
Review Article
Austin J Anal Pharm Chem. 2024; 11(2): 1172.
Spectrophotometric and HPLC – MS / MS for Determination of Silodosin in Pharmaceutical Formulation
Dilip Maheshwari¹; Chandramauly Sharma²*; Tushar Tyagi³; Yadvendra Agrawal4
1Department of Quality Assurance, LJ Institute of Pharmacy, LJ University, Ahmedabad – 388210, India
2Department of Chemistry, LJ School of Applied Sciences, LJ University, Ahmedabad – 388210, India
3National Forensic Science University, Gandhinagar, India
4Centre of Excellence in Macromolecules and Nanotechnology, L J University, Ahmedabad – 388210, India
*Corresponding author: Chandramauly Sharma, Department of Chemistry, LJ School of Applied Sciences, LJ University, Ahmedabad – 388210, India. Email: drcrsharma10@gmail.comt
Received: October 09, 2024; Accepted: October 29, 2024 Published: November 05, 2024
Abstract
A simple, selective, and sensitive spectrophotometric and HPLC – MS / MS method for determining microgram amounts of silodosin is described. Silodosin is converted into (R)-1-(3-hydroxypropyl)-5-(2-((2-(2-(2,2,2-trifluoroethoxy) phenoxy)ethyl)amino)propyl)-2,3-dihydroindoline-7-carboxohydroxamic acid, which gives a purple violet colour (λmax = 510 nm) with ferric chloride in an acidic medium. This forms the basis for the quantitative determination of ethionamide in both its pure form and tablet form. The influence of commonly used excipients is also studied. The HPLC determination was performed on a C18 column using an acetonitrile and water mobile phase. Both methods were validated for linearity, precision, accuracy, and robustness. The HPLC method demonstrated good linearity over the spectrophotometric range of 3–18 μg/mL.
Keywords: Silodosin; HPLC – MS / MS; Spectrophotometric; Hydroxamic acid
Introduction
Silodosin is used to treat bothered by Lower Urinary Tract Symptoms (LUTS) secondary to Benign Prostatic Hyperplasia (BPH) [1]. Silodosin determines smooth muscle relaxation in bladder and prostate tissues, increases bladder blood flow in conditions of chronic bladder ischemia and regulates the activity of transcriptional factors responsible for stromal growth and prostate hyperplasia [2].
Silodosin, a potent a1A-adrenergic receptor antagonist, stands out for its unprecedented selectivity. Unlike its counterparts, it specifically targets a1A receptors, making it an attractive therapeutic option. Silodosin effectively tackles Lower Urinary Tract Symptoms (LUTS) associated with Benign Prostatic Hyperplasia (BPH). Its rapid onset of action improves voiding and storage symptoms, enhancing overall quality of life. Notably, this medication prioritizes cardiovascular tolerability, making it a promising choice for patients in need. In summary, silodosin holds the key to alleviating BPH-related urinary woes [3-11].
Silodosin is a new type of alpha-blocker that has a unique ability to selectively target a1A-adrenergic receptors, compared to a1B and a1D receptors [3,4]. It works effectively as an a1A-adrenergic receptor blocker and does not have major side effects [5]. Silodosin is a safe and effective treatment for relieving symptoms of lower urinary tract issues related to Benign Prostatic Hyperplasia (BPH) [6]. When taken orally, silodosin quickly improves symptoms in men with BPH, including voiding and storage symptoms, and increases the maximum urinary flow rate [7-9]. It works by improving bladder storage function and relieving the obstruction caused by an enlarged prostate [10]. Silodosin is particularly beneficial for patients who need a treatment that is gentle on the cardiovascular system [11].
Literature shows that various methods have been developed and validated for estimating silodosin in bulk and pharmaceutical forms. These methods include UV-Vis Spectrophotometry, Uv-Spectroscopic and RP-HPLC method, and a Spectrofluorimetric method. These techniques have been used to accurately measure silodosin in pure form, pharmaceuticals, and biological samples [12-14].
Several innovative methods have been developed for the analysis of silodosin. A new, specific, and stable method using Ultra-High- Performance Liquid Chromatography (UHPLC) was developed and validated. This method, compatible with mass spectrometry, accurately determines silodosin and its related substances. In addition, a simple, sensitive, precise, and specific method using highperformance thin layer chromatography was developed and validated. This method is particularly useful for determining silodosin in bulk and in pharmaceutical dosage forms. Lastly, a new eco-friendly method using High-Performance Liquid Chromatography (HPLC) with dual detection modes was presented. This method allows for the versatile quantification of dutasteride and silodosin in pharmaceutical formulations. These advancements significantly contribute to the field of pharmaceutical analysis. [15-17].
Various analytical methods are described in the literature for examining Silodosin. These include a selective spectrofluorimetric method with enhanced sensitivity for determining Silodosin in dosage forms and human plasma, RP-HPLC for analysing the kinetics of Silodosin degradation, and liquid chromatography/Fourier transform mass spectrometry for characterizing the degradation products of Silodosin under stress conditions. Stability-indicating spectrophotometric methods are used to determine Silodosin in the presence of its degradation product. Additionally, an LC-ESI-MS/ MS method is employed to study the forced degradation behaviour of Silodosin and evaluate the anticancer activity of Silodosin and its major degradation products [18-21].
In simpler terms, researchers have developed two methods for analysing silodosin, a drug used to treat urinary problems. The first method uses capillary gas chromatography to detect and measure the amount of two genotoxic impurities in silodosin, which are harmful substances that can damage genetic material. This method also includes an in-silico toxicity assessment, which is a computer-simulated evaluation of the potential harmful effects of these impurities. The second method, guided by a quality by design approach, uses capillary electrophoresis to determine the chiral purity of silodosin, which is a measure of the balance between its two mirror-image forms. These methods ensure the safety and effectiveness of silodosin [22-23].
Chromatography is known as the most effective method for analysis.
In the present spectrophotometric method is based on the ferric hydroxamate method for determination of amides as
R – CONH2 + NH2OH R – CONHOH + NH3
The HPLC is a more commonly used tool in quality control as a result spectrophotometric and HPLC methods are developed for silodosin.
Materials and Methods
Chemicals and Reagents
Aristo Pharmaceutical gave a free Silodosin API sample. All analysis reagents were AR grade. SDFCL (Mumbai) supplied Ortho Phosphoric acid.
Reagents Preparation for UV method
• Hydroxylamine hydrochloride (2.0 M): Dissolve 35 gm of hydroxylamine in 250 mL of distilled water.
• NaOH Solution (3.5 M): Dissolve 14 gm of NaOH in 100 mL of distilled water.
• HCl Solution (3.5 M): Dissolve 30 mL of HCl in 100 mL of distilled water.
• Drug solution: Dissolve 1 mg of the drug in 100 mL of ethanol.
• FeCl3 (0.1 M) in 0.1 M HCl: Dissolve 8 gm of FeCl3 in 100 mL of 0.1 M HCl solution.
• Alkaline solution: Mix 50 mL of Hydroxylamine hydrochloride with 50 mL of NaOH solution. The ratio is 50:50.
Appratus
A Shimadzu HPLC-LC-2010CHT with a UV detector to develop, optimize, and validate Silodosin. Separation was done using a C18 column (250 × 4.6 mm, 5 μm). The solution’s pH was adjusted using a Systronics MK VI pH meter. Filtration used 0.45 μm nylon membranes.
Solvents were degassed with an ultrasonicator. The Silodosin API was weighed with a WENSARTM DAB 220 balance. The optimal mobile phase was a mix of Methanol, Acetonitrile, and 10 mM Phosphate buffer (pH adjusted to 4 using 1% OPA) in a 40:40:20 ratio. The flow rate was 1 mL/min, the column temperature was 25 ± 1, the injection volume was 10 μL, and the detection wavelength was 225 nm. A Shimadzu UV-1800 spectrophotometer with UV Probe software was used, with a double beam and 1.0 cm quartz cells.
Preparation of the Standard Solutions
Accurately weigh 10 mg of Silodosin and transfer it into a 100 mL volumetric flask. Dissolve it in Mobile Phase and dilute to mark with the same solvent to obtain a standard stock solution of 100 μg/mL. From this solution, pipette out volumes of 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mL and transfer each to a 10 mL volumetric flask. Fill each flask up to the mark with Mobile Phase, resulting in concentrations of 5, 10, 15, 20, 25, and 30 μg/mL respectively. For the Spectrophotometric method, accurately weigh 10 mg of Silodosin reference standard and transfer it to a 100 mL volumetric flask. Dissolve it in a solution. Prepare concentrations of 3, 6, 9, 12, 15, and 18 μg/mL.
Preparation of the Sample Solutions
In the HPLC method, an accurately weighed amount of Silodosin capsule powder, equivalent to 8.0 mg of Silodosin, was transferred to a 100 mL volumetric flask and dissolved in the mobile phase. The final prepared solution had a concentration of 80 μg/mL. For the UV Spectrophotometric method, 12.5 mL of alkaline solution is added to 1 mL of the drug solution. This mixture is heated for 5 minutes and then cooled in an ice bath. Next, 5 mL of HCl (3.5M) and 5 ml of FeCl3 (0.1M) are added. The absorbance is then measured at 591 nm using UV Spectrometry (UV-1800 Shimadzu).
Method Validation
The methods were validated according to International Conference on Harmonization (ICH) guidelines Q2 (R1) for validation of analytical procedures [9]. The System Suitability Test was carried through for both the methods evaluating theoretical plates and asymmetry.
Linearity
The calibration curve was generated using five concentrations of the standard solution (5-30 μg/ml for the HPLC method and 3-18 μg/ml for the UV spectrophotometric method). Linearity was assessed using linear regression analysis, calculated by the least square regression method.
Precision
Assay precision was assessed through repeatability (within-day), intermediate precision (intraday), and reproducibility (inter-day). Repeatability involved analysing samples at the same concentration on the same day, with a repeatability of 10 μg/mL for the HPLC method and 6 μg/mL for the UV spectrophotometric method (n = 6). Intermediate precision was evaluated by analysing samples at three different concentrations and three different times within the same day, with concentrations of 5, 10, and 15 μg/mL for the HPLC method and 3, 6, and 9 μg/mL for the UV spectrophotometric method (n = 3). Reproducibility was assessed by analysing samples at three different concentrations on three different days, with concentrations of 5, 10, and 15 μg/mL for the HPLC method and 3, 6, and 9 μg/mL for the UV spectrophotometric method (n = 3).
Accuracy
Accuracy in an analytical method reflects how closely the test results match the true value. In the UV spectrophotometric method, accuracy was gauged by recovering known amounts of silodosin reference standard added to the samples initially. The accuracy was evaluated through three replicate determinations at three concentration levels: 11.08, 12, and 13.02 μg/mL. The absolute means obtained were 99.83%, 99.66%, and 100.22%, indicating the method's accuracy within the desired range, as depicted in Table 2. For the HPLC method, accuracy was determined by assaying three concentrations of the sample solution (18, 20, and 22 μg/mL) in triplicate. The absolute means obtained were 100.16 %, 100.41 %, and 99.92 %, as illustrated in Table 3.
Parameter
HPLC Method
UV Method
Concentration range
5 – 30 μg/mL
3 – 18 μg/mL
Regression equation
y = 18104x + 133239
y = 0.0357x - 0.0702
Correlation coefficient (R²)
0.9976
0.9972
Standard error of slope
18104
0.0357
Standard error of intercept
133329
0.0702
Limit of detection (μg/ml)
0.39
0.04
Limit of quantification (μg/mL)
1.18
0.14
Table 1: Linearity study Data.
Precision Parameter
HPLC Method
UV Method
Con.
(μg/mL)Mean Peak Area
R.S.D. (%)
Con.
(μg/mL)Mean Absorbance
R.S.D. (%)
Repeatability
(n = 6)10
314613
0.811
6
0.136
0.579
Intermediate precision
(n = 3)5
234991
0.883
3
0.0517
0.678
10
314201
0.868
6
0.1367
0.335
15
394773
0.564
9
0.2463
0.204
Reproducibility
(n = 3)5
235205
0.965
3
0.0605
0.595
10
315897
0.957
6
0.1403
0.653
15
396381
0.675
9
0.2371
0.345
Table 2: Precision tests Data.
Method
Level of drug taken (%)
Mean percent recovery (%)
S. D
HPLC
80
100.16
0.257
100
100.41
0.276
120
99.92
0.44
UV
80
99.83
0.03
100
99.66
0.215
120
100.22
0.047
Table 3: Recovery tests Data.
Method
Condition
Variation
% Assay
HPLC
pH
4
99.84
3.5
99.51
Flow rate
1.0 mL/min
100.2
0.8 mL/min
99.3
UV
Detection wavelengths
591
100.1
594
99.7
Table 4: Robustness study Data.
Robustness
The HPLC method's robustness was evaluated by analysing samples under different conditions, including slight variations in pH (ranging from 3.5 to 4.0) and flow rate (0.8 to 1 mL/min). This assessment included studying the impact on retention time, Silodosin quantification, and peak parameters. Meanwhile, the robustness of the UV spectrophotometric method was determined by making small adjustments to the wavelength.
Results and Discussion
HPLC Method
The choice of mobile phase was guided by factors like peak symmetry, run time, ease of preparation, and cost. Figure 2 displays a typical chromatogram resulting from analysing both a standard and a sample solution of Silodosin using this method. The chromatogram illustrates a symmetrical peak for Silodosin, with a retention time of 2.9 minutes, enabling quick drug determination, vital for routine analyses. Calibration curves were constructed by plotting peak area against concentration, showing excellent linearity within the 5-30 μg/mL range. The linear equation, y = 18104x + 133239, and the highly significant correlation coefficient (r = 0.9976) underscore the method's reliability (Table 1).
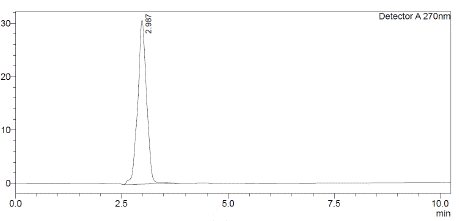
Figure 1: chromatogram of sample solution (80 μg/mL).
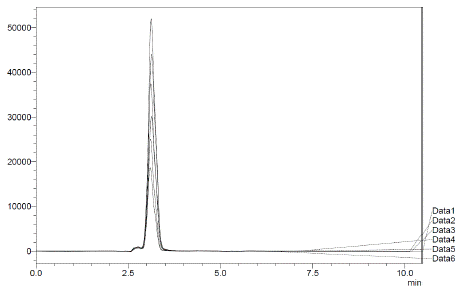
Figure 2: Overlay chromatogram of standard solutions (5 – 30 μg/mL).
The sensitivity of method was indicated by LOD and LOQ values of 0.3 and 1.18 μg/mL, respectively, reflecting high sensitivity. Method precision was assessed through repeatability, intermediate precision, and reproducibility, expressed as R.S.D (%) of multiple measurements. Results showed a repeatability R.S.D of 0.81%, an intermediate precision mean R.S.D of 0.77%, and a reproducibility mean R.S.D of 0.87%. The method's accuracy was determined, with a mean recovery ranging from 99.92% to 100.41%, indicating agreement between the true and found values (Table 3). Specifically designed for assessing Silodosin in tablets, the HPLC method exhibited specificity, with no interfering peaks observed even when simulated excipient samples were added to the solutions. The method was also found to be robust against variations in pH and flow rate.
Spectrophotometric Method
The impact of altering the concentration of hydroxylamine hydrochloride was examined at 591 nm. During this investigation, the concentration of silodosin was maintained constant, while the concentration of hydroxylamine hydrochloride was varied. The proposed spectrophotometric method offers quick and convenient quantification of Silodosin in capsules without needing extensive sample preparation. Additionally, it employs simple instrumentation compared to other techniques. The absorption spectra of Silodosin revealed a λmax of 270 nm, which was utilized in the analysis. Calibration curves were established within the expected concentration range (3 - 18 μg/mL), yielding the representative equation y = 0.0357x - 0.0702, with a correlation coefficient of 0.9972 (Table 1). LOD and LOQ were determined as 0.04 and 0.14 μg/mL, respectively, confirming the method's efficacy for Silodosin determination. Precision assessment resulted in a repeatability R.S.D of 0.57%, an intermediate precision mean R.S.D of 0.40%, and a reproducibility mean R.S.D of 0.53%. Accuracy evaluation revealed a mean recovery ranging from 99.66% to 100.22%, indicating agreement between the true and measured values (Table 3). Lastly, the method demonstrated specificity in determining Silodosin in capsules (Figure 4).
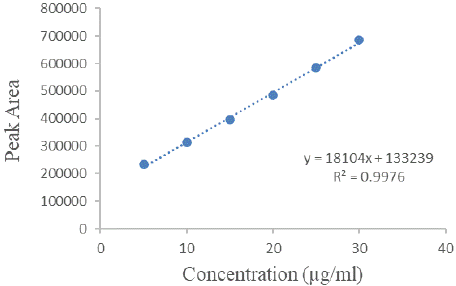
Figure 3: Calibration curve for HPLC method.
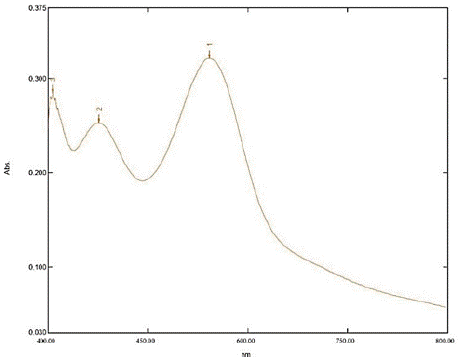
Figure 4: Spectrum of sample solution (μg/mL).
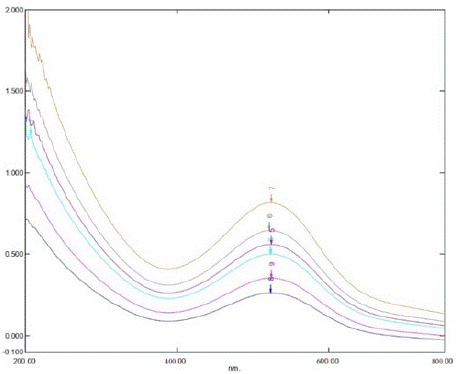
Figure 5: overlay spectrum of standard solutions (3 – 21 μg/mL).
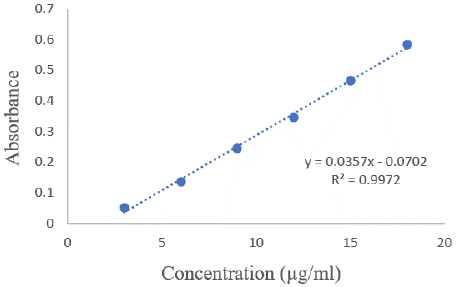
Figure 6: Calibration curve for UV method.
Conclusion
In this study, High-Performance Liquid Chromatography (HPLC) and UV-Spectrophotometric methods were successfully developed and validated for analysing silodosin, an a1-adrenoceptor antagonist used in treating Benign Prostatic Hyperplasia (BPH). Both methods demonstrated excellent linearity, precision, accuracy, and robustness, meeting ICH guidelines. The HPLC method showed superior specificity and resolution, making it suitable for complex matrices and trace-level silodosin analysis in pharmaceutical formulations. Conversely, UV-Spectrophotometry proved simpler and more cost-effective for routine analysis, providing rapid quantification at Silodosin's maximum absorbance wavelength of 280 nm. Comparative evaluation of HPLC and UV-Spectrophotometric methods revealed HPLC's enhanced sensitivity and selectivity, while UV-Spectrophotometry offered practical advantages in instrument availability, ease of operation, and affordability. Therefore, the method choice should consider specific analytical needs, sample complexity, and resource availability. Both HPLC and UV-Spectrophotometric methods are suitable for silodosin quantification in pharmaceutical formulations, ensuring quality control and regulatory compliance. This research contributes valuable insights to analytical chemistry, aiding analysts and researchers in selecting the most appropriate method for silodosin analysis based on their specific needs and constraints.
Author Statements
Conflicts of Interest
Authors have declared that there is no conflict of interest.
References
- Jindan Luo, Wang Xiao, Xie Liping. Evolving role of silodosin for the treatment of urological disorders–A narrative review. Drug Des Dev Ther. 2023: 2861- 2884.
- Assaly, Rana, Julie Faugeroux, Miguel Laurin, Sandrine Compagnie, Laurent Alexandre, François Giuliano, and Delphine Behr-Roussel. Silodosin improves functional consequences of lower urinary tract obstruction secondary to benign prostate hypertrophy, a proof-of-concept study in the spontaneously hypertensive rat supplemented with testosterone. BMC urology. 2020; 20: 1-8.
- Rossi M, Roumeguère T. Silodosin in the treatment of benign prostatic hyperplasia. Drug Des Devel Ther. 2010; 27: 291–297.
- Müderrisoglu A Elif, Jean JMCH de la Rosette, Martin C Michel. Potential side effects of currently available pharmacotherapies in male lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Expert Opinion on Drug Safety. 2023; 22: 1213-1224.
- O’Quin Collyn, Kathryn L White, John R Campbell, Sarah H Myers, Shilpadevi Patil, Debbie Chandler, et al. Pharmacological approaches in managing symptomatic relief of benign prostatic hyperplasia: a comprehensive review. Cureus. 2023; 15: e51314.
- Cui Y, Zong H, Zhang Y. The efficacy and safety of silodosin in treating BPH: a systematic review and meta-analysis. Int Urol Nephrol. 2012; 44: 1601–1609.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Silodosin in the treatment of the signs and symptoms of benign prostatic hyperplasia: a 9-month, openlabel extension study. Urology. 2009; 74: 1318–1322.
- Keating GM. Silodosin: a review of its use in the treatment of the signs and symptoms of benign prostatic hyperplasia. Drugs. 2015; 75: 207–217.
- Montorsi F, Gandaglia G, Chapple C, Cruz F, Desgrandchamps F, Llorente C. Effectiveness and safety of silodosin in the treatment of lower urinary tract symptoms in patients with benign prostatic hyperplasia: A European phase IV clinical study (SiRE study). International Journal of Urology. 2016; 23: 572–579.
- Matsukawa Y, Gotoh M, Komatsu T, Funahashi Y, Sassa N, Hattori R. Efficacy of silodosin for relieving benign prostatic obstruction: prospective pressure flow study. The Journal of urology. 2013; 189: S117–121.
- Lepor H, Hill LA. Silodosin for the treatment of benign prostatic hyperplasia: pharmacology and cardiovascular tolerability. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2010; 30: 1303–1312.
- Tp AN, Rajasekaran A. Method development and validation for the estimation of sildosin in bulk and pharmaceutical dosage forms using UV-VIS spectrophotometry. Asian J Pharm Clin Res. 2012; 5: 150–152.
- Kishore P. Validated Estimation of Silodosin in Pure, Pharmaceuticals and in Biological Sample by Uv-Spectroscopic and Rp-Hplc Method (Doctoral dissertation, Adhiparasakthi College of Pharmacy, Melmaruvathur).
- Prachi Bhamre SJ. Spectrofluorimetric method for the determination of silodosin in bulk and pharmaceutical dosage form. Indo Am J Pharm Res. 2014; 4: 5106–5110.
- Shaik JV, Saladi S, Sait SS. Development of stability-indicating UHPLC method for the quantitative determination of silodosin and its related substances. J Chromatogr. Sci. 2014; 52: 646–653.
- Sayana PS, Iyer RS, Shibi A, Harischandran S. Development and validation of HPTLC method for quantification of silodosin in bulk and pharmaceutical dosage form. The Pharma Innovation. 2012: 1.
- Wadie M, Abdel-Moety EM, Rezk MR, Marzouk HM. A novel eco-friendly HPLC method with dual detection modes for versatile quantification of dutasteride and silodosin in pharmaceutical formulation, dissolution testing and spiked human plasma. Microchem J. 2024; 197: 109753.
- Omar MA, Mohamed AI, Derayea SM, Hammad MA, Mohamed AA. Selective Spectrofluorimetric Method with Enhanced Sensitivity for Determination of Silodosine in Dosage Form and Human Plasma. Application to Stability Studies and Content Uniformity Testing. J Fluoresc. 2017; 27: 473-482.
- Boltia SA, Abdelkawy M, Mohamed TA, Mostafa NN. Validated Chromatographic and Spectrofluorimetric Methods for Analysis of Silodosin: A Comparative Study with Application of RP-HPLC in the Kinetic Investigation of Silodosin Degradation. J AOAC Int. 2020; 103: 946-957.
- Pandeti S, Narender T, Prabhakar S, Reddy TJ. Characterization of degradation products of silodosin under stress conditions by liquid chromatography/Fourier transform mass spectrometry. Rapid Commun Mass Spectrom. 2017; 31: 572-582.
- Boltia SA, Abdelkawy M, Mohammed TA, Mostafa NN. Validated stabilityindicating spectrophotometric methods for the determination of Silodosin in the presence of its degradation products. Spectrochim Acta A Mol Biomol Spectrosc. 2018; 202: 131-145.
- Panchakarla RK, Ravi PR, Buddha MS, Mullangi S, Kondapalli VG. In silico toxicity assessment and trace level quantification of two genotoxic impurities in silodosin using capillary gas chromatography. J Anal Sci Technol. 2023; 14: 15.
- Modroiu A, Krait S, Hancu G, Scriba GK. Quality by design-guided development of a capillary electrophoresis method for the chiral purity determination of silodosin. J Pharm Biomed Anal. 2023; 222: 115117.
- International Council for Harmonization of Technical Requirements of Pharmaceuticals for Human Use, Validation of Analytical Procedure Q2(R1). 2005.