 1351-1356.
- Grykiewicz G, Silfirski P. Curcumin and curcuminoids in the quest for medicinal status. Acta Biochim Pol. 2012; 59: 201-212.
- Esatbeyoglu T, Huebbe P, Insa MA, Dawn Chin E, Wagner AE, et al. Curcumin—From Molecule to Biological Function. Angew Chem Int Ed. 2012; 51: 5308-5332.
- Gupta S, Prasad S, Ji HK, Patchva S, Webb LJ, et al. Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep. 2011; 28: 1937-1955.
- Priyadarsini KI. Chemical and structural features influencing the biological activity of curcumin. Curr Pharm Des. 2013; 19: 2093-2100.
- Priyadarsini KI. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from organic solutions, bio-mimetics and living cells. J Photochem Photobiol. C. 2009; 10: 81-96.
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003; 23: 363-398.
- Arvapalli DM, Sheardy AT, Allado K, Chevva H, Yin Z, et al. Design of Curcumin Loaded Carbon Nanodots Delivery System: Enhanced Bioavailability, Release Kinetics and Anticancer Activity. ACS Applied Bio Materials. 2020; 3: 8776-8785.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: Problems and promises: Full Text Finder Results. Mol Pharm. 2007; 4: 807-818.
- Heger M, van Golen RF, Broekgaarden M, Michel MC. The Molecular Basis for the Pharmacokinetics and Pharmacodynamics of Curcumin and Its Metabolites in Relation to Cancer. Pharmacol. Rev. 2013; 66: 222-307.
- Serafini MM, Catanzaro M, Rosini M, Racchi M, Lanni C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol Res. 2017; 124: 146-155.
- Adiwidjaja J, McLachlan AJ, Boddy AV. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin Drug Metab Toxicol. 2017; 13: 953-972.
- Van den Bekerom MPJ, Sjer A, Somford MP, Bulstra GH, Struijs PAA, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating acute ankle sprains in adults: benefits outweigh adverse events. Knee Surg Sports Traumatol Arthrosc. 2015; 23: 2390-2399.
- Scheiman JM, Yeomans ND, Talley NJ, Vakil N, Chan FKL, et al. Prevention of Ulcers by Esomeprazole in At-Risk Patients Using Non-Selective NSAIDs and COX-2 Inhibitors. Am J Gastroenterol. 2006; 101: 701-710.
- Sabzwari SR, Qidwai W, Bhanji S. Polypharmacy in elderly: a cautious trail to tread. J Pak Med Assoc. 2013; 63: 624-627.
- Abdulla A, Adams N, Bone M, Elliott AM, Gaffin J, et al. Guidance on the management of pain in older people. Age Ageing. 2013; 42: 1-57.
- Sabzwari SR, Qidwai W, Bhanji S. Polypharmacy in elderly: a cautious trail to tread. J Pak Med Assoc. 2013; 63: 624-627.
- Rostom A, Dube C, Wells GA, Tugwell P, Welch V, et al. Prevention of NSAID-induced gastroduodenal ulcers. Cochrane Database Syst Rev. 2000; 4: CD002296.
- Vandraas KF, Spigset O, Mahic M, Slørdal L. Non-steroidal anti-inflammatory drugs: use and co-treatment with potentially interacting medications in the elderly. Eur J Clin Pharmacol. 2010; 66: 823-829.
- Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;- 284: 1247-1255.
- Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004; 364: 675-684.
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000; 343: 1520-1528.
- Rahme E, Bardou M, Dasgupta K, Toubouti Y, Ghosn J, et al. Hospitalization for gastrointestinal bleeding associated with non-steroidal anti-inflammatory drugs among elderly patients using low-dose aspirin: a retrospective cohort study. Rheumatology (Oxford). 2007; 46: 265-272.
- Stillman MJ, Stillman MT. Appropriate use of NSAIDs: considering cardiovascular risk in the elderly. Geriatrics. 2007; 62: 16-21.
- Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N, Chatsuwan T. Curcumin suppresses gastric NF-κB activation and macromolecular leakage in Helicobacter pylori-infected rats. World J Gastroenterol. 2010; 16: 4039.
- Saroj K. Pal, Pulok K, Mukherjee BP. Saha, Studies on the Antiulcer Activity of Moringa oleifera Leaf Extract on Gastric Ulcer Models in Rats. Phytotherapy Research. 1995; 9: 463-465.
- Pillai NR, Santhakumari G. Effects of nimbidin on acute and chronic gastroduodenal ulcer models in experimental animals. flanta Med. 1984; 50: 143-146.
- Aguwa Cletus N, Ramanujam TR. Antiulcer effects of trimipramine using various laboratory models. Jpn J Pharmacol. 1984; 36: 125-129.
- Jain SK, Gill MS, Pawar HS, Suresh S. Novel Curcumin Diclofenac Conjugate Enhanced Curcumin Bioavailability and Efficacy in Streptococcal Cell Wall-induced Arthritis. Indian J Pharm Sci. 2014; 76: 415-422.
- Li X, Bradford BU, Dalldorf F, Goyert SM, Stimpson SA, et al. CD14 mediates the innate immune responses to arthritopathogenic peptidoglycan-polysaccharide complexes of Gram-positive bacterial cell walls. Arthritis Res Ther. 2004; 6: 273-281.
- Kannan K, Ortmann RA, Kimpel D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiol. 2005; 12: 167-181.
- Koga T, Kakimoto K, Hirofuji T, Kotani S, Ohkuni H, et al. Acute joint inflammation in mice after systemic injection of the cell wall, its peptidoglycan, and chemically defined peptidoglycan subunits from various bacteria. Infect Immun. 1985; 50: 27-34.