
Review Article
Austin J Anal Pharm Chem. 2024; 11(1): 1167.
Application and Medicinal of Terpenoids
Ana Borges1,2; Filipa Mandim1,2; Sandrina A Heleno1,2; Ricardo C Calhelha1,2*
¹Centro de Investigação da Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolónia, Portugal.
²Laboratório Associado para a Sustentabilidade e Tecnologia em Regiões de Montanha (SusTEC), Instituto Politécnico de Bragança, Campus de Santa Apolónia, Portugal
*Corresponding author: Ricardo C Calhelha Centro de Investigação da Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal. Email: calhelha@ipb.pt
Received: January 17, 2024 Accepted: February 17, 2024 Published: February 24, 2024
Abstract
There has been a rise in interest in terpenoids due to their variety of chemical forms and their distinct biological features, providing a range of applications in various industries, and enabling a significant economic, social, and environmental value. A systematic review was conducted based on the results of the collected scientific articles using Web of Science, Scopus, Scielo, Science Direct, and PubMed databases. This article examines the characteristics of terpenoids and their biological effects, concentrating on their anti-aging effects. Additionally, it discusses the various industrial applications and drawbacks of their application. Thanks to their activity, terpenoids play a significant part in various industries, making them important compounds with a wide range of applications. They are also frequently used and have excellent development prospects. emphasizing in particular their anti-aging qualities. Future research should focus on terpenoids’ broad toxicity, their catalytic mechanism, bioavailability, pharmacodynamics, biomarkers, extensive examinations of their bioactive qualities, and their usage in various industries in light of their effectiveness.
Keywords: Terpenoids; Essential oils; Emerging extraction processes; Bioactivities; Aging
Introduction
Since ancient times, medicinal plants have been essential for treating and preventing human diseases [1,2]. Endowed with a wide variety of properties, plant species have been extensively studied in an attempt to identify which compounds are responsible for the multifaceted properties. In addition, the current sustainable policies related to the adequate use of resources and all their potential, are differentiating factors for the importance currently given to the natural resources research area [2,3]. Recently, pharmaceutical companies have been paying more attention and importance to this area of study. These companies intend to identify possible molecules of interest, which can be used in the development of new drugs. Medicines with high therapeutic efficacy, economically viable and accessible, safe, and with reduced adverse effects are some of the main objectives [1-3].
Plants produce a wide variety of compounds through their primary and secondary metabolism. Concerning the secondary one, terpenoids, alkaloids, tannins, saponins, and phenolic acids, among others, are some of the classes of compounds of interest [4-6]. Those classes have been studied due to the varied bioactive properties they have been exhibiting (e.g., anticancer, anti-inflammatory, antiviral, antimicrobial) [3]. Among the mentioned secondary metabolites classes, terpenoids are one of the largest and most structurally diverse [2-5]. This group of compounds plays a crucial role in the physiological processes, environmental reactions, and plant growth and development [3]. Terpenoids are derived from isoprene and can be found in a variety of chemical forms [3,4,7]. They exhibit a linear hydrocarbon or a cycle chain configuration, with diverse chemical variations of the substituent groups [4,8]. Several studies in the literature emphasize the multifaceted properties of terpenoids, which as a consequence of their multiple configurations, result in distinct biological features [5,8-10]. These characteristics are the main reason for the wide range of applications in several industrial areas (e.g., pharmaceutical, food, and agricultural industries) and therefore, for their economic valorization [3,5]. The growing knowledge about the potential associated with terpenoids has been the main contributor to the increased interest associated with this bioactive class [2-5]. Its consequent exploitation and increased applicability are important economic contributions to its valorization and their produced species [3].
This review article gathers recent findings regarding terpenoids characteristics and biological effects. As a result of major concerns and a higher incidence of conditions associated with aging, particular consideration was given to terpenoids' anti-aging effects (namely skin aging, degenerative diseases, and cancer). Additionally, industrial applications and the associated side effects of their use will also be discussed. Scientific data bases such as Web of Science, Scopus, Scielo, Science Direct, and PubMed were used for collecting scientific articles and chapters. The keywords “terpenoids”, “essential oils”, “bioactivities”, “antiaging” and “emerging extraction processes” were used isolated and in combined form.
Characterization and Biosynthesis
Plants synthesize a vast variety of metabolites through their primary and secondary metabolism. Primary metabolites such as sugars, proteins, and lipids, are found in all species and are necessary for fundamental processes of growth and development of the plant. On the other hand, secondary metabolites have a great diversity and structural complexity, are synthesized in response to external stimulus, and are essential for the survival and perpetuation of the species [6]. Terpenes are a class synthesized through secondary metabolism. Terpenoids correspond to modified terpenes where methyl groups are moved or removed, or additional functional groups (usually oxygen-containing) are added [6,8,10]. They present a great complexity and structural diversity. To date, more than 90,000 terpenes have been identified, being one of the largest classes and with a higher structural variety [10].
Terpenes are classified according to the number of isoprene units [6,8,10]. Table 1 contains the different terpenoid classes and their main natural sources and biological properties known.
Class
Number of Carbons
Natural Sources
Properties
References
Hemiterpenoids
5
Found in plants and leaves of many trees (conifers, willows, conifers).
Flavors, fragrances, anti-inflammatory, neuroprotective, cytotoxic, and apoptogenic
[4,6,8,9,11,12]
Monoterpenoids
10
The main components of fruits are essential oils a and volatile fraction of Turpentine
Aroma or odor, anti-tumor, antibacterial, antioxidant, generation of aging protection
[8,13–27]
Sesquiterpenoids
15
Found particularly in higher plants, marine organisms, and fungi
Anti-inflammatory, anti-allergic, antinociceptive, antioxidant, anti-cancerous, gastrointestinal protector, antibacterial, local anesthetic, generation of aging protection
[8,13–27]
Diterpenoids
20
Widely distributed in plants (emphasizing coffee and spices), and fungus
Antioxidant, anti-aging, anti-cancer, treatment of neurodegenerative and cardiovascular conditions, metabolic disorders, antiviral, antimicrobial, antiparasitic, antiprotozoal, plant protection, generation of aging protection
[8,13–16,18–23,25–27]
Sesterterpenoids
25
Frequently reported from bacteria, fungi, lichens, insects, marine invertebrates, and some higher plant families
Aroma, odor, phytotoxic, antimicrobial, nematocidal, cytotoxic, antiviral, and anti-inflammatory
[8,13,15,18,20,22,25–28]
Triterpenoids
30
Biosynthesized by bacteria, plants, fungus, and animals
Anti-aging, anti-cancer, neurodegenerative, cardiovascular, metabolic disorders; mitigate obesity and hyperlipidemia, antiviral, antimicrobial, antiparasitic, immunomodulator agent, generation of aging protection
[8,13–16,18–22,25–27]
Tretraterpenoids
40
Found in roots, leaves, seeds, fruits, and flowers
Antioxidant, anti-aging, food colorant, generation of aging protection
[8,14–16,19–22,25–27]
Polyterpenoids
> 40
Found in a variety of natural compounds (for example hardwoods)
Use in the food industry due to the resistant to change in viscosity, color, and oxidation (thermally stable, low odor and volatility)
[3,8,9,11,12,29–31]
Table 1: Classification of terpenoids, distribution in nature, and main properties.
Although terpenoids have a wide structural variation, all of them are synthesized from two universal precursors of five carbon: dimethylallyl diphosphate (DMAPP), and isopentenyl dyphosphate (IPP) [3,6]. In plants, these two molecules can be synthesized from two independent biosynthetic pathways: the classic mevalonic acid (MVA) and deoxylulose phosphate pathway (DMAPP) (Figure 1) [6,10]. The first gives origin to IPP from acetyl-CoA units, the condensation of three molecules of acetyl-SCoA results in the ester Β-hydroxy-Β-methylglutaryl-CoA formation. This ester after reactions of hydrolysis and an enzymatic reduction originates the mevalonic acid [6]. Successive phosphorylation reactions of hydroxyl groups, followed by decarboxylation and elimination of a pyrophosphate group originate the intermediate IPP [6]. DMAPP, in turn, comprises seven enzymatic steps and is formed after the removal of a C-2 proton from that isoprene unit by an isomerase. In turn, 1-deoxy-D-Xylulose-5-Phosphate (DXP), the intermediate involved in the non-mevalonate biosynthetic pathway, is formed from pyruvic acid and D-glyceraldehyde, with the coenzyme Thiamine diphosphate (TPP) as a mediator (Figure 1) [6].
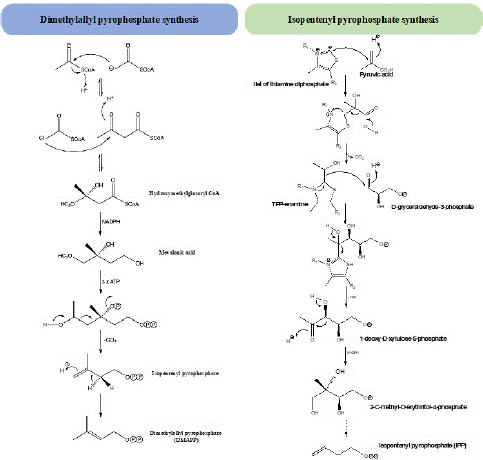
Figure 1: Synthesis of the universal precursors of terpenoids.
The biosynthesis of terpenoids occurs through "tail-head" condensation between the DMAPP and IPP units, and with phenyl-transferase as the catalytic enzyme (Figure 2). This condensation generates the C10 chain of Geranyl Pyrophosphate (GPP), the precursor of monoterpenes. Its successive condensation with IPP units gives rise to the different terpene precursor chains, namely the farnesyl pyrophosphate (C15), geranylgeranyl pyrophosphate (C20), and geranylfarnesyl pyrophosphate (C25) chains, precursors of sesquiterpenes, diterpenes, and sesterterpenes, respectively. In turn, the condensation of two farnesyl pyrophosphate (C30) or two geranylgeranyl pyrophosphate (C40) chains, are precursors of triterpenes and tetraterpenes, respectively (Figure 2) [6].
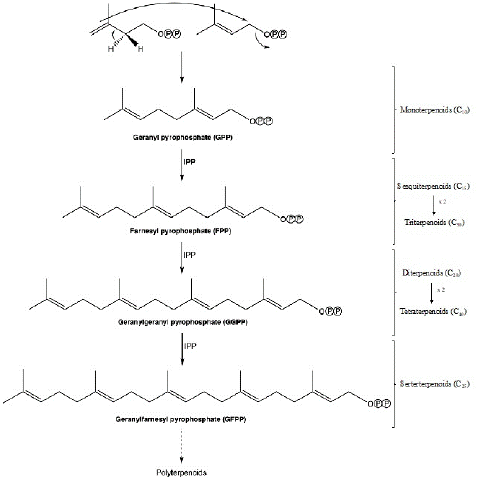
Figure 2: Synthesis of terpenoids.
Bioactive Properties
Terpenoids have been exhibiting an extensive spectrum of bioactive properties [31-34]. Those properties have been demonstrated by several authors who consider these compounds promising bioactive substances in the treatment and prevention of several diseases [3,35,36]. As a result of the multifaceted potential that has been proven in various studies, terpenoids could play a significant role in discovering and developing new therapeutic options. The studies and principal findings of the last few years are summarized in the following sections.
Anti-Inflammatory Properties
Recent research has demonstrated that terpenoids exhibit the capacity to reduce inflammation. Studies describe that terpenoids can inhibit the inflammatory process through the suppression of several related inflammatory processes [37,38].
Inflammation is a protective reaction of live tissue with a circulatory system to a variety of damaging causes, and it is intimately associated with several diseases [19,39]. Numerous events take place as a result of cellular processes that are essential to the inflammation process, such as oxidative stress and autophagy, as well as from the excessive production of pro-inflammatory cytokines and inflammatory mediators, such as interleukin-1Β (IL-1Β), IL-6, tumor necrosis factor-alpha (TNF-a), nitric oxide (NO), produced by non-reduced NO synthase (iNOS), and prostaglandin E2 (PGE-2) synthesized by cyclooxygenase-2 (COX-2) [39]. In addition, a key transcription factor called the nuclear-κB (NF-κB) factor plays a crucial part in the production of proinflammatory genes during inflammation [39].
Curcuma kwangsiensis was studied in vitro and in vivo for its anti-inflammatory and antinociceptive effects. These assays of various layers (methanol (ME), ethyl acetate (EA), and aqueous (AQS)) from C. kwangsiensis were achieved by carrageenan-induced paw edema and acetic acid-induced writhing animal models, administrated in male mice randomly assigned to groups. The results showed that ME and EA significantly inhibited the paw edema in comparison to the control group (p<0.01/0.05), while AQS showed no significant inhibitory effect. The inhibitory ratios of ME and EA amounted to 35.3/34.0%, and 41.3/31.7% at the dose of 200/100 mg/kg, respectively (p<0.01/0.05). Furthermore, aspirin (ASP) was used as the positive control and played a similar role in reducing paw edema with a suppression rate of 69.7% at the dose of 200 mg/kg. The above data suggested that ME and EA exhibited significant anti-inflammatory effects equivalent to that of ASP. Based on the in vivo bioactivity evaluation, the EA layer was selected for phytochemical investigation next. In this layer, all the isolated compounds were evaluated for their anti-inflammatory effects via detecting inflammatory mediator releases (COX-2, IL-1Β, and TNF-a) in RAW 264.7 macrophage cells induced by LPS. LPS alone significantly increased the COX-2 (98.5±3.0 versus 79.6±1.9 ng/L), IL-1Β (95.6±2.6 versus 76.5±1.4 ng/L), and TNF-a (141.1±1.6 versus 115.8±1.4 ng/L) production compared to the normal group, respectively. However, inflammatory cytokine secretions decreased after the treatment of sesqui- and diterpenoids. The majority of all the isolates exhibited excellent anti-inflammatory activities by inhibiting LPS-stimulated COX-2, IL-1Β, and TNF-a production at a concentration of 20 μg/mL in RAW 264.7 macrophage cells, equivalent to dexamethasone (DXM) (p<0.05/0.01) [40].
In the same way, Nepeta bracteate compounds were isolated and investigated for their anti-inflammatory activity. The activity was evaluated in lipopolysaccharide-stimulated RAW 264.7 macrophages using the MTT colorimetric method. The compounds were dissolved in dimethyl sulfoxide (DMSO) and diluted appropriately just before cell treatments. Cells were incubated with the extract at indicated concentrations, with DMSO not exceeding 0.1% in all experiments. The cells were cultured in DMEM (Dulbecco's Modified Eagle Medium) with 10% FBS (Fasting Blood Sugar) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) at 37°C with 5% CO2. NO release was measured as an indicator of the nitrite concentration. This test showed that all the abietane diterpenoids displayed different degrees of inhibition effect. Among them, compounds nepetabrate B and nepetabrate D displayed the greatest anti-inflammatory activities with IC50 values of 19.2 and 18.8 μM and moderate cytotoxic activities with IC50 values of 36.3 and 41.4 μM, further proving the correlation between inflammation and cancer [41].
Also, all compounds from the fruits of Arenga pinnata (Wurmb) Merr were investigated and evaluated for their anti-inflammatory activity. The cells were treated with LPS (Sigma, 1 μg/mL), and then pretreated with various concentrations. After stimulation, the supernatants of cells were got through centrifugation (3000 g/min, 10 min) and hatching with the equal volume of Griess reagent (1% sulfanilamide in H2O and 0.1% naphthylenediamine in 5% phosphoric acid). The outcomes showed that all of them exhibited different degrees of suppression on NO production, and Pinnasesquiterpene A and Linchuniinone exposed moderate suppressive effects against NO generation in lipopolysaccharide-stimulated RAW 264.7 cells [42].
Moreover, all of the compounds from the twigs and leaves of Abelia macrotera were isolated and studied for the inhibitory effects on NO production in LPS-induced RAW 264.7 cells. Compound methyl 4,5-di-O-caffeoylquinate showed an obvious inhibitory effect on LPS-induced NO production in RAW 264.7 cells with IC50 values of 23.77±1.61 μM, whereas compounds 3Β-hydroxyurs-12-en-28, 20Β-olide, ursolic acid, 2a-hydroxyursolic acid, asiatic acid, ilelatifol B, 2a,3-dihydroxy-3Β-(trans-p-coumaroyloxy)urs-12-en-28-oic acid, vomifoliol, roseoside, lamiuamplexoside C, methyl 3,4-di-O-caffeoylquinate, and methyl 3,5-di-O-caffeoylquinate showed a moderate me dium inhibitory effect. Plus, the anti-inflammatory activity of ursane triterpenoids with 3-pcoumaroyloxy group was significantly improved compared to isolated ursane type triterpenoids. Compound 3Β-hydroxyurs-12-en-28,20Β-olide had moderate anti-inflammatory activity, whereas compound 20Β-hydroxyursolic acid had none, and compound 2a-hydroxyursolic acid had stronger anti-inflammatory activity than the compound asiatic acid [43].
As well, the isolates from Croton laui leaves were isolated, identified, and evaluated for their anti-inflammatory and cytotoxic activities. The inhibition of Nitric Oxide (NO) production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages was used to evaluate the anti-inflammatory activities of the different compounds. Dexamethasone (Sigma, USA) was used as a positive control. From this research, compounds 6S-crotoeurin C and crotoeurin C exhibited inhibitory activities of Lipopolysaccharide (LPS)-induced Nitric Oxide (NO) production in RAW 264.7 macrophages with IC50 values of 1.2 and 1.6 μM, respectively. While dexamethasone served as a positive control with an IC50 value of 0.19 μM [44].
The extracts of Pittosporum qinlingense twigs, fruits, and leaves were also investigated. They described the inhibitory effects of the different compounds on Lipopolysaccharide (LPS)-induced Nitric Oxide (NO) production in BV-2 microglial cells. The chosen positive control was quercetin. With IC50 values of 12.56±0.12, 1.58±0.14, and 11.43±0.14, respectively (the positive control had an IC50 of 2.56 μM), the compounds Pitqinlingoside O, Pitqinlingoside P, and Arvoside C showed considerably greater inhibitory action in these data. Additionally, with IC50 values of 25.67 and 28.74 μM, the substances Pitqinlingoside N and Boscialin had mild inhibitory effects. The remaining substances, on the other hand, had poor anti-inflammatory effects in macrophages with IC50 values greater than 50 μM. Pitqinlingoside P could significantly reduce LPS-induced COX-2 and iNOS expressions, according to Western blot analysis [45].
Furthermore, the various chemicals in Artemisia vulgaris L. leaves were extracted and assessed for their anti-inflammatory effectiveness. By measuring the expression of the inflammatory mediator NO in LPS-induced RAW264.7 cells, all isolates were assessed for their anti-inflammatory efficacy. The results showed that artemvulactone E had a strong anti-inflammatory impact with an IC50 value of 0.9±0.2 μM. The positive control group was the dexamethasone group. Additionally, western blotting tests showed that artemvulactone E may dose-dependently lower LPS-induced COX-2 protein expression [46].
Still, a few plant species employed in Zimbabwean poultry ethnomedicine were assessed for their properties. The anti-inflammatory activity was assessed by the analysis of the lipoxygenase inhibitory activity. The S. singueana extract, which had an IC50 value of 1.72±0.28 μg/ml, and the B. madagascariensis extract, which had an IC50 value of 4.41±0.37 μg/ml, had the best anti-lipoxygenase activity, according to the results. The excellent anti-lipoxygenase activity was also detected in the E. abyssinica extract [47].
Senna tora (L.) Roxb. was explored for its potential as a source of drug candidate. The anti-inflammatory activity was determined by Bovine Serum Albumin (BSA) denaturation and red blood cell (RBC) hemolysis inhibition in vitro. The extracts prevented hemolysis of the RBC membrane in a concentration range from 31.058±3.145% to 89.029±1.186%, with an IC50 value of 28.309 μg/mL. The extracts' resistance to BSA denaturation ranged from 32.617±0.890% to 91.731±0.949, and their IC50 value was 22.980 μg/mL. When the anti-inflammatory activity was compared to that of ibuprofen, it demonstrated anti-inflammatory activity with an IC50 range of 4.956 μg/mL for RBC hemolysis inhibition and 38.260±2.081 to 97.116±0.679 for BSA denaturation inhibition [48].
In addition, the fibrous root of Alangium chinense (Lour.) Harms compounds were investigated. The obtained compounds were evaluated for their anti-inflammatory activity against cyclooxygenase (COX-2). Results showed that the analysis of the different compounds have an inhibitory effect against COX-2 with IC50 values of 49.19±0.76, 23.29±0.99, 47.78±1.33, 44.44±0.12, and 20.43±4.72 μM [49].
An overview of the different studies made about the anti-inflammatory properties of terpenoids is presented in Table 2.
Source
Experimental Model
Main Results
References
Curcuma kwangsiensis
ICR male mice (22–24 g) and LPS-stimulated RAW 264.7 macrophage cell
The pharmacological evaluation of various layers (ME, EA, AQS) from C. kwangsiensis supported its traditional use for relieving inflammation. The in vivo study showed that intragastrically administrated ME and EA significantly inhibited the carrageenan-induced paw edema in comparison to the control group, especially the EA layer with better anti-inflammatory activity. The in vitro study results indicated that most of sesqui- and diterpenoids isolated from the EA layer significantly inhibited IL-1β, COX-2, and TNF-a production at a concentration of 20 μg/mL in LPS-induced RAW 264.7 macrophage cells
[40]
Nepeta bracteate
Lipopolysaccharide-stimulated RAW 264.7 macrophages using the MTT colorimetric method
For the first time, nine abietane diterpenoids, including four new compounds and one new amide alkaloid, were isolated from the traditional medicine Nepeta bracteata Benth., elucidating its active components and laying the groundwork for future clinical applications. All isolates were also examined for their cytotoxic and anti-inflammatory effects. Compounds nepetabrate B and nepetabrate D demonstrated potential biological activity. Both substances are active chemicals that could be useful for research
[41]
Arenga pinnata
Murine RAW 264.7 macrophage cells treated with LPS (Sigma, 1 µg/mL)
The chemical research of A. pinnata fruits. caused the isolation and identification of 26 compounds, including 2 undescribed terpenoids and 24 known compounds, among them the absolute configuration of arenterpenoid D. Furthermore, in the NO production bioassay of compounds, all of them exhibited different degrees of suppression on NO production, and compounds Pinnasesquiterpene A and Linchuniinone exposed moderate suppressive effects against NO generation in lipopolysaccharide-stimulated RAW 264.7 cells
[42]
Abelia macrotera
LPS-induced RAW 264.7 cells
In total, 17 compounds were discovered in the twigs and leaves of A. macrotera. These findings indicate that the anti-inflammatory activity of 28-COOH and 20-OH in ursolic triterpenoids can be increased after esterification, and the presence of 23-OH will weaken the anti-inflammatory activity of the compound. Furthermore, compound methyl 4,5-di-O-caffeoylquinate may play an anti-inflammatory role by combining with Cathepsin G & Chymase, and HPG D
[43]
Croton laui
Inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages
The present results showed that clerodane diterpenoids could partly account for the traditional uses of C. laui in the treatment of inflammation-related diseases, indicating that this plant might have the potential for further investigation as an anti-inflammatory agent.
[44]
Pittosporum qinlingense
Lipopolysaccharide (LPS)-induced nitric oxide (NO) production in BV-2 microglial cells using the Western blot analysis
The investigation of P. qinlingense led to the isolation of seven sesquiterpenoid glycoside esters, two monoterpenoids, two triterpenoids, two lignans, and others. Pitqinlingoside O, Pitqinlingoside P, and arvoside C presented significant nitric oxide production inhibition in LPS-induced BV-2 microglial cells
[45]
Artemisia vulgaris L
Expression of the inflammatory mediator NO in LPS-induced RAW264.7 cells
Eight undescribed sesquiterpenoids and two undescribed triterpenoids were isolated from the leaves of A. vulgaris, together with thirteen known terpenoids. Biological activity research indicated that the compound Artemvulactone E has significant anti-inflammatory activity, by reducing LPS-induced COX-2 protein expression dose-dependently, according to western blotting experiments
[46]
Bobgunnia Madagascariensis; Adenia gummifera; Senna singueana; Aloe chabaudii; Aloe greatheadii; Agave si-salana; Albizia gummifera; Erythrina abyssinica; Euphorbia matabelensis; Tridactyle bicaudate
Evaluation of the anti-lipoxygenase (15-LOX) activity of the different extracts
This study showed the different biological activities of the different plants. Regarding anti-lipoxygenase activity, extracts of B. madagascariensis, S. singueana, T. bicaudata, and E. matabelensis were more active than toxic (selective index >5) indicating the anti-inflammatory potential
[47]
Senna tora (L.) Roxb. leaves
Heat-induced hemolysis of Red blood cell (RBC) and Bovine serum albumin (BSA) protein denaturation assays
The findings of this study concluded that Senna tora (L.) Roxb. leaves contain various activities due to the presence of some biologically active phytochemicals, such as terpenoids. The extract showed dose-related anti-inflammatory action by preventing RBC hemolysis and BSA denaturation, according to the anti-inflammatory activity assays. Collectively, this may contribute to the development of antibacterial agents for humans
[48]
Alangium chinense
Fluorometric assay of COX-2 inhibitors
In summary, one new sesquiterpene and four known compounds were obtained from the roots of A. chinense. The new compound 1-carbonyl-2,8-dihydroxy-11-oxabicyclo [4,4,1] germacra-2(3),4(5),6(7), 8(9)-tetraene and oleanane-type triterpenoids showed strong inhibiting effects to COX-2, which may reveal the material basis of anti-inflammatory
[49]
Table 2: Anti-inflammatory properties of terpenoids.
Antibacterial Properties
Terpenoids have a wide range of biological activity, and studies have shown that they also have antibacterial properties. These substances might be very significant in several areas, including food chemistry, pharmacology, and pharmaceutics [50,51].
The antibacterial action against microorganisms that cause foodborne illness was observed. Escherichia coli, Salmonella enterica, and Staphylococcus aureus each had minimum inhibitory concentration assay (MIC50) and Minimum Bactericidal Concentration assay (MBC) values that ranged from 0.420 to 1.598 mg/mL and 0.673 to 3.432 mg/mL, respectively. The MBC was found to be at a concentration of 3.432 mg/mL for each of the studied terpenoids. The three terpenoids without a hydroxyl group—pinene, limonene, and myrcene—showed antibacterial activity, with limonene exhibiting the highest effects at a dosage of 0.421 mg/mL. Among the four terpenoids containing hydroxyl groups—geraniol, linalool, nerol, and terpineol—nerol and geraniol showed comparable antibacterial activity. Gram-positive bacteria were found to be marginally more susceptible than Gram-negative bacteria based on the results of MIC50 and MBC values. The antibacterial activity at the selected MIC50 levels was also assessed using the time-kill curve test. The antibacterial activity of all seven prominent terpenoids was observed to plateau between 16 and 24 hours, whereas the growth control demonstrated a sharp rise in antibiotic activity. As a result, they discovered that after 16 hours, the seven main terpenoids in wine showed effective antibacterial action against all three bacterial species at their set MIC50 [52].
Also, Eclipta prostrata (L.) L. terpenoid compounds were examined. The liquid growth inhibition method was used to assess the antibacterial activity against two Gram-positive strains, Staphylococcus aureus, and Bacillus subtilis. Only substances exhibiting growth inhibition rates more than 50% were subjected to further testing at concentrations higher than 50 μM to determine their IC50 values. The positive control utilized was cephalosporin. Only compound 3-O-(6-O-crotonyl-D-glucopyranosyl)-16-hydroxy-olean-12-en-28-oic acid 28-O-D-glucopyranosyl ester was shown to have antibacterial activity against S. aureus, with an IC50 value of 37.36 μM, according to the data [53].
Additionally, Lavandula Atlantica essential oils (LAEO) were studied to determine their antibacterial effectiveness against resistant microorganisms. This activity was estimated by measuring the inhibitory diameters of nine strains of bacteria: Methicillin-resistant Staphylococcus aureus, Escherichia Coli, Enterobacteraerogenes, Pseudomonasaeruginosa, klebsiella pneumonia, Klebsiellaoxytoca, Salmonella spp., Acinetobacterbaumanii, Enterobactercloacae. The activity of the substances under investigation was first assessed using the disc diffusion method, and then the MIC and MBC concentrations were calculated using the microdilution method. The outcomes demonstrate that LAEO was effective against all examined strains, with MIC values for the investigated bacteria varying from 3.13 mg/L to 25 mg/L. The same study also found that against Escherichia coli, Acinetobacter baumanii, and Enterobacter cloacae, all terpenoids were even more effective than Gentamicin (control) [54].
Likewise, the potential of several Paeonia ostii T. organs as antibacterial agents was investigated. Eight Gram-positive and Gram-negative bacteria were used as test subjects for the antibacterial activity. The quantitative test showed that P. ostii hydrosols effectively inhibited the growth of Streptococcus hemolytis- Β and Staphylococcus aureus. The inhibition zones and Minimum Inhibitory Concentrations (MICs) of freshly developed leaf hydrosols were 10.65-17.5 mm and 0.78-12.5 mg/mL, respectively, showing more pronounced antibacterial effects than those from other organs [55].
In the same lane, the antibacterial effect of the terpenoid compounds from Ferula haussknechtii was studied by measuring their MIC values against S. aureus, B. cereus, P. aeruginosa, E. coli, clinical isolate of H. pylori, b-lactamase producing clinical strain of K. pneumonia and clinical isolate of vancomycin-resistant E. faecium. According to the results, the overall inhibitory activities of the compounds were higher against the tested Gram-positive bacteria than the Gram-negative bacteria. All of the compounds had a significant effect on S. aureus but a moderate effect on K. pneumonia and P. aeruginosa. Among these compounds, Hawraman 8-p-hydroxybenzoyl-tovarol had the widest antibacterial spectrum (antibacterial effect on B. cereus with the MIC of 16 μg/mL) [56].
Lemnalia sp., a soft coral found in the Xisha region, was the subject of research. Five bacterial strains were used, including Bacillus subtilis, Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella paratyphi, to assess the antibacterial activities by MIC assay. Gentamicin served as a positive control. The various compounds displayed different concentrations, being Nardosinoid A, Nardosinoid B, and em-nal-1(10)-ene-7,12-diol, displayed moderate antibacterial activities against Bacillus subtilis (MICs 4-8 μg/mL), and Nardosinoid B and Lemnal-1(10)-ene-7,12-diol also showed moderate activity against Staphylococcus aureus (MICs 4-16 μg/mL) [57].
As before mentioned, the properties of a few plant species employed in Zimbabwean poultry ethnomedicine were assessed. Regarding the antibacterial activity, the Minimum Inhibitory Concentrations (MICs) of the acetone extracts against three pathogens (Staphylococcus aureus, Escherichia coli, Salmonella Enteritidis), as well as two clinical strains (Escherichia coli and Salmonella Gallinarum), recovered from hens, were used to determine the antibacterial activity. Erythrina abyssinica displayed the best antibacterial activity against both strains in these experiments, with MIC values ranging from 0.02 to 0.156 mg/ml. All plants demonstrated antibacterial activity. First, the effects of terpenes (carvacrol, thymol, nootkatone, eugenol, limonene, carvone, and geraniol) on the growth of each bacterial strain were investigated [47].
In a different approach, the antibacterial activity of several terpenoid combinations against various bacterial species were investigated. Four terpenoid combinations—C1 (carvacrol and thymol), C2 (carvacrol, thymol, and eugenol), C3 (carvacrol, thymol, and nootkatone), and C4 (carvacrol, thymol, eugenol, and nootkatone)—were used against eight bacterial strains (Salmonella enteritidis, Escherichia coli, Acinetobacter baumannii, Bacillus cereus, Enterococcus faecalis, Staphylococcus aureus, Listeria monocytogenes, Corynebacterium diphtheriae). Growth suppression was observed to be more effective when the terpenoids were used in combinations rather than alone. The various combinations of terpenoids effectively inhibited the growth of all of the examined bacterial strains; however, gram-negative bacteria (such as A. baumannii, E. coli, and S. enteritidis) were more effectively inhibited than gram-positive bacteria. The bactericidal action of all combinations against E. coli and S. enteritidis was complete at 1 mM, regardless of how the terpenoids were combined. At 0.5 mM, C4 demonstrated potent bactericidal action against C. diphtheriae and S. enteritidis. The other bacilli were more sensitive than B. cereus, in comparison. Little bactericidal activity was seen for gram-positive cocci (E. faecalis and S. aureus) with C1, and combinations of three or four terpenoids at 1 mM were required for bactericidal activity against E. faecalis and S. aureus [58].
As aforementioned, the potential of Senna tora (L.) Roxb. as a source of drug candidate was explored. The antibacterial activity was evaluated by agar-well diffusion methods. The two-fold serial dilution method was used to test the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) against Bacillus infantis, Exiguobacterium sp., Staphylococcus aureus, and Enterococcus sp., Escherichia coli, Vibrio cholerae, Salmonella typhi, Pseudomonas aeruginosa, and Haemophilus influenzae. The findings revealed that, at rates ranging from 62.5 to 500 mg/mL, the compounds of the extract can prevent the proliferation of bacteria, with inhibition zones ranging from 14±1 to 23±1 mm for Gram-positive bacteria and 11.5±0.5 to 22±1 mm for Gram-negative bacteria. This result demonstrates that the compounds of the extract of Senna tora (L.) Roxb. leaves have antibacterial effectiveness across a broad range of microorganisms. The MBC values ranged from 3.270±1.133 to 6.541±2.266 mg/mL, which were higher than those for MIC [48].
Furthermore, the extracts from Xerophyta spekei and Grewia tembensis were studied. Tests for antibacterial activity against Salmonella Typhi, Bacillus subtilis, Staphylococcus aureus, and Escherichia coli included disc diffusion, minimum inhibitory concentrations, and bactericidal concentrations. G. tembensis exhibited effects on S. aureus only with Mean Zone Inhibition (MZI) of 07.07±0.07 to 12.33±0.33 mm and 08.33±0.33 to 11.67±0.33 mm for stem bark and leaf extracts respectively. While X. spekei extract had effects on S. aureus with MZI of 07.67±0.33 to 14.67±0.33 mm and B. subtilis with MZI of 09.67±0.33 to 14.33±0.33 mm [59].
An overview of the different studies made about the antibacterial properties of terpenoids is presented in Table 3.
Source
Experimental Model
Main Results
Referenes
Myrcene, limonene, geraniol, linalool, nerol, a-pinene, and terpineol compounds
Reducing power assays - Minimum inhibitory concentration assay (MIC50) and Minimum bactericidal concentration assay (MBC) – and time-kill curve test, against Escherichia coli, Salmonella enterica, and Staphylococcus aureus
The seven predominant wine terpenoids displayed effective antibacterial activity against typical foodborne pathogenic bacteria, at a predetermined MIC50 after the 16-h timepoint. Proving that they could be new potential sources of natural antibacterial and antioxidant agents for use in the food industry
[51]
Screening of these isolates in an array of bioassays revealed antibacterial, cytotoxic, and a-glucosidase inhibitory activities for selective compounds. According to the results, only compound 3-O-(6-O-crotonyl-D-glucopyranosyl)-16-hydroxy-olean-12-en-28-oic acid 28-O-D-glucopyranosyl ester showed to have antibacterial activity against S. aureus
Eclipta prostrata L.
Liquid growth inhibition method against Staphylococcus aureus and Bacillus subtilis
All studied terpenoids were even more active than Gentamicin against escherichia Colii, Acinetobacterbaumanii, and Enterobacter cloacae, proving that LAEO compounds have an
[53]
Lavandula Atlantica
Disc diffusion method, and MIC and MBC concentrations by microdilution method against Methicillin-resistant Staphylococcus aureus, Escherichia Coli, Enterobacteraerogenes, Pseudomonasaeruginosa, klebsiella pneumonia, Klebsiellaoxytoca, Salmonella spp., Acinetobacterbaumanii, Enterobactercloacae
effect on the antibacterial power. So, the use of this essential oil or its constituent in the formulation of drugs is recommended
[54]
Paeonia ostii T.
Standard broth-microdilution method, examination of minimum inhibitory concentrations (MICs), and Oxford cup technique, against Staphylococcus aureus, Streptococcus hemolytis-β, Propionibacterium acnes, Listeria monocytogenes, Pseudomonas aeruginosa, Escherichia coli, Proteus vulgaris, and Salmonella enterica subsp. enterica
The hydrosols from different P. ostii organs possessed antibacterial activities against some ordinary skin-infecting and food-borne bacterial pathogens. Consequently, the P. ostii hydrosols may be used as organic antibacterials in food, nourishment, pharmaceutical, and cosmetic industries to improve the safety of corresponding products
[55]
Ferula haussknechtii
MIC values against S. aureus, B. cereus, P. aeruginosa, E. coli, clinical isolate of H. pylori, b-lactamase producing clinical strain of K. pneumonia, and clinical isolate of vancomycin-resistant E. faecium
These compounds were assayed for antibacterial activity and the results showed that all of them were generally more active against S. aureus compared with other species. Overall, the study demonstrated that F. haussknechtii may be appropriate natural antibacterial agents and potent lead compounds
[56]
Lemnalia sp.
MIC values against Bacillus subtilis, Staphylococcus aureus, MRSA, Pseudomonas aeruginosa and Salmonella paratyphi
The various compounds were tested against gram-positive and gram-negative bacteria. The results showed that the compounds had activity against them, notably against Bacillus subtilis and Staphylococcus aureus
[57]
Bobgunnia Madagascariensis; Adenia gummifera; Senna singueana; Aloe chabaudii; Aloe greatheadii; Agave sisalana; Albizia gummifera; Erythrina abyssinica; Euphorbia matabelensis; Tridactyle bicaudata
MICs of the acetone extracts were determined using a serial two-fold dilution method using bacterial strains: S. aureus, S. Enteritidis, E. coli, and clinical strains: E. coli and Salmonella Gallinarum
This study showed the different biological activities of the different plants. Regarding the antibacterial activity, three extracts were noted: E. abyssinica extract which had significant antibacterial activity; S. singueana acetone extract which had moderate antibacterial activity and A. greatheadii, which had reasonable antibacterial activity
[47]
Pure compounds supplied by FUJIFILM Wako Pure Chemical Corporation, Tokyo, Japan (Carvacrol, thymol, eugenol, nootkatone, perillyl alcohol, limonene, cineole, carvone, and geraniol)
Evaluation of the growth inhibitory effect of the different compounds, individually and in combination, against bacterial strains: S. enteritidis, E. coli, A. baumannii, B. cereus, S. aureus, E. faecalis, L. monocytogenes, and C. diphtheriae
This study revealed that combinations of terpenoids have activities against a spectrum of bacteria. The most effective bactericidal activity was observed for gram-negative bacteria and in combinations. Thus, this provides new candidates for the development of antibacterial
[58]
Senna tora (L.) Roxb. leaves
Agar well diffusion, MBC, and MIC assays, against Bacillus infantis, Exiguobacterium sp., Staphylococcus aureus, Enterococcus sp., Escherichia coli, Vibrio cholerae, Salmonella typhi, Pseudomonas aeruginosa, and Haemophilus influenza
The findings of this study concluded that Senna tora (L.) Roxb. leaves contain various activities due to the presence of some biologically active phytochemicals, such as terpenoids. Based on the antibacterial activity assays, the extract can prevent the proliferation of bacteria, with visible inhibition zones. Collectively, this may contribute to the development of antibacterial agents for humans
[48]
Xerophyta spekei (whole plant without roots) and Grewia tembensis (leaves and stem barks)
Disc diffusion, minimum inhibitory concentrations, and bactericidal concentrations tests against Salmonella Typhi, Bacillus subtilis, Staphylococcus aureus, and Escherichia coli
The studied plant extracts demonstrated antibacterial potentials with X. spekei extract, exhibiting activity on S. aureus and B. subtilis. While both the stem bark and leaf extracts of G. tembensis exhibited activity on S. aureus alone. Overall, these current findings indicate that the studied extracts can be potential candidates to extract therapeutic antibacterial agents for managing and treating bacterial illnesses
[59]
Table 3: Antibacterial properties of terpenoids.
Source
Experimental Model
Main Results
References
Alpinia eremochlamys, Etlingera flexuosa, and Etlingera acanthoides
Viral-ToxGlo colorimetric method against HIV-infected MT-4 cells
The screening of antiviral activity showed that the ethanol extract of E. acanthoides and A. eremochlamys rhizomes have the potency to inhibit the replication of HIV-1 on MT-4 cells in vitro. E. acanthoides rhizome showed the best antiviral activity with the lowest IC50 value, less cytotoxicity on MT-4 cells, and the highest selectivity index
[60]
Lippia alba
MTT colorimetric assay for Zika virus (ZIKV) in VERO cells, and molecular docking assay to identify the interaction between β-caryophyllene and ZIKV enzyme
This plant is widely used in ethnomedicine and popular medicine, primarily in the form of infusions, syrups, and poultices. The essential oil of Lippia alba demonstrated an antiviral action against ZIKV and minimal cytotoxicity. The primary component of essential oils, Β-caryophyllene, was found to be tested for its ability to suppress ZIKV replication only at the beginning of the viral life cycle. The molecular docking experiments showed that Β-caryophyllene had a stronger affinity for the NS2B-NS3 protein complex and the NS5 protein
[61]
Arthrospira maxima, Chlorella vulgaris, Dunaliella salina, and Haematococcus pluvialis
Tissue Culture Infectious Dose 50 (TCID50) in Vero cells against Mayaro virus (MAYV)
The findings of this study demonstrated that all microalgal extracts had levels of MAYV inactivation that were higher than those of the reference compound ribavirin. As a result, the extracts have a significant potential for use in the treatment of Mayaro Fever, which is currently not managed by any medications or vaccines
[62]
Lemnalia sp.
Inhibitory cytopathic effects (CPE) assay against influenza A virus (H1N1) and Herpes Simplex Virus Type 1 (HSV-1)
The various compounds were tested against H1N1 and HSV-1 virus. The results showed that the compounds parathyrsoidin I and linardosinene E had inhibitory actions against influenza A virus H1N1
[57]
Diterpenic Mannich bases compounds
Hemagglutination assay against influenza virus A/Puerto Rico/8/34 (H1N1) in MDCK cells and SARS-CoV-2 pseudovirus in BHK-21-hACE2 cells
Collectively, the data suggested the potency of diterpenic Mannich bases as effective anti-influenza and anti-COVID-19 compounds.
[63]
Lippia multiflora and Zingiber officinale essential oils
Cell culture cytopathic inhibition test against poliovirus type I and enterovirus type I
From results obtained regarding the antiviral assay, Zingiber officinale significantly inhibited type I enterovirus’ (EV-1) activity as compared to Lippia multiflora. However, in general, these two essential oils could be considered a source of natural therapeutic agents in the treatment of viral infections
[64]
Table 4: Antiviral properties of terpenoids.
Hypoglycemic Effect
High blood sugar levels are a defining feature of diabetes, a metabolic disease [19,65]. Diabetes patients' chronically excessive blood sugar levels can harm and be dysfunctional in a variety of tissues [19]. Medicine and insulin are used to treat this condition, but they have several adverse effects, including gastrointestinal problems like diarrhea, flatulence, and abdominal pain. Phytochemicals are the natural substances from which new and more potent medications for the treatment of diabetes can be developed, according to the present study findings [19]. Thus, research into some naturally occurring, plant-derived bioactive chemicals are deemed vital to control and treat diabetes mellitus with fewer complications and negative effects [65].
Terpenoids' action plays a significant part in the creation of new medicines and enhancements to current therapeutic options [19].
The antihyperglycemic activity of the ethanol extract of Annona diversifolia leaves (EEAd), chloroformic (CHCl3Fr), ethyl acetate (EtOAcFr), aqueous residual (FrAcR), secondary 5 (Fr5) fractions, and two acyclic terpenoid isolates from this plant, farnesol and farnesal were explored. Using the oral sucrose and lactose tolerance (OSTT and OLTT, respectively) and Intestinal Sucrose Hydrolysis (ISH) tests, the potential as a-glucosidase inhibitors of products were assessed. Additionally, tests for Oral Glucose Tolerance (OGTT), Intestinal Glucose Absorption (IGA), and Urine Glucose Excretion (UGE) were used to assess the potential as Sodium-Glucose cotransporter 1 protein (SGLT-1) inhibitors. Male normoglycemic and streptozocin-induced diabetes type 2 (SID2) mice were used to assess the impact on blood glucose levels. At two and four hours, all treatments in OSTT and OLTT had appreciable action. Half maximum effective concentrations (CE50) for ISH were calculated to be 565, 662, and 590 μg/mL, 682, and 802 μM, respectively. At two hours in the OGTT, all therapies demonstrated meaningful action. IGA calculated respective CE50 values of 1059, 783, and 539 μg/mL, 1211, and 327 μM. In comparison to canagliflozin, farnesol, and farnesal compounds significantly reduced the amount of glucose excreted in UGE Fr5 [66].
As before mentioned, Eclipta prostrata L. terpenoid compounds were examined. The a-glucosidase inhibitory activity was studied by the calculation of the different concentrations of the absorbance recorded. Only the isolates with > 50% inhibition ratio were sent on to be evaluated at an IC50 concentration after all the isolates had initially been tested at a primary concentration of 100 μM. Being 103 times more active than the positive control acarbose, the tetracyclic triterpenoid demonstrated very significant inhibition against a-glucosidase with an IC50 of 0.82 0.18 μM [53].
In a different approach, the efficacy of Ganoderma Lucidum Ethanol Extract (GLEE) against metabolic syndrome (MetS) complications in rats was evaluated. Thirty male rats were randomized into six different groups (containing 25 MetS and 5 normal rats). Animals were sacrificed following two weeks of GLEE treatment post-MetS induction. Biochemical and histological studies were performed on blood, pancreas, hearts, livers, and kidneys. A digital glucometer was used to measure blood sugar levels. Results showed that GLEE, compared to the MetS control, which had a 40% increase in blood sugar compared to the normal control, effectively reversed MetS-induced hyperglycemia in a dose-dependent manner up to 4-folds. Comparable to the impact achieved in the other GLEE group, where a 3.9-fold decline followed, the combined action of glibenclamide and atenolol caused a 3.4-fold decrease in hyperglycemia [67].
In the same lane, the in vivo hypoglycemic, antihyperglycemic, and antidyslipidemic effects of the solvent fractions of Hagenia abyssinica leaf extract were investigated. In normal, oral glucose-loaded, and streptozotocin-induced diabetic mice, the anti-diabetic effects of the solvent fractions were assessed. After administering three different doses of the solvent fractions (100, 200, and 400 mg/kg), the hypoglycemic, antihyperglycemic, antidyslipidemic, and effect on body weight change were assessed. Both the aqueous and the ethyl acetate fractions of H. abyssinica leaves demonstrated considerable (P<0.05) hypoglycemic efficacy in normoglycemic mice. At 60 and 120 minutes after oral glucose loading, the two doses of the aqueous fraction—200 mg/kg (p<0.05) and 400 mg/kg (p<0.001)—showed a significant antihyperglycemic effect, whereas the ethyl acetate fraction also demonstrated a significant antihyperglycemic effect at 60 min (P<0.05 for 200 mg/kg and P<0.001 for 400 mg/kg) and 120 min (P<0.01). With the exception of 100 mg/kg of the aqueous and chloroform fractions, all doses of the solvent fractions significantly (P<0.05) decreased blood glucose levels in diabetic mice receiving a single dose of treatment. Additionally, daily administration of the aqueous fraction for several weeks dramatically decreased hyperglycemia, prevented weight loss, and enhanced [68].
Also, the anti-diabetic properties of a terpenoid-rich extract from Dillenia indica L. bark (TRDI) in Palmitic Acid-induced Insulin Resistance (PA-IR) in C2C12 myotube and a streptozotocin (STZ)-induced diabetic mouse model were studied. TRDI's IC50 value of 3.03±1.01 μg/mL, which was 92-fold greater than the value for the positive control, acarbose (IC50 = 279.49±μg/mL), substantially inhibited a-glucosidase activity. Furthermore, TRDI increased glucose transporter 4 (GLUT4) translocation to the plasma membrane (PM), which increased glucose absorption. TRDI also stimulated the insulin receptor substrate-1 (INS-1), downregulated Phosphoinositide-Dependent Kinase-1 (PDK1), and protein kinase B (Akt) in both normal and PAIR C2C12 cells as well as in STZ-induced diabetic mice [69].
Using activity-guided fractionation as a strategy, was investigated the potential antidiabetic effects of Salvia polystachya Cav. and its isolated products, particularly its effect as an a-glucosidase and sodium-glucose cotransporter 1 (SGLT1) inhibitor using in vivo (in mice), ex vivo (treatments in the intes tine portions), and in silico (molecular docking of ursolic acid, oleanolic acid, acarbose on a-Glucosidase enzyme, and canagliflozin on SGLT1 Cotransporter) assays. All of the therapies during the tests for glucose tolerance decreased the postprandial peak, much like the control medications. Ursolic Acid (UA) and Oleanolic Acid (OA) were determined to have IC50 values of 739.9 and 726.3 μM, respectively, during the Intestinal Sucrose Hydrolysis (ISH). Calculated IC50 values for UA and OA during Intestinal Glucose Absorption (IGA) were 966.6 and 849.3 μM, respectively. Finally, during the molecular docking studies, UA and OA demonstrated ?G values on -glucosidase enzymes of -6.41 and -5.48 kcal/mol-1, respectively. Both UA and OA displayed ?G values of -10.55 and -9.65 during SGLT1 [70].
Hypericum perforatum's terpenoid-based bicyclic dihydropyran enantiomers ((±)-Hyperpyran A) with hypoglycemic action were explored. The compounds' hypoglycemic potential was examined in human liver cancer cell line HepG2 and mice with normal liver cell line AML12. The glucose concentration in the supernatant of AML12 and HepG2 cells was determined using a glucose test kit following the manufacturer's instructions after treatment with 40 μM of the tested compounds for 24 hoPositiveitive control was utilized, which was metformin (150 μM). The findings showed that (+)-Hyperpyran A compound moderately promoted glucose absorption activity in hepatocytes [71].
An overview of the different studies made about the hypoglycemic effect of terpenoids is presented in Table 5.
Source
Experimental Model
Main Results
References
Annona diversifolia
Assessment for the potential a-glucosidase inhibitors: Oral sucrose (OSTT) and lactose tolerance (OLTT) and intestinal sucrose hydrolysis (ISH) tests
These results provide information about the possible mechanisms of action of farnesal and farnesol, confirming their antihyperglycemic activity mediated by the inhibition of a-glucosidase and selective inhibition of SGLT-1. Additionally, the ethanolic extract obtained from the leaves of A. diversifolia was found effective in vivo for controlling fasting and postprandial blood glucose levels in animal models of diabetes mellitus. Thus, leaves from A. diversifolia represent a good phytotherapeutic agent for the treatment of this disease. So, the results reported from this study provide a starting point for the development of new drugs for the treatment of diabetes mellitus
[66]
Assessment for the potential as sodium-glucose cotransporter 1 protein (SGLT-1) inhibitors: tests for oral glucose tolerance (OGTT), intestinal glucose absorption (IGA), and urine glucose excretion (UGE)
In male normoglycemic and streptozocin-induced diabetes type 2 (SID2) mice
Eclipta prostrata L.
Calculation of the different concentrations of the absorbance recorded
Screening of these isolates in an array of bioassays revealed antibacterial, cytotoxic, and a-glucosidase inhibitory activities for selective compounds. Of all the compounds tested, the C30H50O2 compound represents an undescribed type of triterpenoid inhibitor and proved to be one of the most potent natural ones, and it thus could serve as a template compound for future anti-diabetes drug development
[53]
Ganoderma lucidum
Thirty male rats were randomized into six different groups (containing 25 MetS and 5 normal rats), following two weeks of Ganoderma lucidum ethanol extract (GLEE) treatment post- metabolic syndrome (MetS) induction. Biochemical and histological studies were performed on blood, pancreas, hearts, livers, and kidneys
Taken together, GLEE showed tremendous biological effects. Results revealed that GLEE (70 mg/kg) reversed significantly (p < 0.05) the MetS-induced hyperglycemia. Besides, rats treated with GLEE did not show any pathological features in the pancreas, heart, liver, and kidneys. This study, therefore, showed that Ganoderma lucidum might be a candidate regimen in the management of MetS
[67]
Hagenia abyssinica
Normal, oral glucose-loaded, and streptozotocin-induced diabetic mice, were administrated with three different doses of the solvent fractions (100, 200, and 400 mg/kg). One-way ANOVA followed by Tukey’s post hoc test was used for data analysis, and p<0.05 was considered a statistically significant
The results of the current study proved that Hagenia abyssinica leaf solvent fractions have antidiabetic effects in normoglycemic, oral glucose-loaded, and streptozotocin-induced diabetic mice. Additionally, the solvent fractions improved the changes in serum lipid profiles and body weight associated with diabetes. As a result, this research supports the use of Hagenia abyssinica in the treatment of diabetes
[68]
Dillenia indica
Palmitic acid-induced insulin resistance (PA-IR) in C2C12 myotube and a streptozotocin (STZ)-induced diabetic mice model
Dillenia indica L. bark (TRDI) competitively inhibited a-glucosidase activity in a dose-dependent manner. Additionally, it enhanced GLUT4 translocation and activated the insulin signaling pathway (e.g., IRS-1 and Akt signaling) in basal and PA-IR C2C12 myotubes. These results may help formulate novel glucose management therapeutics in the future using Dillenia indica in the treatment of diabetes
[69]
Salvia polystachya
Tests of the hyperglycemia activity in mice of ethanolic extract from Salvia polystachya (EESpS), ethyl acetate fraction (EtOAcFr), secondary-6-fraction (SeFr6), ursolic acid (UA), and oleanolic acid (OA)
In diabetic rats, blood glucose levels were decreased by the ethanolic extract from Salvia polystachya, EtOAcFr, SeFr6, and UA and OA discovered in the EtOAcFr. Studies conducted in silico, ex vivo, and in vivo all supported this activity. Inhibition of the a-glucosidase enzyme and the SGLT1 cotransporter is thought to play a role in the antidiabetic effect of the products made from the stems of S. polystachya. This study confirms S. polystachya's phytochemical and pharmacological origins and utility as a source of prospective anti-diabetic drugs
[70]
a-glucosidase inhibition evaluated with oral sucrose and starch tolerance tests (OSuTT and OStTT), intestinal sucrose hydrolysis (ISH) assay, and molecular docking studies using acarbose as a control
SGLT1 inhibition was evaluated with oral glucose and galactose tolerance tests (OGTT and OGaTT), an intestinal glucose absorption (IGA) assay, and molecular docking studies using canagliflozin as the control
Hypericum perforatum
Human liver cancer cell line HepG2 and mice normal liver cell line AML12 using a glucose test kit
The compounds (+)-Hyperpyran A and (-)-Hyperpyran A were investigated for their hypoglycemic activity. The results showed that compound (+)-Hyperpyran A exhibited a moderate promotion of glucose uptake activity in hepatocytes
[71]
Table 5: Hypoglycemic effect of terpenoids.
Antioxidant Activity
One of the most studied bioactivities of natural compounds, as well as the capability to prevent oxidative stress and several diseases, is the ability of natural extracts to scavenge free radicals [72,73]. An imbalance between prooxidant and antioxidant species causes oxidative stress, which is a surprisingly significant and frequent occurrence that damages macromolecules and interferes with cellular regulation and redox signaling. Here, significantly elevated free radical levels and concurrently low antioxidant levels cause oxidative stress to have a considerable impact on the organism as a whole [74]. Finding out how free radicals contribute to the formation and progression of the disease has garnered increasing attention in recent years [74]. Particular focus has been placed on cancer, cardiovascular illness, and accelerated aging, as well as neurological disorders such as Alzheimer's and Parkinson's disease [74-76]. Results from the vast majority of research that has been conducted link free radicals to the development of diseases [74].
The presence of hydroxyl groups in phenolic substances determines their ability to scavenge reactive radicals, which in turn determines their antioxidant potential [75]. One of the most desirable biological qualities of natural compounds is their capacity to scavenge free radicals. Finding novel substances with high antioxidant activity has become a focus of a growing number of studies. This pattern has become particularly notable in the fields of biology, pharmacognosy, and pharmacotherapy. Interest in this topic is sparked by knowledge of the harmful effects of free radicals on the human body, the diseases caused by the activity of the reactive forms, and the fact that antioxidants can eliminate the reactive species [74].
The antioxidant terpenoids from the red alga Laurencia tristicha were investigated. By measuring the hydroxyl free radical's (OH) scavenging activity using Electron Paramagnetic Resonance (EPR) spectroscopy, antioxidant activity was determined. The 1,10-epoxy moiety was discovered to be essential for the antioxidant activity because all sesquiterpenes with this moiety were found to be active while those without it were found to be less active. The molecules with acetyl groups connecting to 1-OH were less active, and the aliphatic hydroxy exhibited less influence, however, the phenolic hydroxy greatly increased the antioxidant activity. Additionally, the anti-oxidant effect was marginally reduced by the Br group on the phenyl ring, while the C-10 configurations had no discernible impact [77].
Also, the bioactivity activity of Pinus merkusii needle and bark was evaluated. The antioxidant capacity of extracts was investigated using the extract's ability in inhibiting DPPH (2,2-diphenyl-1-picrylhydrazyl) radical. The scavenging activity values are expressed as IC50 for each extract. Pine needles and bark extract both exhibited the same tendency in dose-dependent ways about their DPPH scavenging abilities. The needle and bark extracts both demonstrated significant levels of scavenging activity, with values of 97,98% and 93,93%, respectively. However, the antioxidant activity (IC50) of P. merkusii bark extract was 59.32±1.74 μg/mL, stronger than needle extract at 68.67±1.47 μg/mL [78].
As before mentioned, the efficacy of Ganoderma Lucidum Ethanol Extract (GLEE) against metabolic syndrome (MetS) complications in rats was evaluated. Thirty male rats were randomized into six different groups (containing 25 MetS and 5 normal rats). In vitro was used the 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) radical scavenging activity of the G. lucidum, Ferric ion Reducing Antioxidant Potential (FRAP), and Total Antioxidant Capacity (TAC) assays to assess the antioxidant profiling of GLEE. Ascorbic acid was used as the positive reference standard. In vivo was used Malondialdehyde (MDA) level, assay for Superoxide Dismutase (SOD) and Catalase (CAT) activity. A dose-dependent total antioxidant capacity and, a near dose-dependent DPPH radical scavenging, and ferric ion reducing ability were exhibited by GLEE. Besides, MDA level measures (pancreas: 37%, heart: 65.58%, liver: 43.17%, and kidneys: 73.2%), SOD activity (pancreas: 33.11%, heart: 26.97%, liver: 13.69%, and kidneys: 25.16%), CAT activity (pancreas: 9.33%, heart: 31.06%, liver: 30.21%, and kidneys: 25.22%), and NRF2 protein level, which were increased in the MetS group, were significantly reduced in the GLEE-treated groups [67].
The essential oil of the Vietnamese plant Alseodaphne velutina Chev was studied. Utilizing the DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), and FRAP (ferric reducing antioxidant power) assays, the antioxidant potential of leaf essential oil was assessed. The results showed moderate to high activity comparable to the well-known antioxidant standard Trolox. Trolox equivalent antioxidant capacity (mg TEAC/g dw), which represents the level of antioxidant activity in comparison to conventional Trolox, is shown after the results. The DPPH assay showed a modest activity (1.08 mg TEAC/g dw). The ABTS+ test had a scavenging capacity of 2.53 mg TEAC/g dw, which was 2.34 times greater than the DPPH test. In comparison to DPPH and ABTS, FRAP analysis showed the strongest ability to degrade Fe3+ into Fe2+ (2.79 mg TEAC/g dw) and the highest antioxidant activity. Additionally, they highlight that according to the results, A. velutina leaf oil's antioxidant activity was probably connected to terpenoids' dominance (89.18%) [79].
As aforementioned, Senna tora (L.) Roxb. was investigated for its potential as a source of therapeutic candidate. H2O2-scavenging tests and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays were used to measure the in vitro antioxidant activity. Senna tora (L.) Roxb. leaves' ethyl acetate extract (EAESTL) demonstrated dose-dependent antioxidant activity with a range from 33.041±1.166% to 91.068±1.950% DPPH inhibition and an IC50 value of 24.425 μg/mL at doses ranging from 2.5 to 200 μg/mL. Ascorbic acid had comparable antioxidant efficacy, DPPH restraint ranging from 40.455±2.019% to 98.190±0.863%, with an IC50 value of 2.585 μg/mL. For EAESTL and ascorbic acid, an H2O2-scavenging assay was also conducted. With an IC50 value of 17.434 μg/mL and concentration-dependent antioxidant activity, EAESTL was shown to scavenge H2O2 with a range of 34.595±1.104% to 93.734±0.336%. Additionally, ascorbic acid had H2O2-scavenging activity that was equivalent to EAESTL, with a range of 39.238±2.040 to 99.154±0.115% and an IC50 value of 1.923 μ g/mL. So, when comparing the outcomes of various scavenging studies, EAESTL and an established antioxidant (ascorbic acid) demonstrated comparable antioxidant activity [48].
Additionally, Carissa edulis and Pappea capensis extracts were tested for their antioxidant activity. Using the iron chelating, hydroxyl radical, DPPH radical, and ferric reduction activities, the extracts' in vitro antioxidant capabilities were assessed. The extracts' catalase, superoxide dismutase, and glutathione reductase activities were also discovered. The antioxidant prop erties of the extracts were concentration-dependent. Additionally, has an impact by increasing the expression of enzymatic antioxidants [80].
An overview of the different studies made about the antioxidant activity of terpenoids is presented in Table 6.
Source
Experimental Model
Main Results
References
Laurencia tristicha
Hydroxyl free radical's (OH) scavenging activity using electron paramagnetic resonance (EPR) spectroscopy
The antioxidant properties of eleven laurane-type sesquiterpenes obtained from L. tristicha were evaluated. The laurane-type sesquiterpenes with 1,11-epoxy moiety showed potential antioxidant activity
[77]
Pinus merkusii
DPPH (2,2-diphenyl-1-picryl-hydroxyl) scavenging activity
Pinus merkusii extracts have the potential as a natural source of antioxidants and antiaging and might be beneficial in these subjects. With particular regard to the antioxidant properties of bark extract that are stronger than the needle extract
[78]
Ganoderma lucidum
Thirty male rats were randomized into six different groups (containing 25 MetS and 5 normal rats), following two weeks of Ganoderma lucidum ethanol extract (GLEE) treatment post- metabolic syndrome (MetS) induction. Biochemical and histological studies were performed on blood, pancreas, hearts, livers, and kidneys
Taken together, GLEE showed tremendous biological effects. GLEE demonstrated a dose-dependent total antioxidant capacity, a nearly dose-dependent DPPH radical scavenging ability, and ferric ion reduction capability. In comparison to the MetS control group, the GLEE-treated group displayed elevated CAT (pancreas and heart) and SOD (the four organs) activity as well as dramatically decreased NRF2 protein levels. This study, therefore, showed that Ganoderma lucidum might be a candidate regimen in the management of MetS
[67]
Alseodaphne velutina Chev.
DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and FRAP (ferric reducing antioxidant power) assays comparable with Trolox
The terpenoids present in the leaf essential oil of A. velutina plants from Vietnam are highly concentrated (89.18%), with b-patchoulene and b-caryophyllene being two of the main constituents. Natural essential oil demonstrated great reducing anti-oxidant power and high free radical scavenging activity, indicating that it may be a viable source of natural antioxidant
[79]
Senna tora (L.) Roxb. leaves
2,2-diphenyl-1-picrylhydrazyl (DPPH)- and H2O2-scavenging tests comparable with Ascorbic acid
The findings of this study concluded that Senna tora (L.) Roxb. leaves contain various activities due to the presence of some biologically active phytochemicals, such as terpenoids. Based on the antioxidant activity assays, when comparing the outcomes of Senna tora (L.) Roxb. leaves' ethyl acetate extract (EAESTL) and ascorbic acid, the extract demonstrated comparable antioxidant activity with the established antioxidant. Collectively, this shows the potential of the extract to fight against oxidants
[48]
Carissa edulis and Pappea capensis
Hydroxyl radical, DPPH radical, and ferric reduction activities
The antioxidant properties of C. edulis and P. capensis extracts have been established. The existence of phytocompounds, which have the capacity to contribute hydrogen atoms or electrons and thereby quench the radicals, is responsible for the actions. Additionally, the phytocompounds are linked to higher levels of enzymatic antioxidants' expression. According to the research, one of the underlying reasons for the therapeutic effects of C. edulis and P. capensis extracts could be their antioxidant properties.
[80]
Activities of catalase, superoxide dismutase and glutathione reductases of the extracts
Table 6: Antioxidant activity of terpenoids.
Anti-Aging Properties
Over time, many of the body's primary systems experience degenerative deterioration as part of the extremely complicated process of aging. Hereditary, behavioral, and environmental factors all have an impact on this unavoidable process [81,82]. The aging process is accompanied by a variety of outward and internal indications and symptoms, such as changes to the skin, and neurological diseases like Alzheimer's disease, cancer, etc [81,83,84].
The majority of this aging process is linked to inflammatory factors and oxidative stress [85]. One of the primary initiating factors causing aging-related damages and concerns is oxidative stress, which is a result of an imbalance between pro- and antioxidants. This is because metabolism produces highly reactive byproducts like reactive oxygen and nitrogen species, which cause cellular damage and apoptosis. Due to their capacity to suppress the generation of free radicals or stop their spread, antioxidants can lower oxidative stress, preventing harmful processes and extending healthy life. Natural anti-aging substances, including vitamins, polyphenols, hydroxy-acids, polysaccharides, and a host of others, are essential for slowing down the aging process [81].
An enormous economic and social burden is being placed on the world by the rise in the aging populace [83]. Anti-aging medicine is a relatively new branch of medicine that is expanding extremely quickly. It is well known that certain nutrients can delay aging and support healthy aging, including specified vitamins, minerals (as micronutrients), essential and branched amino acids, polyunsaturated fatty acids, probiotics, and plant metabolites like polyphenols and terpenoids [81].
The scientific community will need to pay close attention to the timely creation of medications that can reduce the aging process, either alone or as multiple agents, as a new era of anti-aging drug discovery dawns [86]. Natural substances give us the motivation to advance in our quest to comprehend and enhance the health span, extending life expectancy and enhancing health and quality of life by reducing the onset of some age-related chronic diseases [81,86].
Skin Aging: The skin is a complex organ that shields the body from the outside environment [8,14]. It has several purposes, including serving as a physical permeability barrier, safeguarding against pathogenic agents, regulating body temperature, enhancing sensation, guarding against Ultraviolet (UV) rays, and promoting regeneration and wound healing [87].
As stated in study (88), skin aging is a situation when the skin is unable to maintain its structural and physiological integrity. There are numerous causes of skin aging, but external factors are the primary ones (for example, ultraviolet UV rays). These variables affected Reactive Oxygen Species (ROS) levels in the body through a variety of routes. The condition known as oxidative stress is characterized by an imbalance between the generation of ROS and its removal, which is frequently observed in older skin. As a result of the up-regulation of several types of collagenases and elastases, the structural protein created by the skin's connective tissue was prone to disintegration in aged skin. They concluded that reducing the effects of free radicals is a crucial strategy for combating skin aging.
Antioxidants are therefore crucial in avoiding skin aging since they can destroy free radicals by providing or accepting an electron to complete the unpaired molecules. Furthermore, antioxidants' anti-inflammatory properties boost their ability to delay the aging process of the skin [72,89].
Researchers in the cosmetic industry have become interested in bioactive natural materials due to their potential use in treating skin-related issues including wrinkles because of their antioxidative capabilities [87,90].
One example is the potential of mushroom active compounds to activate the skin’s immune system and reduce cell apoptosis, resulting in delaying the process of senescence as skin aging [91]. Moreover, study [90] proved that lupeol (terpenoid) can be recommended as an antiwrinkle agent, and therefore be recommended to be incorporated in topical formulations.
According to study [92], terpenoids are effective enhancers from natural sources that are useful in transdermal medicine delivery because they have low toxicity, high bioavailability, and are easily absorbed via the skin. As a result, they can help the primary medicine penetrate through the skin when employed as excipients in formulations. As an illustration, they provide invasomes, which are now the most widely used nanosystem formulation using terpenoids as excipients. They are made of phosphatidylcholine, ethanol, and a variety of terpenoids. They function as penetration-enhancing vesicles because of their flexibility and deformability, which facilitate penetration across epidermal layers.
The cosmetics business and dermatological research are both focused on developing anti-aging solutions. Although the development of modern skincare products necessitates a thorough understanding of ingredients, natural product chemistry, and skin biology, there has been a steady rise in research into the use of biodegradable materials, largely as a result of growing environmental concerns and the ecological effects of using synthetic alternatives [89].
Degenerative Diseases: Alzheimer's disease is a chronic, progressive neurological condition that most frequently affects elderly people and is associated with dementia and cognitive decline [93,94]. This is the most typical type of dementia that is currently recognized [95]. The condition has a significant economic burden in addition to its detrimental consequences on human health and quality of life [94,96].
The second most common neuro-degenerative ailment to cause morbidity and mortality in elderly populations is Parkinson's disease. As the disease progresses, symptoms start to become more noticeable. This illness is characterized by a neuro-degenerative disorder of the central nervous system, by loss of nerve cells in the area of the brain [97].
One of the main causes of these disorders is neuroinflammation. The so-called "damage signals" are where the processes for inflammatory process in the human brain begin. Palliative care can halt the evolution of cognitive symptoms and stop any worsening of the patient's symptoms, but there is currently no effective medication that can cure the condition. Massive efforts are put towards finding medications that target molecular pathways and stop progression as well as different disease modifying therapies. Intervention therapy employing natural products high in antioxidant and flavonoid content is now more important than ever due to the limitations of current preventive methods [93].
We can uncover compounds with anti-aggregate activity, as well as compounds with antioxidative and anti-inflammatory actions when searching for nutraceutical bioactive principles [94]. The investigation of anti-inflammatory and neuroprotective phytochemicals, such as terpenoids, phenolic derivatives, alkaloids, glycosides, and steroidal saponins, reveals therapeutic potential for the amelioration and prevention of severe neurodegeneration [93]. Terpenoids are the largest and most diversified collection of chemical molecules among several natural products [98]. Terpenoids are more likely to be discovered to have substantial anti-dementia activity [98] as a result of the investigation of these compounds, making them prospective neuroprotective agents [97].
Numerous secondary plant metabolites have reportedly been found as potential therapeutic candidates for use in the management of various forms of dementia, according to study [95]. Based on both in vitro studies of this investigation, the recommended terpenoids had low cytotoxicity, and carvone stood out as having the strongest acetylcholinesterase inhibitory efficacy.
Aromatherapy using essential oils is one of the treatments in use and has been shown to improve cognitive performance in dementia patients. These substances pass the blood-brain barrier, are absorbed through the skin, and enter the systemic circulation. Consequently, topical treatment or inhalation may have a nervous system effect that is not just psychological. The hydrocarbon terpenoids with the shortest molecular size and highest lipophilicity had the highest expected penetration. The size of molecules, which poses issues with distribution to the brain, is one key drawback of innovative multitarget compounds created for the treatment of Alzheimer's disease, according to this research. Therefore, they explain the necessity for smaller molecules with specific biological effects and more desired physicochemical features and pharmacokinetics [99].
Anticancer
Worldwide, cancer is recognized as a serious public health issue and a life-threatening disease [100-102]. It is the biggest cause of death globally and is seen as one of the main barriers preventing the rise in life expectancy [103]. It is characterized by unchecked cell growth that invades the tissues around it and the development of tumor masses [100,104]. This disease can manifest itself in various parts of the body, such as breast cancer (abnormalities in the proliferation of breast cells and is the most common cancer with high mortality in women), brain cancer (glioblastoma multiforme that grows and develops rapidly), colon cancer (develops in the large intestine), liver cancer (aberrant growth of liver tissue that mutates and develops a tumor), uterus cancer (a malignant tumor that develops commonly in the uterus in women with menopause or over 50 years of age), lung cancer (malignancy in the lung tissue originating from cells inside and outside of the lungs), leukemia (the body produces excess white blood cells), and many others [24].
Despite decades of research into the disease, there is still a need for highly effective anticancer medications with low tolerance and fewer side effects [100]. Chemotherapy, radiation therapy, and surgical therapy are some of the therapeutic options. These treatments, however, result in significant tissue damage and other unfavorable side effects [23,24]. The main challenges in treating cancer include chemoresistance, extreme toxicity, recurrence, and metastasis [102]. To expand the number of efficient, risk-free, and affordable cancer treatments, it is therefore still important to develop new therapeutic agents [102,104,105].
A new approach to treating cancer is therefore urgently required [104,106]. The trend towards using natural products has sparked the emergence of novel bioactive metabolites that may be targeted for specialized medicinal applications [23]. The majority of pharmaceuticals used now in therapy are derived from natural substances [107]. Drugs made from natural sources continue to occupy a major role, despite advancements and the creation of synthetic pharmaceutical chemistry [103,104,107]. Due to their wide chemical variety, there is a higher likelihood of discovering novel compounds with distinctive structures and possible biological activities [103,107].
Many researchers are interested in natural substances like terpenoids [107]. Terpenoids, which make up the majority of the secondary metabolites generated by plants, are frequently thought of as medicines [23]. Terpenoids have excellent anticancer properties, according to a large number of studies [23,24,100,101]. Their anti-tumor actions, which are among their many biological characteristics and include anti-proliferative, apoptotic, anti-angiogenic, and anti-metastatic activities, are particularly interesting [103,107].
Apoptosis is the main method by which terpenoids cause cell death [103]. Terpenoids, specifically autophagy, have been linked to different types of cell death [103]. Numerous in vitro and in vivo investigations have been conducted to comprehend the 'terpenoid-induced autophagy phenomena in cancer cells [103]. The complex balancing act between activating or silencing certain proteins, with the result being expressed through connected signaling pathways, is what causes the latter crosstalk. Targeting autophagic signaling pathways may offer an innovative therapeutic option for the treatment of cancer since mounting data suggest that autophagy plays a significant role in the development of cancer. It's interesting to note that terpenoids have been shown to activate the molecular processes that cause cancer cells to undergo autophagic cell death. The function of autophagy remained unclear, though. Furthermore, some terpenoids also have an anticancer impact by preventing or accelerating several stages of cancer development. For instance, they can stop carcinogenesis in its early stages by causing cell cycle arrest, preventing cancer cell differentiation, and activating apoptosis [102].
Authors have looked into a few instances, like study [107] which highlighted the potential of salvicine, sesquiterpene lactones, and diterpenoids as alternative cancer treatment options because of their preferential selectivity over particular tumors and cell lines in addition to acting on particular signaling pathways. Parthenolide also exhibits anticancer properties against a variety of tumor types, including colorectal, melanoma, pancreatic, breast, prostate, cervical, renal, and thyroid cancers [103]. Additionally, study [101] demonstrates that the terpenoids isolated from Curcumae Rhizoma, such as (β -elemene, Furanodiene, Furanodienone, Germacrone, Curcumol, Curdione, etc.), are promising anticancer agents based on data from various cancer cell lines, animal models, and clinical trials, as well as their mechanisms of inhibiting cell proliferation, autocrine growth factor production, and DNA synthesis.
The use of terpenoids does have some restrictions, though, as other authors have noted. Study [102] highlights the disconnect between terpenoids' preclinical evidence and clinical outcomes, in addition to their poor absorption and low bioavailability. Terpenoids were reportedly explored in a relatively small number of experimental settings in preclinical models. The difficulties presented by the diverse genetic composition of the human population and the complexity and heterogeneity of cancer arise as a result when terpenoids are finally tested in cancer patients. Given the efficacy of terpenoids, they concluded that, before undertaking extensive clinical studies, future research should concentrate on thorough preclinical toxicity, bioavailability, pharmacodynamics, biomarkers, and wide investigations of tumor suppression using appropriate animal models. Study [101] also emphasized the scant research done to compare the anticancer properties of various terpenoids.
Given the benefits of these phytoconstituents, terpenoids may be used with other chemotherapeutic medications and radiation therapy to improve their therapeutic efficacy as well as provide new options for chemopreventive strategies [104].
Other Biological Activity
In addition to the previously mentioned anti-inflammatory, antibacterial, antiviral, hypoglycemic, antioxidant, and anti-aging properties, terpenoids also have other biological and pharmacological activities.
Some studies have mentioned its involvement in processes like plant growth (used as signal molecules to mediate plant defense in response to herbivorous insects and patho-genic bacteria’s invasion) [3,11,12,108]; development, and defense, immunoregulation, activity in the olfactory system (sensed in the olfactory epithelium could be the lateral/ventral areas, which project their axons from sensory neurons to the lateral/ventral domain in the olfactory bulb) [34,51]; gastroprotective activity (modulate gastric acid secretion, enhance mucosal defense mechanisms, and inhibit the growth of Helicobacter pylori bacteria) [34,38]; food additive (approved to be used as flavorings and food preservatives) [12,38,51]; antiparasitic activity (terpenoids have shown promise in combating parasitic infections, including malaria, by interfering with parasite growth, replication, and survival [3,12]; and cardioprotective activity (have significant therapeutic effects on various cardiovascular diseases, such as regulating vascular function, inhibiting cardiomyocyte hypertrophy, and inhibiting thrombosis) [3,10,109].
Industry Applications
The preference for natural products has sparked the identification of new bioactive metabolites that may be targeted for a variety of applications [23].
Terpenoids, which are the most prevalent plant secondary metabolites and have a variety of structural characteristics as previously mentioned, comprise the largest family of natural products and have widespread applications in various fields [16,83,110].
Indeed, terpenoids have been crucial to numerous aspects of human life [111], and their application can be grouped into five main industries [112]: pharmaceutical, cosmetic, food, agriculture, and other industries. An overview of the use of terpenoids in different industries is presented in Figure 3.
Figure 3: Overview of the use of terpenoids in the different industries.
Terpenoids have a substantial effect on human health due to their utility in the pharmaceutical industry [14,16,20,113-116] and their abundance in bioactive components [112]. Numerous studies have emphasized the vast range of biological and pharmacological activities that demonstrate the activity shown by terpenoids has a significant role in the creation and development of new medications and improvements in available treatment choices [15,17,24,25,39,52,92,111,112,117-120].
The fragrance sector is one of the major markets for these kinds of chemicals [14]. Terpenoids are often used in the creation of cosmetics [14,16,19,39,112,118,121], especially perfumes [14,24,39,115,118], as they have wide market potential and provide economic advantages [19].
The food industry is one of these compounds' other important markets [14,25,39,81,86,114-116,118]. Terpenoids in herbs and spices are frequently employed to preserve food because of their microbicidal and insecticidal properties [14,39,111,112,122]. Becoming an alternative eco-friendly food preservative [39]. They are also responsible for the flavor found in food, beverages (tea), alcoholic drinks, and wine [19,52,111,112].
Plus, some terpenoids play an important role in the agriculture industry [14,19,118]. They are used as pesticides [14,19,25], as insecticides [112,117], and as repellents [112] and they also contribute to the producers' defense against or attraction to beneficial organisms [14,20]. Additionally, they serve several physiological and ecological roles in plant life through direct and indirect plant defenses, by luring pollinators, and through various interactions between the plants and their surroundings (such as acting as vectors for the transfer of pollen, for example) [19,25,27,116].
Moreover, some researchers have found that terpenoids have applications in synthetic and bulk chemicals (39,115), rubber products [39], and some industrial raw materials [19,39], as well as being precursor compounds to biofuel [14,16,19,26,39,114,115,121].
Terpenoids have a variety of functions in numerous industries, as was already indicated, and have grown to have significant economic value [19]. However, many continue to emphasize the need for greater research on this chemical so that industrialization and production of it in the future can be safer and more advantageous [19,39,116,121].
Disadvantages
Despite the terpenoid’s diverse uses and great demand [115], their promising ability to prevent and treat various diseases [92], and their wide range of applications [115], a few studies have revealed certain disadvantages to their use.
The difficulties mentioned included their low water solubility [21,92,123,124], difficult stability [92,125], difficult extraction [22,102,115,126], low bioavailability [21,27,92,102,124], high production costs [115], and organoleptic effects [39], as well as other side effects that could restrict their use in clinics [92,124], including irritant index [92], poor absorption [21,102], unfavorable effects on reproductive functions [13], and gastrointestinal upsets [13].
Specifically, research [115] talks about how difficult it is to chemically synthesize this family of compounds. Additionally, it draws attention to the fact that making these compounds requires several complex synthetic procedures, which raises the cost of manufacture. It further clarifies that the extraction of terpenoids chemicals from natural sources is time-consuming, yields are low, and important resources are used up excessively.
In addition, study [21] points out that terpenoids have high polarity and poor bioavailability due to their structure, which limits their biofilm permeability and absorption [57]. Therefore, they concluded that chemical or pharmaceutical methods must improve the dissolution and absorption ability.
The efficiency of terpenoids [102] has led many to point out the need for more research into this substance to comprehend the biological features' underlying mechanisms in a way to overcome these issues [23,39,102,110,115,124,127].
Conclusions
The preference for natural products has sparked the discovery of novel metabolites that may be targeted for certain therapeutic purposes. The majority of secondary metabolites produced are terpenoids, which have been found to have several important health benefits, including anti-inflammatory properties, antitumor and anticancer effects, antibacterial and antiviral properties, antimalarial properties, the ability to prevent and treat cardiovascular diseases, the promotion of transdermal absorption, and anti-aging properties.
Terpenoids play a significant part in various industries thanks to their activity, making them important compounds with a wide range of applications. They are also frequently used and have excellent development prospects. emphasizing in particular their anti-aging qualities. Due to age-related medical issues like cancer, neurological illnesses, and skin aging, the considerable increase in the world's aging population is placing economic and social responsibilities.
Terpenoids may have geroprotective qualities, and the discovery and application of effective geroprotective strategies can lead to an increase in health span and the prevention or amelioration of age-related diseases. This substance has a strong potential to launch a new class of anti-aging medications. To aid in the creation of viable interventions to lessen the detrimental effects on health, further research on the biological process of aging is required.
Their low water solubility, difficult stability, difficult extraction, low bioavailability, high production costs, and organoleptic effects are some of these compounds’ drawbacks. They also have additional side effects that may limit their use in clinics, such as irritant index, poor absorption, unfavorable effects on reproductive functions, and gastrointestinal upsets. Researchers also have certain concerns about this compound's biosynthesis, extraction, and production processes. Future research should focus on terpenoids' broad toxicity, their catalytic mechanism, bioavailability, pharmacodynamics, biomarkers, extensive examinations of their bioactive qualities, and their usage in various industries in light of their effectiveness.
Future breakthroughs in cutting-edge high-throughput approaches will be necessary to find novel and efficient natural candidates that prolong lifespan and delay aging and related disorders, such as terpenoids.
Author Statements
Author Contributions
Ana Borges and Filipa Mandim: Conceptualization, writing—original draft preparation; Sandrina A. Heleno and Ricardo C. Calhelha: Conceptualization, formal analysis, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2020). National funding by FCT, P.I., through the scientific employment program contract for the contract of R. Calhelha (CEEC Institutional).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Rasul MG. Conventional Extraction Methods Use in Medicinal Plants, their Advantages and Disadvantages. International Journal of Basic Sciences and Applied Computing. 2018; 2.
- Prakash V. Terpenoids as cytotoxic compounds: A perspective. Pharmacognosy Reviews. Wolters Kluwer Medknow Publications. 2018; 12: 166-76.
- Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X. Advances in Pharmacological Activities of Terpenoids. Vol. 15. Natural Product Communications. SAGE Publications Inc. 2020.
- Abdallah Q II, W.j. A Glimpse into the Biosynthesis of Terpenoids. KnE Life Sciences. 2017; 11: 81-98.
- El-Baba C, Baassiri A, Kiriako G, Dia B, Fadlallah S, Moodad S. Terpenoids’ anti-cancer effects: focus on autophagy. Apoptosis. Springer. 2021; 26: 491-511.
- Nagegowda DA, Gupta P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Science. Elsevier Ireland Ltd; 2020; 294: 110457.
- Setyorini D, Antarlina SS. Secondary metabolites in sorghum and its characteristics. Sociedade Brasileira de Cienciaa e Tecnologia de Alimentos. 2022; 42.
- Ludwiczuk A, Skalicka-Wozniak K, Georgiev MI. Terpenoids. Pharmacognosy: Fundamentals, Applications and Strategy Elsevier Inc. 2017; 233–66.
- Zeng T, Liu Z, Liu H, He W, Tang X, Xie L. Exploring Chemical and Biological Space of Terpenoids. J Chem Inf Model. 2019; 59: 3667-3678.
- Liu X, Wang S, Cui L, Zhou H, Liu Y, Meng L. Flowers: precious food and medicine resources. (Food Science and Human Wellness. KeAi Communications Co. 2023; 12: 1020-52.
- Boncan DAT, Tsang SSK, Li C, Lee IHT, Lam HM, Chan TF. Terpenes and terpenoids in plants: Interactions with environment and insects. International Journal of Molecular Sciences MDPI AG. 2020; 21: 1–19.
- Huang Y, Xie FJ, Cao X, Li MY. Research progress in biosynthesis and regulation of plant terpenoids. Vol. 35. Biotechnology and Biotechnological Equipment. Taylor and Francis Ltd. 2021.
- Proshkina E, Plyusnin S, Babak T, Lashmanova E, Maganova F, Koval L, et al. Terpenoids as potential geropro-tectors. 2020; 9: 1–51.
- Avalos M, Garbeva P, Vader L, Wezel GP, Dickschat JS, Ulanova D. Biosynthesis, evolution and ecology of microbial terpenoids. Natural Product Reports. Royal Society of Chemistry. 2022; 39.
- Boncan DAT, Tsang SSK, Li C, Lee IHT, Lam HM, Chan TF. Terpenes and terpenoids in plants: Interactions with environment and insects. International Journal of Molecular Sciences MDPI AG. 2020; 21: 1–19.
- Belcher MS, Mahinthakumar J, Keasling JD. Title: New frontiers: Harnessing pivotal advances in microbial engi-neering for the biosynthesis of plant-derived terpenoids. 2020.
- Koyama S, Heinbockel T. The effects of essential oils and terpenes in relation to their routes of intake and appli-cation. International Journal of Molecular Sciences MDPI AG. 2020; 21: 1558.
- Amirzakariya BZ, Shakeri A. Bioactive terpenoids derived from plant endophytic fungi: An updated review. Phytochemistry. Elsevier Ltd. 2011; 197: 113130.
- Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X. Advances in Pharmacological Activities of Terpenoids. Vol. 15. Natural Product Communications. SAGE Publications Inc. 2020.
- Zeng T, Liu Z, Liu H, He W, Tang X, Xie L. Exploring Chemical and Biological Space of Terpenoids. J Chem Inf Model. 2019; 59: 3667-3678.
- Liu Y, Yang L, Wang H, Xiong Y. Recent Advances in Antiviral Activities of Triterpenoids. MDPI; 2022. 15: 1169.
- Abdallah Q II, Wj. A Glimpse into the Biosynthesis of Terpenoids. KnE Life Sciences. 2017; 3: 81-98.
- Prakash V. Terpenoids as cytotoxic compounds: A perspective. Pharmacognosy Reviews. Wolters Kluwer Medknow Publications. 2018; 12: 166.
- Mustapa MA, Guswenrivo I, Zuhrotun A, Ikram NKK, Muchtaridi M. Anti-Breast Cancer Activity of Essential Oil: A Systematic Review. MDPI. 2022; 12.
- Kiyama R. Estrogenic terpenes and terpenoids: Pathways, functions and applications. European Jour-nal of Pharmacology Elsevier BV. 2017; 815: 405–15.
- Nagegowda DA, Gupta P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Science. Elsevier Ireland Ltd. 2020; 294: 110457.
- Huang Y, Xie FJ, Cao X, Li MY. Research progress in biosynthesis and regulation of plant terpenoids. Biotechnology and Biotechnological Equipment. Taylor and Francis Ltd. 2021; 35: 1799-1808.
- Ghoran SH, Taktaz F, Mozafari AA, Tunçtürk M, Sekeroglu N, Kijjoa A. Uncommon Terpenoids from Salvia Spe-cies: Chemistry, Biosynthesis and Biological Activities. Molecules: MDPI. 2022; 27: 1128.
- Belcher MS, Mahinthakumar J, Keasling JD. Title: New frontiers: Harnessing pivotal advances in microbial engineering for the biosynthesis of plant-derived terpenoids. 2020; 65: 88-93.
- Kiyama R. Estrogenic terpenes and terpenoids: Pathways, functions and applications. European Jour-nal of Pharmacology Elsevier BV. 2017; 815: 405–15.
- Liu Y, Yang L, Wang H, Xiong Y. Recent Advances in Antiviral Activities of Triterpenoids. MDPI. 2022; 15: 1169.
- Abbas F, Ke Y, Yu R, Yue Y, Amanullah S, Jahangir MM. Volatile terpenoids: multiple functions, biosynthe-sis, modulation and manipulation by genetic engineering. Planta. Springer Verlag. 2017; 246: 803-816.
- Gnat S, Lagowski D, Nowakiewicz A. Major challenges and perspectives in the diagnostics and treatment of dermatophyte infections. Journal of Applied Microbiology. 2020; 129: 212–32.
- Koyama S, Heinbockel T. The effects of essential oils and terpenes in relation to their routes of intake and application. International Journal of Molecular Sciences MDPI AG. 2020; 21: 1558.
- Khongthaw B, Chauhan PK, Dulta K, Kumar V, Ighalo JO. A comparison of conventional and novel phytonutrient extraction techniques from various sources and their potential applications. Journal of Food Measurement and Characterization Springer. 2023; 17: 1317–42.
- Silva IMM, Silva RM, Paula VB, Estevinho LM. Biological activities of endophytic fungi isolated from Annona muricata Linnaeus: a systematic review. Brazilian Journal of Biology. 2024; 84: e259525.
- Conte R, Marturano V, Peluso G, Calarco A, Cerruti P. Recent advances in nanoparticle-mediated delivery of anti-inflammatory phytocompounds. International Journal of Molecular Sciences MDPI AG. 2017; 18: 709.
- Masyita A, Mustika Sari R, Dwi Astuti A, Yasir B, Rahma Rumata N, Bin ET. Terpenes and terpe-noids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem X. 2022; 13: 100217.
- Masyita A, Mustika Sari R, Dwi Astuti A, Yasir B, Rahma Rumata N, Bin ET. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem X. 2022; 13: 100217.
- Yuan HL, Zhao YL, Ding CF, Zhu PF, Jin Q, Liu YP. Anti-inflammatory and antinociceptive effects of Cur-cuma kwangsiensis and its bioactive terpenoids in vivo and in vitro. J Ethnopharmacol. 2020; 259: 112935.
- Zhang M, Chen M, Hou Y, Fan C, Wei H, Shi L. Inflammatory and cytotoxic activities of abietane terpenoids from nepeta bracteata benth. Molecules. 2021; 26: 5603.
- Wu JT, Algradi AM, Liu Y, Huo JH, Li XM, Yang BY. Two new terpenoids with anti-inflammatory activity from the fruits of Arenga pinnata (Wurmb) Merr. Nat Prod Res. 2022; 36: 5753–61.
- Xu Y, Xie H, Chen H, Xiong L, Sa K, Wang X. Terpenoids and Phenylpropanoids Isolated from the Twigs and Leaves of Abelia macrotera and Their Anti-Inflammatory Activities. Chem Biodivers. 2022; 19: e202200870.
- Li F, Zhang DB, Li JT, He FJ, Zhu HL, Li N. Bioactive terpenoids from Croton laui. Nat Prod Res. 2021; 35: 2849–57.
- Deng JL, Huang R, Zhang QG, Sha BT, Wang MC, Deng SY. Further sesquiterpenoids from Pittosporum qinlingense and their anti-inflammatory activity. Fitoterapia. 2022; 162: 105292.
- Liu T, Chen X, Hu Y, Li M, Wu Y, Dai M. Sesquiterpenoids and triterpenoids with anti-inflammatory effects from Artemisia vulgaris L. Phytochemistry. 2022; 204: 113428.
- Jambwa P, Nkadimeng SM, Mudimba TN, Matope G, McGaw LJ. Antibacterial and anti-inflammatory activity of plant species used in traditional poultry ethnomedicine in Zimbabwe: A first step to developing alternatives to antibiotic poultry feed additives. J Ethnopharmacol. 2023; 300: 115687.
- Rahman MM, Al Noman MA, Khatun S, Alam R, Shetu MMH, Talukder EK. Evaluation of Senna tora (L.) Roxb. leaves as source of bioactive molecules with antioxidant, anti-inflammatory and antibacterial potential. Heliyon. 2023; 9: e12855;
- Guo ZH, Huang JY, Xiao T, De YW. Terpenoids as anti-inflammatory substances inhibiting COX-2 isolated from the fibrous roots of Alangium chinense (Lour.) Harms. Nat Prod Res. 2023; 37: 2824–9.
- Venkata ALK, Sivaram S, Sajeet M, S PM, Srilakshman G, Muthuraman MS. Review on terpenoid mediated na-noparticles: significance, mechanism, and biomedical applications. Na-noscience and Nanotechnology. Institute of Physics. 2022; 3: id.033003.
- Wang CY, Chen YW, Hou CY. Antioxidant and antibacterial activity of seven predominant terpenoids. Int J Food Prop. 2019; 22: 230–8.
- Wang CY, Chen YW, Hou CY. Antioxidant and antibacterial activity of seven predominant terpenoids. Int J Food Prop. 2019; 22: 230–8.
- Yu SJ, Yu JH, Yu ZP, Yan X, Zhang JS, Yue SJ. Bioactive terpenoid constituents from Eclipta prostrata. Phytochemistry. 2020; 170: 112192.
- Sayout A, Ouarhach A, Rabie R, Dilagui I, Soraa N, Romane A. Evaluation of Antibacterial Activity of Lavandu-lapedunculata subsp. atlantica (Braun-Blanq.) Romo Essential Oil and Selected Terpenoids against Resistant Bacteria Strains–Structure–Activity Relationships. Chem Biodivers. 2020; 17: e1900496.
- Xin Z, Yang W, Duan Y, Wang W, Niu L, Sun D. Bioactive components and antibacterial activities of hy-drolate extracts by optimization conditions from Paeonia ostii T. Hong & J X Zhang Ind Crops Prod. 2022; 188: 115737.
- Arjmand Z, Hamburger M, Dastan D. Isolation and purification of terpenoid compounds from Ferula hauss-knechtii and evaluation of their antibacterial effects. Nat Prod Res. 2023; 37: 1617–24.
- Liu J, Xia F, Ouyang H, Wang W, Li T, Shi Y. Nardosinane-related antimicrobial terpenoids from Lemnalia sp. soft coral Phytochemistry. 2022; 196: 113088.
- Yamaguchi T. Antibacterial effect of the combination of terpenoids. Arch Microbiol. 2022; 204: 520.
- Nyalo PO, Omwenga GI, Ngugi MP. Antibacterial properties and GC-MS analysis of ethyl acetate extracts of Xerophyta spekei (Baker) and Grewia tembensis (Fresen. Heliyon. 2023; 9: e14461.
- Zubair MS, Khairunisa SQ, Widodo A, Nasronudin P, R. Antiviral screening on Alpinia eremochlamys, Etlingera flexuosa, and Etlingera acanthoides extracts against HIV-infected MT-4 cells. Heliyon. 2021; 7: e06710.
- Ac NS, Sm M, Mm M, Nv S, Dm L. Antiviral activity on the Zika virus and larvicidal activity on the Aedes spp. of Lippia alba essential oil and β-caryophyllene. Ind Crops Prod. 2021; 162.
- Ribeiro MCM, Salles TS, Moreira MF, Barbarino E, Valle AF, Couto MAPG. Antiviral activity of microalgae extracts against Mayaro virus. Algal Res. 2022; 61: 102577.
- V. TE, X M, Ob K, Aa S, Gd P, Am K. Synthesis and evaluation of diterpenic Mannich bases as antiviral agents against influenza A and SARS-CoV-2. Phytochem Lett. 2022; 51: 91-96.
- José-Rita BJ, Bertin GK, Ibrahime SK, Yannick K, Erick-Kévin BG, Riphin KL. Study of the chemical and in vitro cytotoxic activities of essential oils (EOs) of two plants from the Ivorian flora (Lippia multiflora and Zingi-ber officinale) and their antiviral activities against non-enveloped viruses. South African Journal of Botany. 2022; 151: 387–93.
- Singh S, Bansal A, Singh V, Chopra T, Poddar J. Flavonoids, alkaloids and terpenoids: a new hope for the treat-ment of diabetes mellitus. Springer Science and Business Media Deutschland GmbH. Journal of Diabetes and Metabolic Disorders. 2022; 21: 941-950.
- Valdés M, Calzada F, Mendieta-Wejebe JE, Merlín-Lucas V, Velázquez C, Barbosa E. Antihyperglycemic effects of Annona diversifolia safford and its acyclic terpenoids: a-glucosidase and selective SGLT1 inhibitiors. Molecules. 2020; 25: 3361.
- Adeyi AO, Awosanya SA, Adeyi OE, James AS, Adenipekun CO. Ganoderma lucidum ethanol extract abrogates metabolic syndrome in rats: In vivo evaluation of hypoglycemic, hypolipidemic, hypotensive and antioxidant properties. Obes Med. 2021; 22: 100320.
- Kifle ZD, Woldeyohanin AE, Sema FD, Debeb SG, Kasahun AE, Demeke CA. In vivo hypoglycemic, antihy-perglycemic and antidyslipidemic effects of the solvent fractions of Hagenia abyssinica leaves in mice. Metabol Open. 2021; 12: 100139.
- Song BR, Alam MB, Lee SH. Terpenoid-Rich Extract of Dillenia indica L. Bark Displays Antidiabetic Action in Insulin-Resistant C2C12 Cells and STZ-Induced Diabetic Mice by Attenuation of Oxidative Stress. Antioxidants. 2022; 11: 1227.
- Ortega R, Valdés M, Alarcón-Aguilar FJ, Fortis-Barrera á, Barbosa E, Velazquez C. Antihyperglycemic Ef-fects of Salvia polystachya Cav. and Its Terpenoids: a-Glucosidase and SGLT1 Inhibitors. Plants. 2022; 11: 575.
- Zhao X, Guo Y, Xu Q, Shi Z, Xiang M, Li H. ±)-Hyperpyran A: Terpenoid-based bicyclic dihydropyran en-antiomers with hypoglycemic activity from Hypericum perforatum (St. John’s wort) Fitoterapia. 2022; 161: 105221.
- Ramadhani F, Girsang E, Florenly F. The bioactive of Pinus merkusii needle and bark extract as antioxidantand anti-aging. JKPK (Jurnal Kimia dan Pendidikan Kimia. 2021; 6: 78.
- Xu DP, Li Y, Meng X, Zhou T, Zhou Y, Zheng J. Natural antioxidants in foods and medicinal plants: Extrac-tion, assessment and resources. International Journal of Molecular Sciences MDPI AG. 2017; 18: 96.
- Sztretye M, Dienes B, Gönczi M, Czirják T, Csernoch L, Dux L. Astaxanthin: A Potential Mitochondri-al-Targeted Antioxidant Treatment in Diseases and with Aging. 2019; 2019: 3849692.
- Gutiérrez-Del-río I, López-Ibáñez S, Magadán-Corpas P, Fernández-Calleja L, Pérez-Valero á, Tuñón-Granda M. Terpenoids and polyphenols as a natural antioxidant agents in food preservation. 2021; Antioxidants. 2021; 10: 1264.
- Setyorini D, Antarlina SS. Secondary metabolites in sorghum and its characteristics. Sociedade Brasileira de Ciencia e Tecnologia de Alimentos. 2022; 42: e49822.
- Zhang J, Shi LY, Ding LP, Liang H, Tu PF, Zhang QY. Antioxidant terpenoids from the red alga Laurencia tristi-cha. Nat Prod Res. 2021; 35: 5048–54.
- Ramadhani F, Girsang E, Florenly F. The bioactive of Pinus merkusii needle and bark extract as antioxidantand anti-aging. JKPK (Jurnal Kimia dan Pendidikan Kimia. 2021; 6: 78.
- Cuong NT, Ban PH, Van CM. Chemical composition and antioxidant activity of the essential oil of Alseodaphne velutina Chev. from Viet Nam Nat Prod Res. 2022; 36: 617-620.
- Muruthi CW, Ngugi MP, Runo SM, Mwitari PG. In vitro antioxidant activities of Carissa edulis ((Forssk) Vahl) and Pappea capensis (Eckyl. & Zeyh) extracts Heliyon. 2023; 9: e12965.
- Bjørklund G, Shanaida M, Lysiuk R, Butnariu M, Peana M, Sarac I. Natural Compounds and Products from an Anti-Aging Perspective. Molecules. 2022; 27: 7084.
- Cai Z, Zhang J, Li H. Selenium, aging and aging-related diseases. Vol. 31. Springer International Publishing. 2019; 31: 1035-1047.
- Okoro NO, Odiba AS, Osadebe PO, Omeje EO, Liao G, Fang W. Bioactive phytochemicals with anti-aging and lifespan extending potentials in caenorhabditis elegans. Molecules. 2021; 26: 7323.
- Kunugi H, Ali AM. Royal jelly and its components promote healthy aging and longevity: From animal models to humans. International Journal of Molecular Sciences MDPI AG. 2019; 20: 4662.
- Barbalho SM, Direito R, Laurindo LF, Marton LT, Guiguer EL, Guiguer EL, et al. Ginkgo biloba in the Aging Process: A. Narrative Review. 2022; 11: 525.
- Liu JK. Antiaging agents: safe interventions to slow aging and healthy life span extension. Vol. 12. Natural Products and Bioprospecting. Springer. 2022; 12.
- Lister INE, Amiruddin HL, Fachrial E, Girsang E. Anti-Aging Effectiveness of Avocado Peel Extract Ointment (Persea americana Mill.) against Hydration, Collagen, and Elasticity Levels in Wistar Rat. J Pharm Res Int. 2021; 33.
- Girsang E, Lister INE, Ginting CN, Khu A, Samin B, Widowati W. Chemical Constituents of Snake Fruit (Salacca zalacca (Gaert.) Voss) Peel and in silico Anti-aging Analysis. Molecular and Cellular Biomedical Sci-ences. 2019; 3: 122.
- Ahmed IA, Mikail MA, Zamakshshari N, Abdullah ASH. Natural anti-aging skincare: role and potential. Biogerontology: Springer. 2020; 21: 293-310.
- Yasmeen S, Gupta P. Interaction of Selected Terpenoids From Dalbergia sissoo With Catalytic Domain of Matrix Metalloproteinase-1: An In Silico Assessment of Their Anti-wrinkling Potential. Bioinform Biol Insights. 2019; 13: 1177932219896538.
- Sujarit K, Suwannarach N, Kumla J, Lomthong T, Mai Sci CJ. Mushrooms: Splendid Gifts for the Cosmetic Industry. 2021; 48.
- Lason E. Topical administration of terpenes encapsulated in nanostructured lipid-based systems. Molecules. 2020; 25: 5758.
- Shal B, Ding W, Ali H, Kim YS, Khan S. Anti-neuroinflammatory potential of natural products in attenuation of Alzheimer’s disease. Front Pharmacol. 2018; 9: 548.
- Maccioni RB, Calfío C, González A, Lüttges V. Novel Nutraceutical Compounds in Alzheimer Prevention. Biomolecules. 2022; 12: 248.
- Wojtunik-Kulesza KA, Targowska-Duda K, Klimek K, Ginalska G, Jówiak K, Waksmundzka-Hajnos M. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017; 15: 332–43.
- Khan M, Yadav S, Banerjee S. Review Article on Effects of Moringa on Central Nervous System. Moringa on CNS 316 Journal of Young Pharmacists. 2021; 13: 315-319.
- Omoboyowa DA, Balogun TA, Omomule OM, Saibu OA. Identification of Terpenoids From Abrus precatorius Against Parkinson’s Disease Proteins Using In Silico Approach. Bioinform Biol Insights. 2021; 15: 11779322211050757.
- Dembitsky VM, Dzhemileva L, Gloriozova T, D’yakonov V. Natural and synthetic drugs used for the treatment of the dementia. Biochemical and Biophysical Research Communications Elsevier B V. 2020; 524: 772–83.
- Agatonovic-Kustrin S, Chan CKY, Gegechkori V, Morton DW. Models for skin and brain penetration of major components from essential oils used in aromatherapy for dementia patients. J Biomol Struct Dyn. 2020; 38: 2402-2411.
- Lee H, Selvaraj B, Lee JW. Anticancer effects of seaweed-derived bioactive compounds. App Sci. 2021; 11: 11261.
- Chen Y, Zhu Z, Chen J, Zheng Y, Limsila B, Lu M. Terpenoids from Curcumae Rhizoma: Their anticancer effects and clinical uses on combination and versus drug therapies. Biomedicine and Pharmacotherapy. 2021; 138: 111350.
- Kamran S, Sinniah A, Abdulghani MAM, Alshawsh MA. Therapeutic Potential of Certain Terpenoids as Anti-cancer Agents: A Scoping Review. Cancers. 2022; 14: 1100.
- El-Baba C, Baassiri A, Kiriako G, Dia B, Fadlallah S, Moodad S. Terpenoids’ anti-cancer effects: focus on autophagy. Apoptosis. 2021; 26: 491-511.
- Gnanaselvan S, Yadav SA, Manoharan SP, Pandiyan B. Uncovering the Anticancer Potential of Phytomedicine and Polyherbal’s Synergism against Cancer. Biointerface Research in Applied Chemistry. 2023; 13: 356.
- Sheikh I, Sharma V, Tuli HS, Aggarwal D, Sankhyan A, Vyas P. Cancer chemoprevention by flavonoids, dietary polyphenols and terpenoids. In: Biointerface Research in Applied Chemistry. AMG Transcend As-sociation. 2021; 2021: 8502–37.
- J ST, P S, G SREEV, Jt S, M BSMF, V SG. Cancer-Fighting Phy-tochemicals: Another Look. J Nanomedine Biotherapeutic Discov. 2019; 9.
- Sülsen VP, Athanassopoulos CM, Padrón JM, Tamura RE. Editorial: Natural compounds as scaffolds for the discovery of new anti-cancer drugs: Focus on terpenoids and flavonoids. (Frontiers in Pharmacology. Frontiers Media. 2022; 13: 984849.
- Proshkina E, Plyusnin S, Babak T, Lashmanova E, Maganova F, Koval L, et al. Terpenoids as potential geropro-tectors. Antioxidants. 2020; 9: 529.
- Zullkiflee N, Taha H, Usman A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules. 2022; 27: 6120.
- Bian G, Deng Z, Liu T. Strategies for terpenoid overproduction and new terpenoid discovery. In: Current Opinion in Biotechnology. Elsevier Ltd. 2017; 48: 234–41.
- Pichersky E, Raguso RA. Why do plants produce so many terpenoid compounds?. New Phytologist. Blackwell Publishing Ltd. 2018; 220: 692-702.
- Liu X, Wang S, Cui L, Zhou H, Liu Y, Meng L. Flowers: precious food and medicine resources. Food Science and Human Wellness. 2023; 12: 1020–52.
- Nuutinen T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Euro-pean Journal of Medicinal Chemistry Elsevier Masson SAS. 2018; 157: 198–228.
- Li ZJ, Wang YZ, Wang LR, Shi TQ, Sun XM, Huang H. Advanced Strategies for the Synthesis of Terpenoids in Yarrowia lipolytica. Journal of Agricultural and Food Chemistry American Chemical Society. 2021; 69: 2367–81.
- Sachito DC, Oliveira LG. Unveiling the Bacterial Sesquiterpenome of Streptomyces sp. CBMAI 2042 Discloses Cyclases with Versatile Performances. J Braz Chem Soc. 2022; 33: 734-742.
- Abbas F, Ke Y, Yu R, Yue Y, Amanullah S, Jahangir MM. Volatile terpenoids: multiple functions, biosynthe-sis, modulation and manipulation by genetic engineering. Planta. Springer Verlag; 2017; 246: 803-816.
- Li J, Li C, Peng X, Li S, Liu B, Chu C. Recent discovery of tyrosinase inhibitors in traditional Chinese medicines and screening methods. Journal of Ethnopharmacology. Elsevier Ireland Ltd. 2023; 303: 115951.
- Rajput JD, Bagul SD, Pete UD, Zade CM, Padhye SB, Bendre RS. Perspectives on medicinal properties of natural phenolic monoterpenoids and their hybrids. Springer International Publishing. 2018; 22: 225-245.
- Bag BG, Barai AC, Hasan SN, Panja SK, Ghorai S, Patra S. Terpenoids, nano-entities and molecular self-assembly. In: Pure and Applied Chemistry. De Gruyter. 2019; 92.
- Nevzorova YA, Grossmann J, Trautwein C. Anti-tumorigenic and anti-angiogenic effects of natural conifer Abies sibirica terpenoids in vivo and in vitro. Biomedicine and Pharmacotherapy. 2017; 89: 386-395.
- Chen L, Pang Y, Luo Y, Cheng X, Lv B, Li C. Separation and purification of plant terpenoids from biotransfor-mation. Engineering in Life Sciences. 2021; 21: 724-738.
- Zullkiflee N, Taha H, Usman A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules. 2022; 27: 6120.
- Conte R, Marturano V, Peluso G, Calarco A, Cerruti P. Recent advances in nanoparticle-mediated delivery of anti-inflammatory phytocompounds. International Journal of Molecular Sciences. 2017; 18: 709.
- González-Cofrade L, Las Heras B, Apaza Ticona L, Palomino OM. Molecular Targets Involved in the Neuro-protection Mediated by Terpenoids. Planta Medica. 2019; 85: 1304-1315.
- Hanuš LO, Hod Y, Cannabis M, Ag C-NSK. Terpenes/Terpenoids in Cannabis: Are They Important?. 2020; 3: 61–73.
- Rastegari AA, Yadav AN, Yadav N, Sarshari NT. Bioengineering of secondary metabolites. In: New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Secondary Metabolites Biochemistry and Applications. Elsevier. 2019; 55–68.
- Zhang C, Hong K. Production of Terpenoids by Synthetic Biology Approaches. Front Bioeng Bitechnol. 2020; 8: 347.