
Research Article
Austin J Anal Pharm Chem. 2023; 10(2): 1160.
Suppressive and Prophylactic Effects of Ethanol and Aqueous Extracts of Phyllantus Amarus Leaf in Plasmodium Berghei-Infected Mice
Olabode Isaiah Ogunsina1,2; Augustine Olusegun Olusola1*
1Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba Akoko, Nigeria
2Department of Pharmacognosy, Dora Akunyili College of Pharmacy, Igbinedion University Okada, Edo State
*Corresponding author: Augustine Olusegun Olusola Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba Akoko, Nigeria. Email: augustine.olusola@aaua.edu.ng, austinolusola@gmail.com
Received: September 08, 2023 Accepted: October 24, 2023 Published: October 31, 2023
Abstract
The increased resistance of the malaria parasite to synthetic medications has increased the hunt for alternative treatment options derived from plants. The suppressive and preventive potentials of Phyllanthus amarus extracts in Plasmodium berghei-infected mice were studied in this work. For five days, albino mice were given extract at three different concentrations (100, 200, and 400 mg/kg/bwt.). The reference medications were 5 mg/kg bd wt. daily doses of artemether-lumefantrine and 5 mg/kg bd wt. daily doses of chloroquine, while the control mice got just the vehicle (normal saline) and were infected but not treated (negative control). The mice in the prophylactic groups were pretreated daily for five days before being challenged with inoculums of 1×107 chloroquine-sensitive bacteria. P. berghei erythrocyte intraperitoneally.
Chemosupression was shown to be dose dependent in the extract-treated groups. The 400 mg/kg bd wt dose was more effective than the 100 and 200 mg kg bd wt doses in terms of parasite clearance. Chemosupression was considerably (p<0.001) greater in the Artemether-Lumefantrine (AL) and Chloroquine (CQ) treated groups than in the extract-treated groups. The Phyllanthus amarus extracts caused a significant decrease in parasitemia counts, which reduced the percentage parasitemia. When the AL and CQ extracts and 400 mg kg bd wt doses were compared to the controls, the Packed Cell Volume (PVC) increase significantly (p<0.05). This study found that Phyllanthus amarus leaf extracts exhibit significant antimalarial properties that could be useful in malaria
Keywords: Artemether-lumefantrine;Chemosuppression; Chloroquine; Phyllanthus amarus; Plasmodium berghei
Introduction
Malaria is derived from the Medieval Italian word mala aria, which means "bad air," and was previously known as ague or marsh fever due to its relationship with marshes. Malaria is currently the most frequent tropical disease in the world. It has been infecting people for nearly 50,000 years and may have been a human pathogen throughout our species' history. During the first half of the twentieth century, malaria afflicted individuals from all walks of life in tropical and subtropical countries, becoming one of the most serious impediments to economic development [1].
Malaria is caused by Plasmodium parasites, which infect and kill human red blood cells [2]. Malaria continues to be a huge global burden on both public health and economic prosperity, with an estimated 217 million infections and 0.7 million fatalities per year [2]. However, the real frequency of malaria is likely to be underestimated in certain significant endemic areas [3]. Malaria affects over 40% of the world's population. An estimated 1.2 billion persons are at high risk of transmission (1 case per 1000 population), with half of them residing in African regions; 80% of such cases are concentrated in 13 countries, with Nigeria, Congo, Ethiopia, Tanzania, and Kenya accounting for more than half [2]. One-fifth of all malaria cases in Africa are reported in Nigeria [4]. Transmission occurs all year in the southern area of Nigeria, but is more seasonal in the north. Plasmodium falciparum, the most fatal infectious agent on the planet, is to blame for nearly all malaria infections in the country. Malaria treatment and control continue to rely mainly on antimalarial medications [5-8]. In Nigeria and throughout Africa, Artemisinin Combination Therapy (ACT) has been utilized as the first-line treatment for uncomplicated malaria. ACTs have been proven to be highly efficient against Plasmodium parasites, with several being moderately effective against early stages of infection and mosquito transmission. Artemether-Lumefantrine (AL) is the most often used ACT in Nigeria and the African continent. Dihydroartemisinin-Piperaquine (DP) is more effective and has the advantages of easier dosage and a longer prophylactic period [9].
Recent reports of Artemisinin resistance along the [10-12] emphasize the importance of alternate treatments. Plasmodium falciparum has developed resistance to routinely used medicines such as chloroquine and ACT. Malaria remains one of the world's most lethal parasite infections. Malaria is most widespread in African and Asian developing or undeveloped countries, where it kills a lot of kids, especially kids under the age of five and pregnant women [9]. The annual mortality rate is currently estimated to be around 405,000 [9]. As a result, finding new antimalarial medications is crucial. Tropical rainforest plants, according to Balick (1996) [13], have higher concentrations of natural chemical defense and greater diversity than plants from any other biome. Because Sub-Saharan African countries, including Nigeria, account for the majority of malaria-related deaths, there is a drive for study on plants native to these countries. This hypothesis is bolstered by the fact that major antimalarial drugs like quinine and artemisinin are derived from plants, Cinchona ledgeria and Artemisia annua, respectively.
Phyllanthus amarus is a Euphorbiaceae-related little erect tropical annual herb shrub with a green capsule that grows up to 10-50cm high and blooms with five white petals and an apical acute anther (Spurge family). Green capsules and smooth, fruiting pedicels define this fruit. The seeds are rugose all the way down [15]. It grows as a weed on uncultivated soil and is called as "dobisowo," "ehinolobe," or "ehin olubisowo" in Nigeria's Yoruba region, "ngwu" by the Igbo tribe, and "buchioro" by the Asaba people [14].
Phyllanthus amarus is utilized for its hepatoprotective, antidiabetic, antihypertensive, analgesic, antiinflammatory, and antibacterial effects, according to an ethnobotanical survey [15]. The herb is also used to cure digestive problems, skin diseases, and colds [16;17]. It possesses anti-diarrheal properties [18]. It inhibits the hepatitis B virus and has antiviral, anticarcinogenic, and antimutagenic effects [19, 20]. It also has antinociceptive, anti-diabetic, and antilipidemic properties [21;15].
Antibacterial, antiviral, chemoprotective, antimutagenic, and hypoglycemic activities are among the pharmacological activities of P. amarus. Immunomodulatory activity was discovered in a methanol extract of P. amarus. Ellagitannins (geraniin and corilagin) have been found as the most powerful antiviral HIV mediators. P. amarus phyllanthin and hypophyllanthin were found to have anticancer characteristics in Swiss albino mice, as well as cytotoxic effects on K-562 cells, as well as hepatoprotective and antioxidant qualities [15]. Thus, the primary purpose of this work was to assess the suppressive and preventive effects of Phyllanthus amarus leaf extracts on Plasmodium berghei-induced malaria models in mice.
Materials and Methods
Extraction of Plants
The Phyllanthus amarus leaves were properly cleansed in flowing tap water to remove soil particles and adherent debris before being washed with sterile distilled water at the Igbinedion University Campus. The leaves were separated and airdried for three weeks at room temperature. The dried leaves were pulverized into a fine powder using a high-speed electric blender. Five hundred grams of crushed leaves were steeped for 24 hours in 1000 ml of distilled water and 100% ethanol, with intermittent agitation. The filtrates were concentrated using a rotary evaporator and freeze dryer after the ethanol and aqueous solutions were filtered through Whatman No.1 filter paper. The extracts' antiplasmodial activity was tested in mice using a Plasmodium berghei-induced malaria model.
Phytochemical Screening of Phyllanthus Amarus Leaf Extracts
To determine the components of Phyllanthus amarus, ethanol and aqueous extracts were subjected to qualitative analysis using established techniques [22].
Acute Toxicity Determination
The acute toxicity tests of the Phyllanthus amarus leaf extract in mice were carried out by determining their effective and median Lethal Dose (LD50) using the Lorke’s method [23].
Experimental Animals
The animal house of the Institute of Advanced Medical Research and Training (IAMRAT), College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria, provided inbred male Swiss albino mice weighing 18-21g (mean weight, 20.4g). A total of 84 male mice were employed, separated into seven groups of three mice each depending on weight, and kept in various plastic cages with saw dust as bedding. They were fed a pelletized Top Feeds meal and had unfettered access to water. The mice were cared for and utilized in the experiments in compliance with National Institute of Health Laboratory animal care guidelines. The animals were acclimatized for five days following grouping before the experiment began.
Experimental Analysis
Inoculum
The chloroquine-sensitive Plasmodium berghei was obtained from the Institute of Advanced Medical Research and Training (IAMRAT) at the University of Ibadan's College of Medicine in Ibadan, Oyo State, Nigeria. In mice, constant re-infestation keeps parasites alive. This was accomplished by calculating the parasitemia percentage of the donor mice's erythrocyte count and diluting them with normal saline in the proportion specified by both calculations. Each mouse received an intraperitoneal injection of Plasmodium berghei parasitized erythrocytes (0.2 mL) infected blood suspension. The parasitaemia % was estimated by dividing the total number of red blood cells by the number of parasitized red blood cells.The formula used for calculating the volume of blood from donor mice is as given below;
Vol of blood from random mice=
Therefore, to inoculate 39.44% P. berghei parasite into 84 mice, it as calculated as follows;
Vol.of blood from random mice = = .47 ml
The volume of normal saline used to dilute the blood of random mice was 0.2 ml X 84 = 16.8 ml.
As a result, 16.8 ml minus 0.47 ml equals 16.33 ml.
Therefore, 0.47 mL of blood from donor mice was diluted with 16.33 mL of normal saline, resulting in 0.2 mL of final inoculum containing 1 x10-7 parasitized red blood cells, which are the usual inoculums for a single mouse infection [24].
Antimalarial drug: To evaluate Plasmodium berghei medication susceptibility in vivo, tablets of chloroquine phosphate 5 mg/kg, CQ (Pfizer, Nigeria) and artemether-lumefantrine 5mg/kg (Greenlife Pharmaceuticals) were utilized. The medication was freshly mixed in distilled water and given orally through an orogastric tube. The drug dose, indicated in milligrams per kilogram of body weight, was adjusted at the time of administration based on the average weight of the mice.
Chemo-suppressive test: In mice, the Peters' 4-day suppressive test was used to treat chloroquine-sensitive P. berghei NK-65 infection [28]. P. berghei standard inoculum containing parasitized erythrocytes was administered intraperitoneally (i.p) to 42 male mice weighing 18-21 g. Some experimental groups were given different doses of the extract (100, 200, and 400 mg/kg/day). The extract was given to the mice orally in a single dose per day using a 1 mL disposable syringe. The positive control group was given 5 mg of chloroquine per kg body weight and 5 mg of artemether-lumefantrine per kg, whereas the negative control group was given an equivalent volume of sterile distilled water, and a group was left uninfected. On the fifth day of the experiment, blood was extracted from each mouse's tail vein and smeared on a microscope slide to create a thin film smear. The blood films were preserved with methanol, stained for 10 minutes with 10% giemsa at pH 7.2, and parasitaemia was examined under a microscope (X100 magnification). The percentage suppression of parasitaemia was computed at each dose level by comparing parasitaemia in infected but untreated controls to parasitaemia in treated mice, and the result was expressed as a percentage.
Repository/prophylactic activity: The mice were put into seven groups of three mice each at random. Mice in group 1 (negative control group) were given distilled water orally at a dose of 10 ml/kg body weight. Group 2 was infected but untreated. Groups 3 and 4 were given 5mg/kg/day body weight chloroquine phosphate and artemether-lumefantrine, respectively. Both were used as positive control groups. Furthermore, groups 5–7 were given ethanol and aqueous fractions at doses of (100, 200, and 400 mg/kg body weight, respectively). The mice were orally treated for three days (D1-D3) before being intraperitoneally injected with 0.2 ml of contaminated blood containing 1×10-7 Plasmodium berghei parasitized Red Blood Cells (RBCs) on day four (D4). The parasitaemia level was determined using thick and thin films made from the tail blood of each mouse 72 hours (Day 7) following parasite inoculation. The relative value approach was used to measure parasite density by counting Plasmodium parasites against 200 or 500 white blood cells and expressing the resultant number of parasites/l by blood assuming a white blood cell count of 8000 per μl of blood [15]. Parasite density was deduced by using the formula:
Staining and Examination of the Thick and Thin Smears
For each mouse sample, two films were created. Each slide had a measured volume of blood of 6 l for thick film and 2 l for thin film: 10% (1:9 ml) for 7 minutes and 3% (3:97 ml) for 45-60 minutes. Working Giemsa stains were made from Giemsa-staining solution that had already been prepared. After fixing the thin smear with 100% methanol, thick and thin blood smears were stained with Giemsa. The full film was first examined at a low magnification (10X40X objective lens) to identify eligible fields with even WBC distribution (10 - 20 WBC/field) [25]. Smears were then inspected with an immersion in X100 oil. Before a thick smear was pronounced negative, at least 100 high power fields were investigated. Plasmodium berghei parasites were counted per 200 or 500 leukocytes, which were used to estimate the parasite density per micro litre of blood.
Statistical Analysis
GraphPad Prism version 8.0 (GraphPad Software, Inc. San Diego, CA, USA) was used to analyze the data, and the findings were expressed as Mean + SEM. The statistically significant differences between and within groups were examined using One-way ANOVA, followed by multiple comparisons of their means using Turkey's post hoc test. At p<0.05, differences were considered statistically significant.
Results
Suppressive Effect of Ethanolic Extract of Phyllantus Amarus on Parasitemia in Mice
Figure 1 depicts the percentage parasitemia in the control and treatment groups. The ethanolic extract reduced the percentage parasitemia level in a dose-dependent, significant (p<0.05) way. The 400 mg/kg/day body weight dose reduced parasitemia considerably (p<0.05) more than the 100 and 200 mg/kg/day body weight doses. Following a 4-day treatment with (100, 200, and 400) mg/kg/day body weight, the ethanolic extracts of P. amarus showed a percentage suppression of 46.19%, 57.10%, and 75.44%, respectively. Chloroquine at 5mg/kg had the highest percentage suppression of 95.32%, whereas Artemether-Lumefantrine at 5 mg/kg bd wt had a chemo-suppression of 91.99%. A daily increase in parasitemia level in the control group was observed. The parasitemia level in the group treated with chloroquine and artemether-lumefantrine were significantly lower than the extract-treated group.
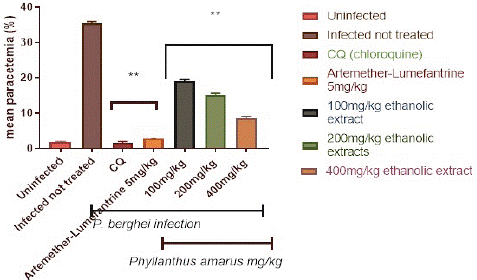
Figure 1: Suppressive effect of ethanolic extract of Phyllantus amarus on parasitemia in mice.
Suppressive Effect of Aqueous Extract of Phyllantus Amarus on Parasitemia in Mice
Figure 2 depicts the percentage parasitemia in the control and treatment groups. The aqueous extract reduced the percentage parasitemia level in a dose-dependent, significant (p<0.05) way. The 400 mg/kg/day body weight dose reduced parasitemia considerably (p<0.05) more than the 100 and 200 mg/kg/day body weight doses. Following a 4-day treatment with (100, 200, and 400) mg/kg/day body weight, the aqueous extracts of P. amarus generated a percentage suppression of 49.76%, 60.87%, and 77.54%, respectively. Chloroquine at 5 mg/kg had the highest percentage suppression of 96.6%, whereas artemether-lumefantrine at 5 mg/kg had a chemo-suppression of 93.8%.
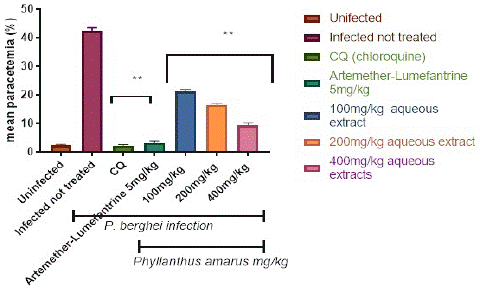
Figure 2: Suppressive effect of aqueous extract of Phyllantus amarus on parasitemia in mice.
Prophylactics Effect of Ethanolic and Aqueous Extract of Phyllantus Amarus on Parasitemia in Mice
Figure 3 depicts the preventive potential of ethanolic and aqueous extracts of P. amarus leaf on P. berghei-infected mice. The plasmodium parasite was discovered in the highest concentration in the P. berghei transfected untreated mice. When compared to the control, the administration of extracts resulted in significant (p<0.001) dose-dependent declines in parasite counts in the treated groups. When compared to controls, significant (p<0.001) decreases in the level of malarial parasites in the blood were only observed in mice pretreated with 100, 200, and 400 mg/kg/day body weight of ethanolic and aqueous extracts of Phyllantus amarus leaf extract on the fifth day of transfection with the percentage parasites as depicted in Figure 3 and Figure 4, following a 7-day prophylactic therapy with (100, 200, and 400) mg/kg/day body weight, the aqueous extracts of P. amarus produced a percentage inhibition of 37.48%, 58.99%, and 81.58%, respectively, whereas the ethanolic extracts possessed 43.31%, 60.05%, and 82.16%. When compared to the control, which had no suppression, the percentage chemo-suppression for each of the dosage increased with increasing number of days’ post transfection for each dosage. Furthermore, the extracts' effect was lower when compared to the reference drugs (5mg/kg chloroquine and 5 mg/kg artemether-lumefantrine), which caused significant (p<0.001) decreases on the 7 days' transfection with percentage parasitemia inhibition of (94.60% and 90.62% of chloroquine and artemether-lumefantrine, respectively). Chloroquine 5mg/kg had the highest percentage suppression of 95.8%, while Artemether-Lumefantrine 5mg/kg had the lowest chemo-suppression of 89.9%).
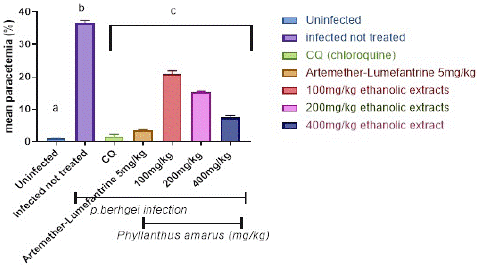
Figure 3: Prophylactics effect of ethanolic extract of Phyllantus amarus on parasitemia in mice.

Figure 4: Prophylactics effect of aqueous extract of Phyllantus amarus on parasitemia in mice.
Packed Cell Volume (PCV) in the Control and Ethanolic Treatment Groups
The Packed Cell Volume (PCV) in the control and treatment groups is depicted in Figure 5. When compared to the extract treated groups, the packed cell volume in the infected but untreated controls was considerably (p<0.05) lower. In the extract-treated groups, PCV improves in a dose-dependent way. After the fifth day of treatment, the extract at (100, 200, and 400) mg/kg/day body weight was shown to be greater in a dosage dependent way. PCV improved much more in the AL and CQ treated groups than in the extract treated groups.
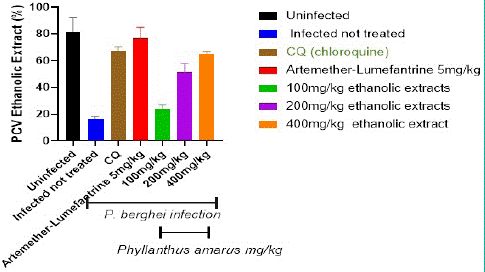
Figure 5: Shows the Packed Cell Volume (PCV) in the control and aqueous treatment groups.
Figure 6 depicts the total Packed Cell Volume (PCV) in the control and treatment groups. When compared to the extract treated groups, the packed cell volume in the infected but untreated controls was considerably (p<0.05) lower. In the extract-treated groups, PCV improves in a dose-dependent way.
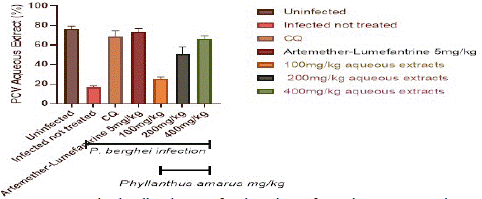
Figure 6: Packed cell volume of P. berghei infected mice treated with Phyllantus amarus leaf.
After the fifth day of therapy, the extract at (100, 200, and 400) mg/kg/day body weight was significantly higher in a dosage dependent manner. PCV increased much more in the AL and CQ treated groups than in the extract treated groups.
Discussion
Malaria is a cytokine-driven inflammatory disease that can result in mortality owing to red blood cell lyses. It is distinguished by oxidative alterations in animals caused by the parasites' various metabolic activities. Although in vivo screening provides a thorough system for assessing medication efficacy in the biological milieu, it is frequently impacted by host characteristics such as drug disposition and other intrinsic drug antiparasitic actions. However, it does not necessarily identify what constitutes a good antimalarial medicine in terms of disposition. Despite its poor animal disposition, artemisinin, for example, is quite efficacious in vivo [27]. Plasmodium berghei is the species of choice for in vivo screening due to its higher accessibility and acceptability in drug. Some genes in the rodent adapted to Plasmodium berghei may have diverged significantly from the human pathogen Plasmodium falciparum.
The in vivo antiplasmodial potential of both the ethanolic and aqueous extracts of P. amarus for the treatment of malaria parasite against P. berghei infection in Swiss albino mice was investigated using a rodent model of malaria. Several classic antimalarial drugs, including chloroquine, halofantrine, mefloquine, and, more recently, artemisinin derivatives, have been found using rodent malaria models [26]. Because P. berghei is used to predict treatment outcomes, it was an appropriate parasite for the investigation. Because this parasite is sensitive to chloroquine, it was utilized as the control medication in this investigation, along with artemether-lumefantrine.
The extracts are so safe, which could explain why these plants have been used safely in the treatment of malaria in Nigeria by traditional people. As a result, the ethanolic and aqueous extracts are a useful solvent for this study. The 4-day suppressive test is a typical antimalarial screening test, and the percentage inhibition of parasitaemia is the most accurate metric. In typical screening investigations, a mean group parasitaemia level of less than or equal to 90% of the mock-treated control animals usually indicates that the test material is active [28]. The results of this study demonstrated a significant decrease in parasitaemia of P. berghei infected mice treated with (100, 200, and 400) mg/kg body weight of each of aqueous and ethanolic leaf extracts of Phyllantus amarus in a dose dependent manner (Figure 1 & 2), whereas chloroquine and artemether-lumefantrine, standard antimalarial drugs, suppressed parasitaemia by 94.8% and The found antimalarial activity is consistent with the plants' traditional use as a herbal medicine against malaria in Nigeria. The significant (p<0.001) reduction in parasite levels in the blood of mice pretreated with (100, 200, and 400) mg kg bwt of Phyllantus amarus extracts after the first day of parasite transfection suggests that Phyllantus amarus has a significant prophylactic effect against malaria infection in P. berghei infected mice. This validates the plant leaf's ethnomedicinal use as an antimalarial drug in Sub-Saharan Africa [29;30]. In accordance with Kamei et al. (2000) [31] submission on the effect of typical antimalarial medicines on P. berghei-infected mice, Chloroquine (CQ) and Artemether/Lumefantrine (AL) utilized in this investigation exerted nearly 100% suppression on the third day at 5 mg kg bwt. The observation that the chemosupression offered by both ethanolic and aqueous extracts of Phyllantus amarus increases with increasing concentration of the extracts, with the 400 mg k bd wt. proving to be more effective than the (100 and 200) mg kg bwt., demonstrates the dose-dependent nature of the antimalarial effect. This effect may be due to short duration of action of the extract occasioned by rapid metabolism and so parasite clearance could not be total. The considerable reduction in Packed Cell Volume (PCV) of mice infected with the parasites could be attributed to the combined effect of plasmodial infection and probable destruction or clearance of plasmodial infected blood cells by the extracts provided [32]. The decrease in PCV, which causes malaria-induced anemia, occurs by increasing the pace at which old red blood cells are damaged and decreasing the rate at which new ones are formed. Plasmodium not only causes parasitized red blood cells to burst, but it also drives macrophage activity in the spleen, which then destroys both parasitized and unparasitized red blood cells [33]. In mice pre-treated with greater doses (400 mg k bwt.) of the extracts, the malaria-induced drop in PCV was totally reversed in a manner reference to the reference drug. This clearly demonstrates the intervention's antimalarial potency, providing validity to the plant's traditional applications in this area.
Conclusion
In conclusion, despite the activities of these extracts appear lower in comparison to standard drugs, they demonstrate significant antimalarial effects in the numerous models tested in this investigation, which can be regarded as support for their ethnomedicinal antimalarial claims. The study of the chemical ingredients responsible for these effects will be fascinating, and it may lead to the identification of novel chemical entities with significant antimalarial characteristics. The recent findings from two complementary in vivo models demonstrate the importance of the plant components utilized to make herbal treatments for the suppression, cure, and prevention of malaria infection.
Author Statements
Acknowledgement
We appreciate the efforts and technical skills of Mr Afolabi Owoloye (Parasitology Unit, Department of Animal and Environmental Biology) and Mr Olatunbosun Akele (Laboratory Unit, Department of Microbiology), Adekunle Ajasin University Akungba Akoko, Nigeria.
Conflict of Interest
There was no declaration of interest and no conflict among the authors in presenting this article for publication.
References
- Sachs J, Malaney P. The economic and social burden malaria. Nature. 2002; 415: 680-5.
- World Health Organization (WHO). Global report on antimalarial drug efficacy and drug resistance [cited May 26 2013]. Available from: http://www.who.int/malaria/publications/atoz/9789241500470/en/. Geneva, Switzerland: World Health Organization Website Available; 2000-2010.
- Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RajuM, Rodriguez PS, et al. Adult and child malaria mortality in India: a nationally representative mortality survey. Lancet. 2010; 376: 1768-74.
- World Health Organization (WHO). World malaria report by roll back malaria partnership. New York; 2005; 9.
- malERA Consultative Group on Drugs. A research agenda for malaria eradication: drugs. PLOS Med. 2011; 8: e1000402.
- Olliaro P, Wells TN. The global portfolio of new antimalarial medicines under development. Clin Pharmacol Ther. 2009; 85: 584-95.
- Yeramian P, Meshnick SR, Krudsood S, Chalermrut K, Silachamroon U, Tangpukdee N, et al. Efficacy of DB289 in Thai patients with Plasmodium vivax or acute, uncomplicated Plasmodium falciparum infections. J Infect Dis. 2005; 192: 319-22.
- World Health Organization (WHO). World malaria report 2010. New York: Roll Back Malaria Partnership; 2010; 6.
- Olusola AO, Ogunsina OI, Olusola AO. Antimalarial Potential of Flavonoid-rich Extract of Lannea acida and chloroquine in Mice Infected with Plasmodium berghei. Int J Sci Eng Res. 2020; 11: 201-206.
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009; 361: 455–67.
- O’Brien C, Henrich PP, Passi N, Fidock DA. Recent clinical and molecular insights into emerging artemisinin resistance in Plasmodium falciparum. Curr Opin Infect Dis. 2011; 24: 570-7.
- Burrows JN, Chibale K, Wells TN, Kelly Chibale, Timothy NC Wells. The state of the art in anti-malarial drug discovery and development. Curr Top Med Chem. 2011; 11: 1226-54.
- Balick MJ. Medicinal resources of the tropical rain forest. New York: Columbia University Press. 1996; 24.
- Adjanohoun E, Aboubakar N, Dramane K, Ebot ME, Ekpere JA, Enoro Orock EG, et al. Contribution to Ethnobotanical and floristic studies in Cameroon: CSTR/OUA. 1996.
- Adeneye AA, Benebo AS, Agbaje EO. Protective effect of the aqueous leaf and seed extract of Phyllanthus amarus on alcohol-induced hepatotoxity in rats. West Afr J Pharmacol Drug Recourses. 2006; 22: 42-50.
- Kokwaro JO. Medicinal plants of East Africa. 1st ed. Nairobi: East Africa Literature Bureau. 1976; 58-9.
- Iwu MM. Modalities of drug administration. In: Hand book of African medicinal ORC press inc. FL. 1993; 309-30.
- Odetola AA, Akojenu SM. Anti-diarrheal and gastrointestinal potentials of the aqueous extracts of Phyllanthus amarus (Euphorbiaceae). Afr J Med Med Sci. 2000; 29: 119-22.
- Thyagarajan SP, Subramanian S, Thirunalasundari T, Venkateswaran PS, Blumberg BS. Effects of Phyllanthus amarus on the chronic calT1ers of hepatitis B vims. Lancet. 1988; 2: 764-6.
- Joy KL, Kuttatu R. Inhibition by Phyllanthus amarus of hepatocarcinogenesis induced by N-nitrosodiethylamine. J Nutr Biochem. 1998; 24: 133-9.
- Kassuya CA, Silvestre AA, Rehder V, Calixto LB. Antiallodynic and antioedematogeni properties of the lignan from Phyllanthus amarus in models of persistent inflammatory and neuropathic pain. Eur J Pharm Sci. 2003; 478: 145-53.
- Trease GE, Evan MC. Textbook of pharmacognosy. 14th ed. London: Tindall press. 1999; 744.
- Lorke D (19830. A new approach to practical acute toxicity testing. Arch Toxicol. 1983; 54: 275-87.
- Okokon JE, Etebong EO, Udobang JA, Obot J. Antiplasmodial and antiulcer activities of Melanthera scandens. Asian Pac J Trop Biomed. 2012; 2: 16-20.
- Owusu-Agyei S, Asante KPoku, Adjuik M, Adjei G, Awini E, Adams M, et al. Epidemiology of malaria in the forest-savannah transitional zone of Ghana. Malar J. 2009; 8: 24-9.
- Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov. 2004; 3: 509-20.
- White NJ. Qinghaosu (artemisinin): The price of success. Science. 2008; 320: 330-4.
- Peters W. Drug resistance in Plasmodium berghei. Vinka and lips. Exp Parasitol. 1967; 17: 80-9.
- Sidibe M, Williams JT. Baobab, Adansonia digitata L. crops for the future, Southampton, UK, ISBN: 9780854327768. 2002; 99.
- De Caluwé E, Halamouá K, van Damme P. Adansonia digitata L.: a review of traditional uses, Phytochemistry and pharmacology. Afr Focus. 2010; 23: 11-51.
- Kamei K, Matsuoka H, Furuhata SI, Fujisaki RI, Kawakami T, Mogi S, et al. Anti-malarial activity of leaf-extract of Hydrangea macrophylla, a common Japanese plant. Acta Med Okayama. 2000; 54: 227-32.
- Tomas AM, van Der Wel AM, Thomas AW, Janse CJ, Waters AP. Transfection systems for animal models of malaria. Parasitol Today. 1998; 14: 245-9.
- Umar MB, Ogbadoyi EO, Ilumi JY, Salawu OA, Tijani AY, Hassan IM. Antiplasmodial efficacy of methanolic root and leaf extracts of Morinda lucida. J Sci Res. 2013; 3: 112-22.