
Research Article
Austin J Anal Pharm Chem. 2015;2(2): 1035.
Development and Validation of HPTLC Method for Estimation of Saroglitazar in Bulk and Pharmaceutical Dosage Form
Patel NM*, Mehta FA, Shah DA and Chhalotiya UK
Department of Pharmaceutical chemistry and analysis, Indukaka Ipcowala College Of Pharmacy, India
*Corresponding author: Nikita M Patel, Indukaka Ipcowala College of Pharmacy, India.
Received: March 17, 2015; Accepted: April 07, 2015; Published: April 09, 2015
Abstract
A simple, sensitive, and precise high-performance thin-layer chromatographic method has been developed for the estimation of Saroglitazar (SAR). The method employed was thin-layer chromatography (TLC). Aluminum plates precoated with silica gel 60 F254 were used as the stationary phase, while the solvent system was n-butanol: ammonia (7:3 v/v). The Rf value was observed to be 0.55 ± 0.02. The spot was densitometrically analyzed at 294 nm. The method was linear in the range of 125-1500 ng/ band. The limit of detection for SAR was found to be 13.09 ng/ band. The limit of quantification for SAR was found to be 39.68 ng /band. The proposed method was validated with respect to linearity, accuracy, precision and robustness. The method was successfully applied to the estimation of SAR in formulation.
Keywords: HPTLC; Saroglitazar; Validation
Introduction
Saroglitazar (SAR) is chemically(S)-a-ethoxy-4-[2-[2-methyl-5- [4-(methylthio) phenyl]-1H-pyrrol-1-yl]ethoxy] benzenepropanoic acid magnesium salt (2:1) has the empirical formula [C25H28NO4S]2Mg with molecular weight 900 (g/ mol) (Figure 1) [1]. Peroxisome proliferator activated receptor (PPAR) α and γ agonists are approved hypolipidemic and anti-diabetic agents respectively and combining them together provides a dual benefit in type 2 diabetes mellitus associated with dyslipidemia. This novel class of dual PPAR agonists is termed as “Glitazars” [2]. SAR is the first glitazar class compound that has been approved as a therapeutic agent [1]. Other compounds of the same chemical class such as Rapaglitazar, Cipoglitazar, Aleglitazar etc. are failed in the clinical trials. SAR is a dual regulator that corrects both the lipid profile and the glycemic indices [3]. SAR is indicated for the treatment of dyslipidemia and has favourable effects on glycaemic parameters in type 2 diabetes mellitus [4]. Structurally, SAR is a non-TZD and non-fibrate molecule and belongs to aryl alkoxy propionic acid class. SAR is a dual PPAR (peroxisome proliferator activated receptor) -α/γ agonist having strong PPAR-α effect with moderate PPAR-γ effect. Agonist action at PPAR-α lowers high blood triglycerides and agonist action on PPAR-γ improves insulin resistance and consequently lowers blood sugar [5]. SAR is indicated for the treatment of diabetic dyslipidemia or hypertriglyceridemia with type 2 diabetes mellitus not controlled by statin therapy [6].
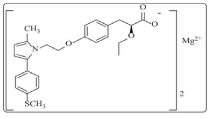
Figure 1: Chemical structure of SAR.
A Literature review regarding quantitative analysis of this drug revealed that two UV spectroscopic methods and one HPLC method are reported [7,8,9].
The LOD in reported UV spectroscopic method is 0.50μg/mL. It is less time consuming method then the other methods.
The LOD in developed HPTLC method was found to be 13.09 ng/ band. So, this method is more sensitive than the UV method.
Both of these methods are specific as no interference found from the excipients in the analysis of formulation.
HPTLC method has the advantage that more number of samples can be analyzed in a short analysis time. Every time new stationary phases can be taken for analysis which prevent problem of carry over. So, for routine quality control analysis, HPTLC method can be used. There is no HPTLC method reported for the estimation of SAR. So attempt have been made to develop HPTLC method.
Experimental
Instruments
HPTLC instrument consists of CAMAG (Muttenz, Switzerland) Linomat V sample applicator with 100 μL applicator syringe (Hamilton, Bonadauz, Switzerland). Chromatography was performed on 10 cm × 10 cm aluminum TLC plates precoated with silica gel 60- F254 (E. Merck, Darmstadt, Germany). CAMAG TLC scanner 4 was used for the densitometric scanning of the developed chromatogram. All drugs and chemicals were weighed on Shimadzu electronic balance (AX 200, Shimadzu Corp., and Japan).
Reagents and chemicals
The standard drug Saroglitazar was obtained as a gift sample from Zydus Cadila Healtcare Ltd, Dabhasa, India. The marketed formulation LYPAGLYN tablets (Each uncoated tablet contains Saroglitazar 4 mg) manufactured by Zydus Cadila Healthcare ltd. was procured from local market. Butanol and Ammonia analytical reagent obtained from SD fine chem, Mumbai.
Chromatographic system
Sample application
Standard and formulation sample of SAR was applied on the HPTLC plates in the form of narrow bands of 5 mm length. The bands were applied 10 mm above from the bottom and 15 mm away from left edge of the plate. Samples were applied under a continuous drying stream of nitrogen gas.
Mobile phase and development
Plate was developed using a mobile phase consisting of n-butanol: Ammonia (7:3 v/v). Linear ascending development was carried out in a twin-trough glass chamber equilibrated with the mobile phase vapors for 30 min. Mobile phase (10 mL) was used for development and allowed to migrate at up to 80 mm. After development, the HPTLC plate was air dried completely.
Densitometric analysis
Densitometric scanning was performed in the absorbance mode under control by win CATS planar chromatography software (CAMAG, Muttenz, Switzerland). The source of radiation was the deuterium lamp, and bands were scanned at 294 nm. The slit dimensions were 5 mm length and 0.45 mm width, with a scanning rate of 20 mm/s. Concentration of the compound was determined from the intensity of diffusely reflected light and evaluated as peak areas against concentrations using a linear regression equation.
Preparation of standard stock solution
1.0 mg of standard SAR was accurately weighed and transferred to 10 ml volumetric flask and dissolved in a few milliliters of methanol. The volume was made up to the mark with methanol to yield a solution containing 100 μg /ml of SAR. From which 0.5 mL of aliquot was transferred to 10 mL volumetric flask. Then volume was made up to the mark with methanol to yield a solution of 50 μg /ml.
Validation
Validation of the developed HPTLC method was carried out according to the International Conference on Harmonization (ICH) guidelines Q2 (R1) [10].
Linearity of calibration curves: Linearity of the method was evaluated by constructing calibration curve at five concentration levels over a range of 125–1500 ng /band for SAR. The calibration curve was developed by plotting peak area versus concentration (n = 5) (Figure 2).
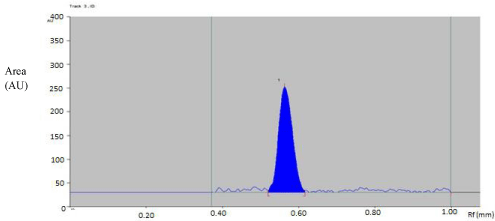
Figure 2: Densitogram of SAR ( 500 ng/band).
Accuracy: The accuracy of the method was determined by calculating recovery of SAR by method of standard additions. Known amount of SAR (0, 125, 250, 375 ng/ band) was taken from the working standard solutions (50 μg/ mL of SAR). It was added to prequantified samples, and the amount of SAR was estimated by measuring the areas and by fitting these values to the straight-line equations of the calibration curves.
Precision: Precision was evaluated in terms of intra-day and inter-day precisions. For precision study, working standard solution of 50 μg/ mL SAR was used for applying bands on the HPTLC plate. Intra-day precision was determined by analyzing sample solution of SAR (125, 500, 1500 ng/ band) at three levels covering low, medium, and high concentrations of the calibration curve three times on the same day (n = 3). Inter-day precision was determined by analyzing sample solution of SAR (125, 500, 1500 ng/ band) and at three levels covering low, medium, and high concentrations over a period of 3 days (n = 3). The peak areas obtained were used to calculate mean and relative standard deviation (% RSD) values.
Repeatability of measurement of peak area was determined by analyzing SAR sample (500 ng/ band) and 6 times without changing the position of plate on the same plate to check the repeatability of injection.
Sensitivity: The limit of detection (LOD) is defined as the lowest concentration of an analyte that can reliably be differentiated from background levels. The limit of quantification (LOQ) of an individual analytical procedure is the lowest amount of analyte that can be quantitatively determined with suitable precision and accuracy.
LOD and LOQ were calculated using the following equation as per ICH guidelines:
LOD = 3.3 × σ/S
LOQ = 10 �× σ/S
Where s is the standard deviation of y-intercepts of regression lines and S is the slope of the calibration curve.
Robustness: Small changes in the chamber saturation time and solvent migration distance were introduced, and the effects on the results were examined. Robustness of the method was determined at concentration level of 500 ng/ band of SAR. The mean and % RSD values of the peak areas were calculated.
Analysis of formulation: Tablet powder equivalent to 0.125 mg SAR was taken in 10 mL volumetric flask. Methanol was added to the above flask, and the flask was sonicated for 15 min. The solution was filtered using Whatman filter paper No. 41, and the volume was made up to the mark with methanol in order to obtain a solution 12.5 μg/ml of SAR. From which 10 μL was applied in the form of band to obtain a conc. of SAR (125 ng/ band).
Results and Discussion
Optimization of the mobile phase
To develop the HPTLC method for the estimation of SAR, selection of the mobile phase was carried out on the basis of polarity. A mobile phase that would give a dense and compact band with an appropriate Rf value for SAR is desired. Various mobile phases were tried at initial stage of method development. The mobile phase such as methanol, toluene, and ethyl acetate were tried initially. Mixture of ethyl acetate: glacial acetic acid (9:1, v/v), methanol: n-Propanol (7:3, v/v), methanol-toluene (7:3, v/v), methanol-toluene-glacial acetic acid (5:4:1, v/v/v), and ethyl acetate-toluene-acetic acid (3:6:1,v/v/v) were tried as mobile phase, but satisfactory results were not achieved (Table 1).
SR No.
Mobile Phase
Observation
1.
Ethyl acetate: Glacial acetic acid (9:1)
Spot moving with solvent front
2.
Methanol : n-propanol (7:3)
Spot moving with solvent front
3.
Methanol: toluene(7:3)
Spreading of spot
4.
Methanol: Toluene: Glacial acetic acid ( 5:4:1)
No clear spot found
5.
Ethyl acetate: Toluene (5:5)
Spreading of spot
6.
n-butanol : Ammonia(7:3)
Clear spot observed.
Table 1: Optimization of the mobile phase.
The mobile phase n-butanol: Ammonia (7:3, v/v) was found to be satisfactory. It was also observed that chamber saturation time and solvent migration distance were crucial in the chromatographic separation. A chamber saturation time of less than 15 min and solvent migration distances greater than 80 mm resulted in diffusion of the analyte band. So, n-butanol: Ammonia (7:3, v/v) mobile phase with a chamber saturation time of 30 min at ambient condition and solvent migration distance of 80 mm was selected as an optimum condition. These chromatographic conditions produced a well-defined, compact band of SAR with Rf 0.55 ± 0.02 (Figure 2).
Selection of detection wavelength
The sensitivity of HPTLC method that uses ultraviolet (UV) detection depends upon proper selection of detection wavelength. An ideal wavelength is the one that gives good response for the drug that is to be detected. In the present study, solution was applied in the form of a band in concentration ranges of 125–1500 ng/ band of SAR, was prepared in methanol. A solution was filled in the syringe, and under nitrogen stream, it was applied in form of band on a single plate. The plate was developed using Butanol: Ammonia (7:3, v/v) at ambient condition and dried in air. The developed plate was subjected to densitometric measurements in scanning mode in the UV region of 200–400 nm, and the overlaid spectrum was recorded using CAMAG TLC Scanner 4. The overlaid spectra showed that drug absorb appreciably at 294 nm (Figure 3).
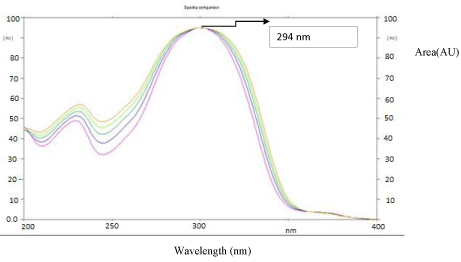
Figure 3: Overlay of absorbance spectra of SAR.
Validation
Linearity and Calibration Curves: Linearity of an analytical method is its ability, within a given range, to obtain test results that are directly, or through a mathematical transformation, proportional to the concentration of the analyte. The method was found to be linear in concentration ranges of 125–1500 ng/ band of SAR (Figure 4). Three dimensional overlay of HPTLC densitogram of Calibration bands of SAR was obtained in above concentration ranges (Figure 5). The regression data showed a good linear relationship over the concentration range studied, demonstrating the suitability of the method for analysis (Table 2).
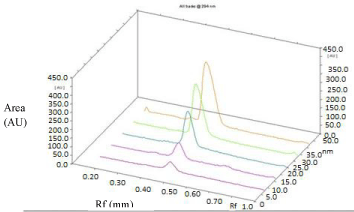
Figure 5: Three dimensional overlay of HPTLC densitogram of Calibration
bands of Saroglitazar.
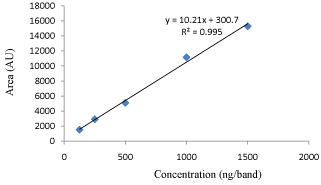
Figure 4: Regression graph of SAR.
Parameters
SAR
Linearity (ng band-1)
125-1500
Correlation coefficient( r2)
0.9952
Slope of regression equation
10.21
Standard deviation of slope
0.179
Intercept of regression
300.7
Standard deviation of Intercept
40.48
Table 2: Regression analysis of calibration curve.
Accuracy: Accuracy of an analytical method is the closeness of test results to the true value (100%). It was determined by the application of analytical procedure to recovery studies, where a known amount of standard is spiked into pre-analyzed sample solutions. % Recoveries was found to be 98.49–102.41% for SAR (Table 3). Values demonstrated the accuracy of the method is in the desired range (90- 110%).
Parameters
SAR
Linearity ( ng band-1)
125-1500
Retention factor
0.55±0.01
Detection limit ( ng band-1)
13.09
Quantitation limit ( ng band-1)
39.68
Accuracy (%)
98.49-102.41
Precision (%RSD)
Intra-day (n = 3)
0.50-1.58
Inter-day (n = 3)
0.63-1.62
Instrument precision (%RSD)
Scanner (n=7)
0.66
Injection (n=7)
0.97
Specificity
Specific
Table 3: Summary of validation parameters of HPTLC.
Precision: Intra-day precision refers to the use of an analytical procedure within a laboratory over a short period of time by the same operator with the same equipment, whereas inter-day precision involves estimation of variations in analysis when a method is used within a laboratory on different days. The RSD values of the response were less than 2% for intra-day and inter-day precision. Repeatability of the scanning device and injection was studied by applying and analyzing SAR samples (500 ng/ band) 6 times. The RSD values obtained were less than 2% (Table 3), which was under the acceptance criteria of ICH method validation guideline (<2%). The results indicated that the method is repeatable and reproducible.
Limit of detection and limit of quantification: Under the experimental conditions used, the lowest amounts of drug that could be detected (LOD) for SAR was found to be 13.09 ng/ band. The limit of quantification (LOQ) for SAR was found to be 39.68ng/ band (Table 3). This indicates that the nanogram quantity of drug can be estimated accurately and precisely which means the method is sensitive.
Robustness: The % RSD values less than 2% were obtained after introducing small, deliberate changes in parameters of the developed HPTLC method, confirming its robustness (Table 4).
Method parameters (condition)
Normal condition
Deliberate changes.
Rf± S.D. (n=3)
Solvent migration distance (mm)
70
75
0.55 ± 0.02
65
0.55 ± 0.02
Chamber Saturation time
(min.)
30
28
0.55 ± 0.02
32
0.55 ± 0.02
Table 4: Results of robustness studies.
Solution stability: The stability of sample solutions was studied at ambient condition for 24h. drug was found to be stable with a recovery of more than 97%.
Analysis of formulation: The formulation was analyzed using the proposed method which gave percentage recovery of more than 98.0% for SAR. A single band at Rf 0.55 ± 0.02 was observed in the chromatogram for SAR, and no interference from the excipients present in the formulation was observed (Table 5).
Formulation
Amount taken(mg)
%Drug recovery
Saroglitazar Tablet
(Lypaglyn)
0.125 mg
100.23± 0.96
Table 5: Analysis of formulation.
Conclusion
Proposed study describes HPTLC method for the estimation of SAR. The method was validated and found to be simple, sensitive, accurate and precise. Statistical analysis proved that method was repeatable and selective for the analysis of SAR without any interference from the excipients. The method was successfully used for determination of drug in its formulation.
References
- Physical and chemical properties of saroglitazar.
- Sharma A, S Amarnath , Kushwah D, S Ramaswamy. Saroglitazar. A novel cardiometabolic agent for diabetic dyslipidemia – A Review. JYP. 2015; 7 : 2-6.
- Lipaglyn – Product Information Booklet.
- Jani RH, Kansagra K, Jain MR, Patel H. Pharmacokinetics, Safety, and Tolerability of Saroglitazar (ZYH1), a Predominantly PPARa Agonist with Moderate PPARγ Agonist Activity in Healthy Human Subjects. Clin Drug Investig. 2013; 33: 809-816.
- “Lypaglyn (saroglitazar) for treating hypertriglyceridemia in type 2 diabetes, India” Drug Development and Technology.
- “Zydus Group launches new diabetic drug.”The Times of India. Jun 6, 2013.
- Amin EH and Maheshwari DG. Development and Validation of UV spectrophotometric method for saroglitazar tablets. J. pharm. Sci. bio- sci. res. 2014; 4: 312-315.
- Method development and method validation of saroglitazar in bulk and pharmaceutical dosage form by UV spectrophotometric method.
- Siddartha B. Method Development and Validation for the estimation of saroglitazar in bulk and pharmaceutical dosage form by RP- HPLC. Int J pharm pharm sci. 2014: 3; 567-575.
- ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures, Text and Methodology, Q2 (R1), Geneva, 2005.