
Research Article
Austin J Anal Pharm Chem. 2024; 11(2): 1173.
Ferric Chloride and DABCO as a Versatile and Effective Catalytic System for Synthesis Thiazolidinedione Phamacophore
Santosh V Padghan¹; Suraj Ade²; Manojkumar Chopade¹*
1Department of Chemistry, Sant Dnyaneshwar Mahavidyalaya, Soegaon, Chhatrapati Sambhaji Nagar 431120, India
2Department of Chemistry, Maulana Azad College, Chhatrapati Sambhaji Nagar, India
*Corresponding author: Manojkumar Chopade, Department of Chemistry, Sant Dnyaneshwar Mahavidyalaya, Soegaon, Chhatrapati Sambhaji Nagar 431120, India. Email: chopademanojkumar@gmail.com
Received: November 23, 2024; Accepted: December 13, 2024;Published: December 20, 2024
Abstract
A mild and efficient procedure for the synthesis of synthetic approach for creating 2, 4-thiazolidinedione derivatives through the Knoevenagel condensation reaction, substituted benzaldehyde with 2,4-thiazolidinedione in ethanol catalysed by Ferric chloride and DABCO as a versatile and effective catalyst. These 2, 4-thiazolidinedione derivatives are significant due to their diverse biological activities’ significant advantages, such as cost-effectiveness, non-toxicity, and operational simplicity and high yield.
Keywords: Knoevenagel condensation; Benzaldehyde; 2, 4-thiazolidinedione; Ferric chloride; DABCO
Introduction
Heterocyclic compounds have been used extensively in the field of medicinal chemistry, where they occupy a central position in the development of new and novel therapeutic agents. Thiazolidinediones are five membered heterocyclic compounds which have attracted considerable attention from chemist over the last 20 years because of their wide range of biological properties including antidiabetic, antibacterial, antifungal, antiproliferative effect on vascular smooth muscle, anti-HIV, antitubercular and anti-inflammatory [1-7]. Additionally some of the rhodamine based compounds shows aldose reductase inhibitory activity [8] and 15-hydroxy prostaglandin dehydrogenase inhibitors [9] (Figure 1). Instead of these biological activities, thiazolidinedione acts as inhibitors of MurD ligase [10].
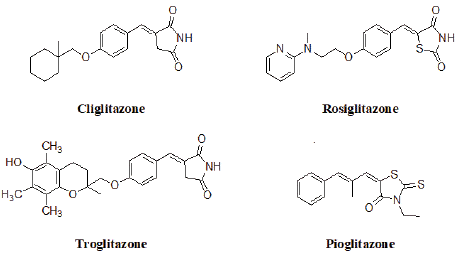
Figure 1: Drug of 2, 4-thiazolidinedione.
Encouraged by all these facts and as part of our continuing research programme dealing with the synthesis of heterocyclic system containing 2, 4-thiazolidinediione moiety we have undertaken the synthesis of 5-arylidine-2, 4-thiazolidinedione. Due to the important pharmacological potential of thiazolidinedione, consequently, a number of synthetic strategies have been reported for the construction thiazolidinedione phamacophore moiety. However, most existing methodology synthesis of thiazolidinedione phamacophore moiety includes KAl(SO4)2.2H2O at 900C [11], baker’s yeast [12], KF-Al2O3 under microwave irradiation [13], glycine under microwave irradiation [14], polyethylene glycol-300 at 100-120°C [15], L-Tyrosine [16], Aqueous media under Ultrasound-Irradiation [17], Mg-doped Ce-Zr solid catalysts [18], Cu(acac)2 [19]. Recentlyionic liquids [bnmim]Cl, C3[min]2.2[Br-] catalyzed synthesis of 5-arylidene-2,4-thiazolidinediones have also been reported [20-21].
However, the above-reported methods suffer from several drawbacks, including prolonged reaction times and the use of environmentally unfavourable solvents. To address these issues, the development of new methodologies in current organic synthesis, mainly focused on the principles of green chemistry, is critical for a sustainable future. Therefore, we decided to explore the potential of our clean and inexpensive catalyst for performing these types of condensation reactions efficiently, aiming to provide a more ecofriendly and cost-effective approach.
The development of FeCl3 and DABCO as a new catalytic system for the Knoevenagel condensation reaction presents a novel approach, utilizing substrates that can serve as valuable intermediates for further transformations. A literature search has revealed some existing reports on the Knoevenagel condensation; however, no previous work has employed ferric chloride FeCl3 and DABCO as a combined catalytic system for this reaction. This innovative methodology has the potential to offer improved efficiency and sustainability in the synthesis of important organic intermediates.
Therefore, we are the first to demonstrate the use of ferric chloride (FeCl3) and DABCO as a catalytic system for the Knoevenagel condensation reaction. To evaluate the applicability of this catalytic methodology, we explored a variety of substituted benzaldehyde, as indicated in Scheme 1. Our final goal was to expand the group of substrates to obtain versatile adducts that can serve as easily transformable intermediates, possessing significant pharmacological activity. This approach aims to develop a more sustainable and efficient method for synthesizing compounds with potential medicinal applications.
Material and Methods
All reactions were carried out in dry solvents, unless otherwise stated. Reactions were monitored by Thin Layer Chromatography (TLC) on silica gel plates (Kieselgel 60 F254, Merck). Visualization of the spots on TLC plates was achieved either by UV light or by staining the plates in 2, 4-dinitrophenylhydrazine/ anisaldehyde and charring on hot plate. All products were characterized by 1H NMR and 13C NMR, IR, Mass and Elemental analysis. 1H NMR and 13C NMR were recorded on Varian Mercury 500 MHz spectrometer. IR spectra were obtained on a Shimadzu FTIR-8400 with samples loaded as thin films on KBr plate, neat or with CH2Cl2 as indicated. Mass spectra were recorded at an ionization potential of 70 eV; Melting points recorded are uncorrected. Column chromatography on silica gel (100-200 mesh) was performed with reagent grade ethyl acetate and hexane as an eluent.
Experimental Section
Typical experimental procedure: In two neck round bottom flask was charged with a mixture of FeCl3 (25 mg, 10 mol%), Benzaldehyde was taken and DABCO (0.010 mg) was added in MeOH 10 mL This mixture was stirred under atmosphere for 10 min then add to it 2, 4-thiazolidinedione and resulting solution was stirred 5h at room temperature till the completion of reaction. The reaction monitored by TLC. The reaction was quenched with a saturated aqueous solution of HCl (2 mL), extracted with ethyl acetate (3 x 20 mL). The combined organic layers were collected and add to it activated charcoal to remove color impurities and filter. The combined organic layer dried using anhydrous Na2SO4, filtered, and the solvent was removed by evaporation. The crude product was purified by crystallization method to afford desired product as colorless solid 5-(4-Chloro-benzylidine)- thiazolidine-2,4-dione (3b) Yield : 89% State : Pale Yellow Solid ,IR (KBr) : 3155, 3049, 879, 2868, 1739, 1691 cm-1, 1HNMR : (500MHz, DMSO)d 13.86 (s, 1H, NH), 8.12 (s, 1H), 7.69-7.57 (m, 4H) 13CNMR : (125MHz, DMSO) d 127.1, 131.8, 132.8, 133.7, 134.8, 134.7, 135.9, ) 171.0, GCMS m/z 237
Result and Discussion
Herein, we performed the Knoevenagel condensation of Benzaldehyde with 2,4-thiazolidinedione in methanol catalysed by Ferric chloride and DABCO as a versatile and effective catalyst the reaction to get 5-arylidene-2, 4-thiazolidinedione with 88 % yield as indicated in Scheme 1.
Optimization of Reaction Conditions
To examine the catalytic efficiency of different transition metal complexes, reaction of benzaldehyde and 2, 4-thiazolidinedione was performed. It is clear that; copper acetyl acetonate promotes reaction more effectively than other transition metal complexes.
The numbers of metal catalysts like CuCl2: DABCO to get desirable product with 82% yield, FeCl3: DABCO, to provided desirable product with 86 % yield, CoCl2: DABCO require longer reaction times with 69% yield, NiCl2: DABCO this catalytic system formation of little amount of side product with less yield (Table 1). The results showed that FeCl3: DABCO is the most efficient catalyst under these conditions and effective for good yield (Table 1 entry 3).
Entry
Catalyst
Time
Yield (%)b
1
CuCl2
12.0 h
45
2
CuCl2: DABCO
8.5 h
60
3
FeCl3 : DABCO
6.0 h
89
4
CoCl2: DABCO
8.0h
69
5
NiCl2: DABCO
9.0 h
65
6
ZnCl2: DABCO
7.0 h
58
Table 1: Study of Several Transition Metal Based Catalysts for Knoevenagel Condensation.
Next, we focused on finding the optimal solvent for synthesizing the target molecule. Initially the model reaction was performed under solvent free condition; low yield of desired product was obtained. The same reaction was performed using different solvents, we performed the model reaction in the presence of various solvents, and the results are summarized in Table 2. We observed that the highest yields were achieved with methanol and ethanol as polar aprotic solvents (Table 2, entries 4 & 5). Among all these solvents, in MeOH maximum yield was obtained hence MeOH was selected as optimal solvent.
Entry
Solvent
Time
Yield (%)b
1
Solvent free
12.0 h
45
2
DCM
8.5 h
60
3
Chloroform
6.0 h
65
4
EtOH
6.0h
84
5
MeOH
5.0 h
89
6
DMSO
7.0 h
58
aReaction conditions: Benzaldehyde(1mmol), 2,4-thiazolidinedione(1mmol), ferric chloride, DABCO, various solvent, were stirred at room temp, bIsolated Yield.
Table 2: Screening of various solvents for the synthesis 2,4 thiazolidinedione Derivattivesa.
To study the substrate scope, optimized reaction conditions were applied to various aromatic aldehydes with 2, 4-thiazolidinedione. Substrates with various functionalities reacted well and afforded high yield of desired 5-arylidine-2,4-thiazolidinedione According to the results in Table 3, Both electron donating and electron withdrawing substituent on benzaldehyde 1a-1i underwent clean reactions affording the desired product with high yields (80-89%). The isolated products were purified by simple recrystallization and their structures were confirmed using FTIR, ¹H NMR, and ¹³C NMR and mass spectroscopy techniques.
Entry
R
Product
Time
Yield %b
1
Benzaldehyde
3a
5.0
84
2
4-Chloro Benzaldehyde 1b
3b
4.5
89
3
4-Bromo Benzaldehyde 1c
3c
4.5
89
4
4-Nitrobenzaldehyde 1d
3d
3.0
85
5
3-Nitrobenzaldehyde 1e
3e
3.5
82
6
2-Chlorobenzaldehyde 1f
3f
6.5
80
7
2,4-Chlorobenzaldehyde 1g
3g
8.0
86
8
4-Methoxy benzaldehyde 1h
3h
8.0
86
9
4-Methyl benzaldehyde 1i
3i
7.0
87
aReaction conditions: substituted Benzaldehyde(1mmol), 2,4thiazolidinedione(1mmol), ferric chloride, DABCO, in MeOH, were stirred at room temp, bIsolated Yield.
Table 3: Ferric chloride catalysed synthesis of 5-arylidine-2,4-thiazolidinedione derivativesa.
Conclusion
In conclusion, a straightforward, efficient, and eco-friendly method has been developed using Ferric chloride and DABCO as a versatile and effective catalyst as a catalyst for synthesizing 5-arylidine-2,4-thiazolidinedione derivatives. This approach offers significant advantages, such as cost-effectiveness, non-toxicity, and operational simplicity. The use of Ferric chloride and DABCO under mild conditions provides an environmentally benign alternative to traditional methods, achieving high yields with a variety of aryl aldehydes. This green catalytic system contributes to sustainable chemistry, offering a promising route for the synthesis of valuable heterocyclic compounds.
Author Statements
Acknowledgement
Authors are thankful to Central instrumental facility, Savitribai Phule University Pune for structural characterization and Department of Chemistry Sant Dnyaneshwar Mahavidyalaya, Soegaon for infrastructural facility.
References
- BCC Cantello, MA Cawthorne, GP Cottam, et al. [[Omega- (Heterocyclylamino) alkoxy]benzyl]-2,4-thiazolidinediones as potent antihyperglycemic agents. Journal of Medicinal Chemistry. 1994; 37: 3977–3985
- IM Labouta, HM Salama, NH Eshba, O Kader, E El-chrbini. Potential antimicrobial: syntheses and in vitro anti-microbial evaluation of some 5-arylazothiazolidones and related compounds. European Journal of Medicinal Chemistry. 1987; 22: 485–489.
- R Ottana, R MacCari, ML Barreca, Bruno G, Rotondo A, Rossi A, et al. 5-Arylidene-2- `imino-4-thiazolidinones: design and synthesis of novel antiinflammatory agents. Bioorganic and Medicinal Chemistry. 2005; 13: 4243–4252.
- JD Peuler, SM Phare, AR Iannucci, MJ Hodorek. Differential inhibitory effects of antidiabetic drugs on arterial smooth muscle cell proliferation. American Journal of Hypertension. 1996; 9: 188–192.
- Rawal RK, Tripathi R, Katti SB, Pannecouque C, DeClercq E. Design and synthesis of 2-(2, 6-dibromophenyl)-3-heteroaryl-1, 3-thiazolidin-4-ones as anti-HIV agents. Eur J Med Chem. 2008; 43: 2800-2806.
- Mallikarjuna BP, Sastry BS, Suresh Kumar GV, Rajendraprasad Y, Chandrashekar SM, Sathisha K. Synthesis of new 4-isopropylthiazole hydrazide analogs and some derived clubbed triazole, oxadiazole ring systems – A novel class of potential antibacterial, antifungal and antitubercular agents. Eur J Med Chem. 2009; 44: 4739-4746.
- Kumar A, Sharma S, Archana A, Bajaj K, Sharma S, et al. Some new 2,3,6-trisubstituted quinazolinones as potent anti-inflammatory, analgesic and COX-II inhibitors. Bio Org Med Chem. 2003; 11: 5293-5299.
- Feseneau P, Cussac M, Morand JM. Synthesis, Activity, and Molecular Modeling of New 2,4-Dioxo-5-(naphthylmethylene)-3-thiazolidineacetic Acids and 2-Thioxo Analogues as Potent Aldose Reductase Inhibitors. J Med Chem. 1998; 41: 4706-4715.
- Wu Y, Karna S, Choi CH. Synthesis and Biological Evaluation of Novel Thiazolidinedione Analogues as 15-Hydroxyprostaglandin Dehydrogenase Inhibitors. J Med Chem. 2011; 54: 5260-5264.
- Nace Z, Tihomir T, Roman S, Rupnik V, Kovac A, et al. Discovery of Novel 5-Benzylidenerhodanine and 5-Benzylidenethiazolidine-2,4-dione Inhibitors of MurD Ligase. Journal of Medicinal Chemistry. 2010; 53: 6584-6594.
- Shelke KF, Sapkal SB, Kakade GK, Sadaphal SA, Shingate BB, Shingare MS. Alum catalyzed simple and efficient synthesis of 5-arylidene-2,4- thiazolidinedione in aqueous media. Green Chemistry Letters & Reviews. 2010; 3: 17-21.
- Pratap UR, Jawale DV, Waghmare RA, Ligampalle DL, Mane RA, UR Pratap, et al. Synthesis of 5-arylidene-2,4-thiazolidinediones by Knoevenagel condensation catalyzed by baker’s yeast. New Journal of Chemistry. 2011; 35: 49-51.
- Yang DV, Yang BY, Chen ZC, Chen SY. A convenient synthesis of 5-arylidenethiazolidine-2,4-diones on potassium fluoride-aluminium oxide. Organic preparations and procedures international. 2006; 38: 81-85.
- Yang BY, Yang DH. Solvent-free synthesis of 5benzylidene-2-thioxothiazolidin- 4-ones and thiazolidine-2,4diones catalysed by glycine under microwave irradiation. Journal of Chemical Research. 2011; 35: 238-239.
- Mahalle SR, Netankar PD, Bondge SP, Mane RA. An efficient method for Knoevenagel condensation: a facile synthesis of 5-arylidenyl 2,4-thiazolidinedione. Green Chemistry Letters & Reviews. 2008; 1: 103-106.
- Thirupathi G, Venkatanarayana M, Dubey PK, Bharathi Kumari Y. Facile and green syntheses of substituted-5-arylidene-2,4-thiazolidinediones using L-tyrosine as an Eco-Friendly catalyst in aqueous medium. Der Pharma Chemica. 2012; 4: 2009-2013.
- Kiran F Shelke, Adinath D Badar, Jankiram B Devhade. An Efficient Synthesis of 5-Arylidene-2,4-ThiazolidinedioneCatalyzed by Boric acid in Aqueous media under Ultrasound-Irradiation. Chemistry & Biology Interface. 2016; 6: 410-415.
- Rathod S, Navgire M, Arbad B, Lande M. Preparation of Mg-doped Ce-Zr Solid Catalysts and Their Catalytic Potency for the Synthesis of 5-Arylidene- 2,4-Thiazolidinediones via Knoevenagel Condensation. Afr J Chem. 2012; 65: 196-201.
- MU Chopade, Santosh V Padghan, BK Magar. Copper acetyl acetonate as a Mild and Efficient Catalytic System for Synthesis of 5-arylidene-2, 4- thiazolidinedione. J of Res Development. 2020; 10: 235-243.
- Shelke KF, Sapkal SB, Madje BR, Shingate BB, Shingare MS. Ionic liquid promoted an efficient synthesis of 5-arylidene-2, 4-thiazolidinedione Bulletin of the Catalysis. Society of India. 2009; 8: 30-34.
- DV Jawale, UR Pratap, DL Lingampalle, RA Mane. Dicationic Ionic Liquid Mediated Synthesis of 5-Arylidine-2,4-thiazolidinediones. Chinese Journal of Chemistry. 2011; 29: 942-946.