
Research Article
Austin J Anal Pharm Chem. 2023; 10(2): 1162.
Elucidation of Alcaloids Contained in the Fec5 Fraction of Bark of Annickia Chlorantha
Aimé Bertrand Madiélé Mabika1,3,4; Arnaud Wenceslas Geoffroy Tamba Sompila4,5*; Arnold Murphy Elouma Ndinga1; Huguette Agnaniet2; Valery Thiery3; Jean Maurille Ouamba1
1Unit of Life and Plant Chemistry (UC2V), Marien Ngouabi University, Brazzaville Congo
2Laboratory of Natural Substances and Organometallic Synthesis (LASUNSO), University of Sciences and Techniques of Masuku, Franceville Gabon
3Coastline Environment and Society (LIENs), University of La Rochelle, France
4National Institute for Research in Engineering Sciences, Innovation and Technology, Scientific City, Brazzaville Congo
5Laboratory of Food and Medical Bioprocesses, National Polytechnic School, Marien Ngouabi University, Brazzaville Congo
*Corresponding author: Arnaud Wenceslas Geoffroy Tamba Sompila Unit of Life and Plant Chemistry (UC2V), Marien Ngouabi University, Brazzaville Congo. Tel: +242 06.662.87.91 Email: arnaud.wens@gmail.com
Received: September 04, 2023 Accepted: October 31, 2023 Published: November 07, 2023
Abstract
The aim of this study was to highlight the presence in the barks of A. chlorantha, new alkaloids not yet identified in the literature. FEC5 fractionation method by semi-preparative HPLC revealed twelve (12) sub-fractions. Of these twelve sub-fractions, five were exploited. These five sub-fractions EC3, EC9, EC10, EC11 and EC12 revealed fifteen (15) alkaloids which were isolated, designated by the letters A to O, and identified mainly by mass spectrometry. The results obtained allowed us to note that the alkaloidal composition of the plant, especially of the barks, is still very poorly elucidated. The isolated alkaloids belong, for the most part, either to the group of protoberberines and tetrahydroprotoberberines or to the bisbenzyltetrahydro isoquinolines which have not yet been reported in the literature. The isolation of palmatine corroborated the results of the literature. These results showed that these sub-fractions are not totally pure. They are a mixture of compounds. The complexity of the alkaloid composition of Annickia chlorantha trunk bark is particularly justified by the number of alkaloids present.
Keywords: Bisbenzyltetrahydroisoquinoleines; Extraction; Fractionation; Isolation; Purification; Tetrahydroprotoberine
Nomenclature: FEC: Fraction Annickia chlorantha; EC: Subfraction Annickia chlorantha; TLC: Thin Layer Chromatography; HPLC: Hight Performance Liquid Chromatography; UV: UltraViolet; NMR: Nuclear Magnetic Resonance; SMHR: High Resolution Mass Spectrometry; DRC: Democratic Republic of Congo; HIV: Human Immunodeficiency Virus
Introduction
Plants have been used in traditional medicine for many millennia. Curiosity is the innate principle of the doctrine that, indicating the possibility of identifying the peculiarities and virtues of each plant by its signature, guided the first men in the choice of new preparations to be tested [1]. In the last decades there has been a growing interest in the study of medicinal plants and their traditional use in different regions of the world [2]. Today, according to the World Health Organization (WHO), nearly 80% of the population depends on traditional medicine for primary health care. Considerable economic advantages in the development of traditional medicine and in the use of medicinal plants for the treatment of various diseases have been noted world [2], hence the need to valorize traditional medicine and especially the molecules extracted from plants. In these plants, we find primary and secondary metabolites. The primary metabolites are in great number whereas the secondary metabolites are molecules having a limited distribution in the plant. The secondary metabolites play different roles, including that of defense against external aggressions. Secondary metabolism products are very numerous; more than 200.000 structures defined [3]. They include an extraordinary structural variety but are produced in small quantities [3]. These molecules mark in an original way, a species, a family or a genus of plant and sometimes allow to establish a chemical taxonomy. Phenolic compounds, terpenoids, steroids and alkaloids are examples of secondary metabolites; they have many pharmaceutical applications [3].
Annickia chlorantha, a plant belonging to the Annonaceae family, is one of the warm and humid climate species of tropical Africa that grows in dense forest usually at low altitude. Geographically, it is found widespread in southern Nigeria, Angola, the Republic of Congo and the Democratic Republic of Congo [4]. A. chlorantha has been the subject of several chemical and pharmacological studies [5] and this revealed that it contains alkaloids. Some parts of the plant, especially the bark and the roots, contain alkaloids with an anti-hepatitis effect. This is a property long recognized in traditional medicine [6]. The extracts of bark of A. chlorantha are used in the manufacture of a drug, having a preventive and curative effect on viral hepatitis [7]. The alkaloids isolated from this plant are active against Trypanosoma cruzi, the American trypanosomiasis pathogen [8]. The plant’s barks are also traditionally used for the treatment of rheumatism, fatigue, tuberculosis and certain ulcers [9], malaria and for deworming [10]. They are also used against diarrhea [10]. In addition, [9] revealed the anti-HIV properties of the aqueous extract of this plant. The use of the sulfur yellow bark provides an excellent dye used in the dyeing of fabrics, skins and mats in some regions, especially in Cameroon, Congo, Gabon and DRC [11,12]. The literature reports that A. chlorantha contains palmatin in the stem and root barks [13]. In 1975, the chemical constituents of barks were elucidated [14]. Several alkaloids were isolated [6,9]. The main alkaloids extracted from the trunk bark are quaternary protoberberines, such as palmatine, berberine, jatrorrhizine and columbamine. Two new alkaloids have been identified, namely aporphine and another alkaloid of the protoberberine type (active anti-HIV alkaloid), 7.8-dihydro-8-hydroxypalmatine have also been isolated from the bark [9].
In the same way, we have set ourselves the goal of extracting, purifying and isolating by chromatographic methods, two alkaloids of the protoberberine type and thirteen alkaloids of the bisbenzyl tetrahydrobenzyl isoquinoline types.
Materiel and Methods
Plant Material
The plant of Annickia chlorantha was collected in November 2012 in the department of Niari precisely in Mossendjo in southwestern Congo. The part used in this study consisted of the bark kept in the dark at room temperature (25 °C) for about five (05) days. The dry matter was then ground with an IKA-WERKE Gmbh-CO-KG, D-79219 Staufen, equipped with a 0.25 mm sieve.
Fractionation and Purification of Dye Extracts
Experimental Approach
The experimental approach (Figure 2) allowed for fractionation and purification of the dye extracts.
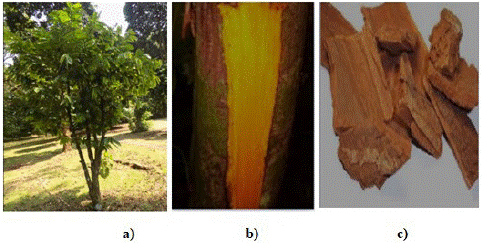
Figure 1: a) Annickia chlorantha plant, b) Annickia chlorantha trunk and c) Annickia chlorantha bark.
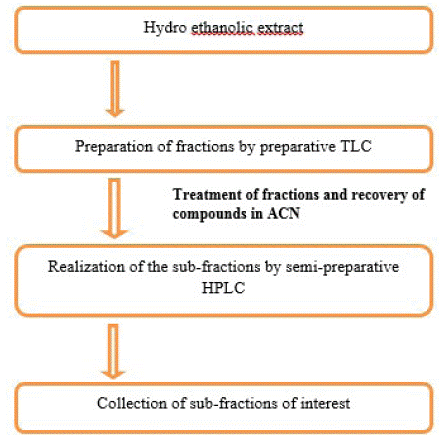
Figure 2: Experimental scheme of alkaloid purification.
Preparative Chromatography on Thin Layer
Preparative chromatography on thin layer was used to separate the different compounds in the plant. The TLC plates used were Merck 60 F 254 on 1-mm-thick silica gel on 20cm x 20cm glass. The extracts were solubilized in methanol =99.8% and then deposited on the plate. The plates were eluted in a saturated tank containing a solvent mixture.
The migration systems were those used in the qualitative analysis by thin layer chromatography. Plates were developed under CAMAG UV lamp with a wavelength ranging from 254 to 366 nm. The silica containing each compound is then recovered with a spatula. The silica is desorbed in a small amount of solvent, and then filtered under vacuum to recover the compounds.
Analysis of Extracts by High Performance Liquid Chromatography (HPLC)
The HPLC system consisted of a Waters 2996 HPLC chain equipped with a W600 pump of the same brand and a diode array UV detector (DAD-UV). The acquisition system was controlled by a Waters software. The Uptisphere C18 300°C, 250×4.6mm 5μm column was from Interchim (France). The mobile phase was composed of the H2O/ACN mixture in gradient elution mode consisting of (H2O: 70, 50, 0, 0, 70, 70) and (ACN: 30, 50, 100, 100, 30, 30). The working flow rate was 1 mL/min with a run time of 60 min divided (0, 10, 35, 45, 53, and 60).
Semi-Preparative High Performance Liquid Chromatography
The purifications were performed using an Agilent 1200 preparative HPLC system. This apparatus is equipped with two G136A pumps and an injection loop (2mL for semi-preparative column), a UV detector with diode arrays (DAD-UV) driven by a Waters software. Compound separations were performed on an Uptisphere Strategy 100 A° semi-preparative column (30 x 250mm, 10μm,) using an Uptisphere RP18e pre-column. The mobile phase was composed of the mixture H2O/AcOH in the proportions 99:1 v/v and ACN/AcOH in the proportions 99:1 v/v.
The gradient mode was the same as the one used in analytical mode, the only changes were observed on the flow rate (10mL/min) and the column. After collection of the fractions, the organic solvent was evaporated using a BUCHI R-201 rotary evaporator at 45°C. Then, the samples were freeze-dried to remove traces of water.
Identification and Structure Determination of Molecules of Interest
Several analytical techniques (UV, NMR, HRMS) were used to obtain the structural information necessary to determine the structures of the isolated compounds. Indeed, the structures were established on the basis of spectroscopic evidence and by comparison of our results with previously published spectral data.
High resolution Mass Spectrometry (HRMS)
The purified molecules were analyzed by Bruker MicrO-Tof-Q 2 mass spectrometry composed of two quadrupoles and an orthogonal time-of-flight analyzer equipped with electrospray or APCI sources. The HRMS was used to determine the elemental composition (gross formula) and molecular weight of the purified product. The purified molecules were analyzed at the Centre Régional de Mesures Physiques de l'Ouest (CRMPO) in Rennes. In this study, the source used was Electrospray (ESI) and the analytical solvent was methanol.
NMR Spectroscopy
The 1H, 13C, COSY1H-1H, COSY 1H-13C and HMBQ nuclear magnetic resonance spectra were recorded on a 400 MHz spectrometer of Jeol brand, at the Centre Commun d'Analyse (CCA) of the University of La Rochelle UMR-7266.
A few milligrams of freeze-dried sample powder were solubilized in d6 DMSO in the 5 mm diameter analytical tubes and then analyzed by 1D and 2D NMR. Chemical shifts were expressed in parts per million (ppm), relative to Tetramethylsilane (TMS), used as an internal reference. The coupling constants (J) were expressed in hertz (HZ). Signal multiplicity was indicated as follows: s (singlet), bs (broad signal), d (doublet), dd (split doublet), ddd (split doublet), t (triplet), m (multiplet), q (quadruplet). ov (overlapped).
Results and Discussion
Fractionation of Extracts, Purification of Fractions and Isolation of Compounds
Fractionation of Annickia chlorantha water/EtOH dye extract, purification of fractions and isolation: The extract obtained after treatment with methanol and 10% HCl was named EEC. EEC underwent a first fractionation by preparative thin layer chromatography to give five (5) fractions FEC1, FEC2, FEC3, FEC4 and FEC5. These fractions were analyzed by reverse phase chromatography coupled with an iodine bar detector (DAD). The fractions FEC4 and FEC5 presented dyeing properties. We focused our attention on the FEC5 fraction because it was obtained in significant quantity (619 mg) and would contain a large number of compounds (Figure 3). From the FEC5 fraction purified by semi-preparative HPLC on a reverse phase silica column with a polarity gradient (gradient 1), we were able to isolate twelve (12) sub-fractions all of yellow color and of which five (5) revealed the dyeing properties (EC3, EC9, EC10, EC11 and EC12).
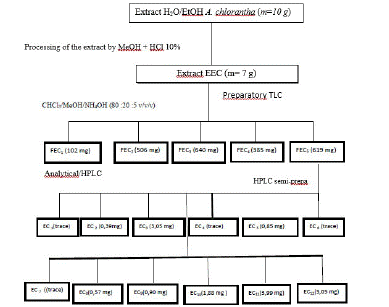
Figure 3: Fractionation, purification and isolation scheme Water/EtOH Annickia chlorantha extract.
HPLC analysis of the FEC5 fraction revealed the chromatographic profile and UV-visible spectra of a few compounds (Figures 4 & 5).
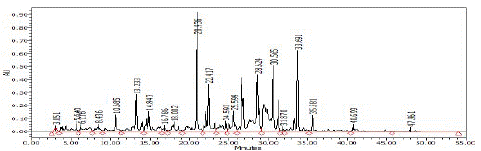
Figure 4: Chromatographic profiles of the FEC5 fraction.
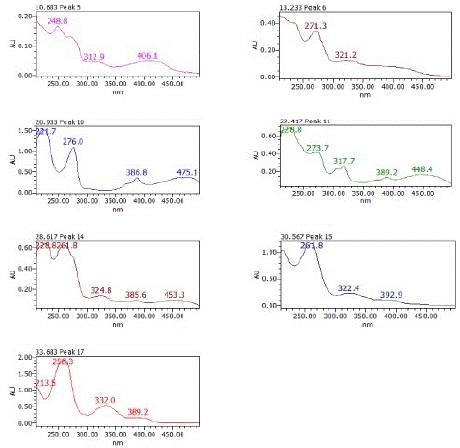
Figure 5: UV absorption spectra of some compounds present in FEC5 (280 nm).
The UV absorption spectrum of some eluted compounds at different retention times showed maxima between 215 and 360 nm characteristic of aromatic bands.
The UV spectra of the compounds present in the FEC5 fraction show absorption maxima characteristic of aromatic bands. Some compounds show a maxima in the visible (406 - 475 nm), characteristic of dye molecules.
The bibliographical study carried out on plants of the genus Annickia revealed that this family contains alkaloids of the protoberberine type in majority [15].
Indeed, these majority compounds of the chlorantha species have presented in their structure aromatic rings. The dyeing principles of the sulfur yellow dye Annickia chlorantha can be attributed to this family of chemical compounds.
The work of [15] on natural dyes, reported that quaternary alkaloids (Palmatine, berberine, jatrorrhizine, columbamine...) isolated from some dye plants belonging to the families Berberidaceae, Rutaceae, Ranunculaceae and papaveraceae constitute the chemical signature of basic yellow dyes.
Elucidation of the Chemical Structures of Some Molecules
Elucidation of the chemical structures of the alkaloids of Annickia chlorantha: Preliminary phytochemical tests indicated the presence of alkaloids in abundance in Annickia chlorantha trunk bark. From the staining extracts, fifteen (15) alkaloids were isolated designated as A to O and identified mainly by mass spectrometry. These alkaloids were extracted from the sub-fractions EC3, EC9, EC10, EC11 and EC12. The results showed that these sub-fractions are not totally pure. They are a mixture of compounds.
As a result, their structure could not be determined. Apart from the compounds of crude formula C18H13NO3 and C21H21NO5, the other alkaloids all belong to the same series and seem to be new in the literature of the species Annickia chlorantha. But at present, it has not been possible to attach them to a known structural type except for the compound of crude formula C21H21NO5 from the EC3 subfraction which corresponds to a known structure oxy-8-palmatine isolated from Annickia chlorantha barks [7,9,14,16].
Compounds Contained in the EC3 Sub-Fraction
The EC3 sub-fraction is a yellow ochre powder soluble in methanol. It is a mixture of four alkaloids. It was impossible from the NMR data of this sub fraction that we elucidate in a simple way the structures of these four alkaloids. However, the NMR spectrum recorded on this impure sub-fraction shows definitely the presence of alkaloids that belong to the series of tetrahydroprotoberberines and bisbenzyl tetrahydro isoquinolines.
Alkaloids A and B: Of crude formulae C33H37N4O6Na and C33H38N4O6Na, the structures could not be determined. Taking into account their molecular weight (theoretical mass) respectively 685 and 686 g/mol, it is possible that these alkaloids are attached to the series of bisbenzyl tetrahydro isoquinolines.
• Alkaloid C: With a molecular formula C18H13NO3Na and a theoretical mass of 314.079 m/z, its mass spectrum presents, in addition to the peak at m/z 314 (molecular peak), a fragmentat m/z 299 (M-15) corresponding to the loss of a CH3;
• Alkaloid D: minority compound of the EC3 sub-fraction, with the molecular formula CH21H21NO5Na and theoretical mass: 390 m/z. It could be easily identified, by examination of its MSMS spectral data and by direct comparison with oxy-8-palmatin, isolated from an Annonaceae, Annickia polycarpa [17].
Its isolation suggested palmatine to be present among quaternary bases in Annickia chlorantha. Its mass spectrum revealed peaks at m/z 375 and m/z 360 corresponding to the loss of two CH3 groups. Figure 6 illustrates the structure of oxy-8-palmatin, elucidated by other authors [14,16].
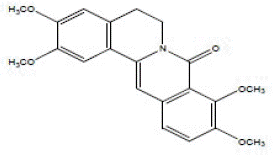
Figure 6: Structure of 8-oxy palmatine.
Compounds Contained in the EC9 Sub-Fraction
The sub-fraction EC9 is a brownish yellow powder soluble in methanol. It is a mixture of two alkaloids. The latter seem to be new compounds and all are attached to the bisbenzyltetrahydroisoquinoline series.
• Alkaloid G: C36H28N2O6Na; theoretical mass: 607.18.
MS data:
[M-.CH3+Na]+. à m/z 592
[M-CH4+Na]+ à m/z 591
[M-2CH3+Na]+ à m/z 577
[M- CONH2] à m/z 563
• Alkaloid H: C36H26N2O6 Na; Theoretical mass : 605.168.
The 1H NMR spectrum of the EC10 sub-fraction shows a characteristic signal 7.38 ppm (2H, m) characteristic of an aromatic ring; a singlet at 3.37ppm due to a methoxy in para or ortho; a singlet of 6 protons at 2.57 ppm due to the two N-methyl which resonate together.
These data do not allow us to attribute the signals to each compound.
Compounds Contained in the EC11 Sub-Fraction
The sub-fraction EC11 is a yellow powder soluble in methanol. It is a mixture of four alkaloids belonging to the same series.
• Alkaloid I : C35H36N2O4Na; Theoretical mass: 571.257
SM data:
[M-.CH3+Na]+. m/z 556,
m/z 293 (M-278), loss of C17H12O3N
• Alkaloid J: C35H34N2O4Na; theoretical mass: 569.241.
MS data:
[M-.CH3+Na]+. à m/z 554,
m/z 292 (M-277) C17H11O3N
• Alkaloid K : C34H30N2O4Na ; Theoretical mass : 553.210
MS data:
[M-CH3+Na]+. at m/z 538
[M-2CH3+Na]+ at m/z 523
[M-3CH3+Na]+. at m/z 508
m/z 495 (M-58) loss of CH3COCH3
m/z 277 (M-276) loss of C17H10O3N
m/z 262 (M-291) loss of C18H13O3N
• Alkaloid L : C36H30N2O4Na ; Theoretical mass : 577.210
The 1H NMR spectrum shows, in addition to the two N-methyl and aromatic proton signals, two singletons of two methoxy at 3.78 and 3.91 ppm. These data do not allow us to assign the signals to each compound.
Compounds Contained in the EC12 Sub-Fraction
The sub-fraction EC12 is a yellow ochre powder soluble in methanol. It is a mixture of three alkaloids belonging to the same group. The alkaloids I and J are found in this sub-fraction. The alkaloid of type I is the majority in the EC12 sub-fraction.
• Alkaloid M : C36H32N2O4Na ; Theoretical mass : 579.223
MS data:
[M-.CH3+Na] at m/z 564
[M-2CH3+Na] at m/z 549
• Alkaloid N : C34H32N2O4Na ; Theoretical mass : 555.225
Alkaloid O : C37H34N2O4Na ; Theoretical mass: 593.225
2 N-methyl (2 singlet at 2.49 and 2.56 ppm) and two methoxy (2 singlet at 3.38 and 3.53 ppm). The characteristic signals 7.45 - 7.13 ppm (4 H, m) and 6.91 - 6.88 ppm (2 H, m) indicate the presence of aromatic rings.
These data do not allow us to attribute the signals to each compound. However they confirm that these compounds are alkaloids.
The group C and D alkaloids are protoberberines, the other alkaloids show mass spectral characteristics like those of the bisbenzyl tetrahydro isoquinoline series alkaloids (Figures 7 & 8).
Their mass spectrum revealed, in addition to the molecular peak, fragmentations characteristic of bisbenzyl tetrahydro isoquinolines with two ether bridges of the berbamine or oxyacanthine type resulting from the loss of the benzyl rings C and C' of the molecule, as well as the ions derived from them. The elimination of the tetrahydroisoquinoline unit A'B' is also noted (Figure 7).
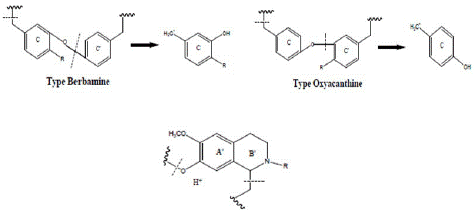
Figure 7: Basic structures of Tetrahydroprotoberine and Bisbenzyltetrahydroisoquinoline.
The alkaloid composition of Annickia chlorantha is found to be more complex than previous work indicates.
Previous study of the barks isolated protoberberine-type alkaloids (Figure 7) that have quaternary ammonium salts [7,13]. These quaternary ammonium salts are abundant and are obviously responsible for the yellow coloring of the bark and probably also for their use in traditional medicine. Taking into account our results, it should be noted that the alkaloid composition of Annickia chlorantha is still very poorly elucidated. The isolated alkaloids belong, for the most part, to the group of bisbenzyl tetrahydro isoquinolines (Figure 7) which has never been reported in previous studies. The isolation of palmatine corroborates with the results of some authors [14,16]. The complexity of the alkaloid composition of Annickia chlorantha trunk bark is particularly justified by the number of alkaloids present. All these alkaloids belong either to the protoberberine and tetrahydroprotoberberine group or to the bisbenzyltetrahydroisoquinoline group.
The isolated and identified alkaloids of the EC3 and EC4 sub-fractions give a C and D type nature which are from Protoberberine types, while, the other isolated alkaloids of the sub-fractions (EC1 to EC15) present mass spectral characteristics such as those of the alkaloids of the Bisbenzyl tetrahydrobenzyl isoquinoline series.
Their mass spectrum reveals, in addition to the molecular peak, classical and characteristic fragments of bisbenzyl tetra hydrobenzyl isoquinolines with two ether bridges of the Berbamine or Oxyacanthine type coming from the Benzylic rings C and C', as well as the ions derived from them. We also note the elimination of the Tetrahydrobenzyl isoquinoline unit A'B' (Figures 7 and 8). These alkaloids are grouped in Table 1:
Sub-fractions
Alkaloids
Raw formulas
Molecular weight (g.mol-1)
Observed peaks m/e
EC1
A
C33H37N4O6
585.737
570(M-CH3), 554(M-OCH3), 394(C11H13O2N)
EC2
B
C33H38N4O6
586.010
571(M-CH3), 555(M-OCH3), 409(C10H12O2N)
EC3
C
C18H13NO3
291.089
276(M-CH3), 260(M-OCH3)
EC4
D
C21H21NO5
367.010
337(M-2CH3), 336(M-OCH3)
EC5
E
C35H26N2O4
538.010
522(M-CH3+ H), 507(M-2CH3+H),
347(M-C11H13O2N)EC6
F
C35H37N2O4
549.207
406(M-C3H7), 491(M-CH3COCH3)
EC7
G
C36H28N2O6
584.190
569(M-CH3), 554(M-2CH3),
393(M-C11H13O2N)EC8
H
C36H26N2O6
582.178
551(M-CH3+H), 537(M-CONH2)
EC9
I
C35H36N2O4
548.267
533(M-CH3), 270(M-C17H12O3N)
EC10
J
C35H34N2O4
546.220
531(M-CH3), 269(M-C17H11O3N)
EC11
K
C34H30N2O4
530.022
515(M-CH3), 500(M-2CH3),
472(M-CH3COCH3), 259(M-C17H10O3N)EC12
L
C36H30N2O4
554.220
263(M-C18H13O3N), 447(M-C7H7O)
EC13
M
C36H32N2O4
556.233
541(M-CH3), 526(M-2CH3),
379(M-C10H12O2N)EC14
N
C34H32N2O4
532.235
517(M-CH3), 428(M-C7H7O),
341(M-C11H13O2N)EC15
O
C37H34N2O4
570.235
555(M-CH3), 433(M-C8H9O2),
393(M-C10H12O2N)
Table 1: Different alkaloids isolated and identified from Annickia chlorantha barks.

Figure 8: Characteristic fragmentation of bisbenzyl tetrahydro isoquinolines.
Conclusion
The alkaloidal composition of Annickia chlorantha turns out to be more complex than previous work indicates. These previous studies on the barks of Annickia chlorantha allowed the isolation of alkaloids of the Protoberberine type which are quaternary ammonium bases. In view of the results obtained, it should be noted that the at1lkaloid composition of Annickia chlorantha was still little known. The alkaloids isolated from this study, belong for the majority to the group of Bisbenzyl tetrahydrobenzyl isoquinolines which has not yet been reported in the literature. Their crude formulas were given on the basis of high resolution mass spectroscopy.
Further studies on the chlorantha species need to be conducted to structurally identify these alkaloids which do not appear to be attached to previously known structures.
Author Statements
Acknowledgements
Our sincere thanks to Professor Mariamme GLABER, head of the AMES team of the Laboratoire Littoral Environnement et Société (LIENs), University of La Rochelle (France). We also thank Mr. Jean Marie MOUTSAMBOTE, Lecturer in Botany at the National School of Agronomy and Forestry (NSAF), Marien Ngouabi University, for the collection and identification of the plant.
We sincerely thank Mrs. Medine Moussounga Keener for her precious help and contribution in the editing of the English version of this article.
Conflict of Interest
The authors declare that we do not have any conflict of interest.
References
- Rodrigues E. Plants of restricted use indicated by three cultures in Brazil (Caboclo-river dweller, Indian and Quilombola). J Ethnopharmacol. 2007; 111: 295-302.
- Muthu C, Ayyanar M, Raja N, Ignacimuthu S. Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J Ethnobiol Ethnomed. 2006; 2: 43.
- Hartmann T. From waste products to ecochemicals: fifty years research of plant secondary metabolism [review]. Phytochemistry. 2007; 68: 2831-46.
- Maydell V HJ. Arbres et Arbustes du sahel. Leurs caractéristiques et leurs utilisations. Eschbom. 1983; 531.
- Ngantchou I, Nono J-J. Activités trypanocides comparées de quelques composés naturels isolés des plantes médicinales camerounaises. Pharm méd Trad Afr. 2004; 13: 133-9.
- Agomo PU, Idigo JC, Afolabi BM. Antimalarial medicinal plants and their impact on cell populations in various organs of mice. Afr J Med Med Sci. 1992; 21: 39-46.
- Tan PV, Nyasse B, Dimo T, Wafo P, Akahkuh BT. Synergistic and potentiating effects of ranitidine and two new anti-ulcer compounds from Enantia chlorantha and Voacanga africana in experimental animal models. Pharmazie. 2002; 57: 409-12.
- Nyasse B, Nkwengoua E, Sondengam B, Denier C, Willson M. Modified berberine and protoberbérines Fron Enantia chlorantha as potential inhibitors of Trypanosoma brucei. Pharmazie. 2002; 57: 409-12.
- Wafo P, Nyasse B, Fontaine C, Sondengam BL. Aporphine alkaloids from Enantia chlorantha. Fitoterapia. 1999; 70: 157-60.
- Sepúlveda-Boza S, Cassels BK. Plant metabolites active against Trypanosoma cruzi. Planta Med. 1996; 62: 98-105.
- Raponda Walker A, Sillans A. Les plantes utiles du Gabon. Edition sepia. 1995; 614.
- Madiélé Mabika AB. des plantes tinctoriales d’Afrique centrale: inventaire ethnobotanique, Caractérisation analytique et biologique et développement des filières de production [thèse] de doctorat. 2015; 258.
- Samir AR, Makboul AM, Moses OF, Gabriel O, Zac G. Boll Fac Sci. 1989; 18: 21-9.
- Hamonnière M, Leboeuf M, Cave A, Paris RR. Alcaloïdes des Annonacées: Alcaloïdes de l’Enantia chlorantha Oliv, pl. méd. phytoch. 1975; 9: 296-303.
- Cardon D. Le monde des teintures naturelles. éditions Belin. 2003; 586.
- Jössang A, Leboeuf M, Cavé A. Alkaloids of Ammonaceae XVII: alkaloids of Enantia polycarpa Engl. et Diels (author’s transl). Planta Med. 1977; 32: 249-57.
- Wright CW, Phillipson JD. Biological screening of traditional preparations from some medicinal plants used antidiabète. Phytother Res. 1990; 4: 127-39.