
Research Article
Austin J Anal Pharm Chem. 2015;2(2): 1036.
Broad Antimicrobial-spectrum of Plantaricin LR14 against Gram-positive and Gram-negative Bacteria
Santosh Kumar Tiwari1,2* and Sheela Srivastava2
1Department of Genetics, Maharshi Dayanand University, Haryana, India
2Department of Genetics, University of Delhi South Campus, New Delhi, India
*Corresponding author: Santosh Kumar Tiwari, Department of Genetics, M. D. University, India
Received: March 27, 2015; Accepted: April 16, 2015; Published: April 17, 2015
Abstract
Bacteriocin, plantaricin LR14 was purified from a natural isolate of Lactobacillus plantarum LR/14 and showed bactericidal mode of action when treated with cells of target bacteria as described in our previous study. Here, antimicrobial activity of purified plantaricin LR14 was monitored in terms of loss of viable count of sensitive strains after treatment. Both time-bound and concentration-based response of plantaricin LR14 on the loss of viable count of target bacteria was observed. While it was active against indicator strain Micrococcus luteus, some other Gram-positive as well as Gram-negative bacteria were also affected. Pathogenic strains including strictly food-borne pathogens such as Listeria monocytogenes, Salmonella, Yersinia enterocolitica, and Bacillus licheniformis were inhibited by plantaricin LR14. The inhibition could also be demonstrated of a clinical urogenic strain of Escherichia coli. Inhibition of Gram-positive as well as Gram-negative bacteria implies that plantaricin LR14 has a broad host-range inhibitory spectrum, which may have applications not only in food safety but in some clinical settings as well.
Keywords: Bacteriocins; Plantaricin LR14; Antimicrobial spectrum; Grampositive; Gram-negative bacteria
Introduction
Most of the Gram-positive bacteriocins are membrane active peptides that increase the permeability of the cytoplasmic membrane. They often show a much broader spectrum of bactericidal activity than the colicins, produced by the strains of Esherichia coli. Bacteriocins of lactic acid bacteria (LAB) are GRAS (Generally Regarded as Safe) status molecules since they are degraded by the proteases present in human intestine and fall within two broad classes, viz the lantibiotics and the non-lantibiotics [1,2]. Nisin, a lantibiotics, produced by several strains of Lactococcus lactis, is a permitted food additive in more than 50 countries including the US and Europe under the trade name Nisaplin. This is based on the fact that it prevents the growth of spoilage bacteria in fermentation products, and canned foods, thus extending their shelf-life [3].
The heterogeneity in the antimicrobial spectrum of bacteriocins led to the establishment of narrow, intermediate and broad spectrum bacteriocins: (i) bacteriocins with a narrow antimicrobial spectrum restricted to strains within the species of the producer microorganism, such as lactococcin A [4] (ii) bacteriocins with intermediate antimicrobial spectrum also inhibiting other genera of LAB and Gram-positive bacteria, including the food-borne pathogens, L. monocytogenes, Staphylococcus aureus, Clostridium perfringens and C. botulinum. This group includes, among others, lactacin F [5] plantaricins S and T [6]; and (iii) broad antimicrobial spectrum bacteriocins inhibit a wide range of Gram-positive including, besides the genera mentioned above, Propionibacterium spp., Clostridium spp and Bacillus spp. Representatives of this group are nisin A, Z [7] and pediocin AcH/PA-1[8]. Bacteriocins of LAB commonly exhibit bactericidal as well as bacteriostatic mode of action against other strains of bacteria. Bacteriocins produced by Lactobacillus plantarum LR/14 and Bacillus subtilis W42 have shown bactericidal activity [9,10].
We have earlier described the production, purification and some novel features of plantaricin LR14 produced by a natural isolate of Lactobacillus plantarum strain LR/14 [9,11,12]. Bacteriocin, plantaricin LR14 was purified to homogeneity using multichromatographic steps [11]. Here, we report the broad antimicrobialspectrum of purified bacteriocin against some pathogenic bacteria which may be useful for industrial applications.
Materials and Methods
Bacterial strains and culture media
L. plantarum LR14 was grown in modified TGYE medium at 37°C for 20h and the bacteriocinogenic activity in culture free supernatant was assayed as described previously [9,11]. The other strains were obtained from the laboratory of Dr. J. S. Virdi, Department of Microbiology, University of Delhi South Campus, New Delhi. Among these strains, E. coli was grown in LB medium, Bacillus sp, B. licheniformis and Enterococcus fecalis were grown in TGYE medium and the rest in TSB (Trypticase Soya Broth) medium. All the media components were procured from Hi-Media, India.
Preparation of purified plantaricin LR14
Plantaricin LR14 was purified to homogeneity described earlier [12,13]. Briefly, it involved the enrichment of culture supernatant by ammonium sulphate precipitation, specific binding and elution by cation-exchange, desalting using gel-filtration chromatography and purity level was assessed using reverse phase-fast protein liquid chromatography (RP-FPLC). The bacteriocin activity of purified preparation was quantified in terms of AU/ml. One activity unit (AU) was defined as the reciprocal of the highest dilution of the bacteriocin causing 50% growth inhibition (50% of the turbidity of the control culture without bacteriocin) [14].
Antimicrobial spectrum
The antimicrobial spectrum of the purified preparation was determined against Gram-positive and Gram-negative bacteria. In each case, a cell suspension of ~7.0 log10 CFU/ml in normal saline was tested with 400 AU/ml and the viability count after 24h was determined by plating on respective medium as mentioned earlier [9].
Kill-kinetics of target cells
A time-bound experiment was done on selected pathogenic strains like E. coli (urogenic), L. monocytogenes, Y. enterocolitica, and B. licheniformis. With these strains, plantaricin LR14 added at the concentration of 200 and 400 AU/ml to the respective cell suspension of ~7.5 to 8.5 log10 CFU/ml and viable count was determined after 4, 8, and 24h of incubation. Set without bacteriocin was considered as control. A concentration-based experiment was also performed on these target bacteria wherein three different higher concentrations (400, 800 and 1000 AU/ml) of purified bacteriocin sample were applied and viable count was monitored after the incubation of 24h.
Statistical analysis
Experiments were performed in triplicate and mean values were plotted along with standard error of means (SEMs). Three independent experiments (n = 3) were performed to monitor the reproducibility of results. The level of statistical significance was analyzed as p = < 0.05.
Results and Discussion
Antimicrobial spectrum
The present investigation has demonstrated the bactericidal nature of plantaricin LR14 on indicator strain M. luteus and other pathogenic bacteria as well. In comparison to the untreated cultures, the killing of target cells by plantaricin LR14 was highly effective. When purified bacteriocin was applied on different pathogenic strains, a variable response was observed. The results, however, were encouraging as inhibition of some food-borne pathogens, L. monocytogenes, Streptococcus flexneri, Y. enterocolitica, B. licheniformis, and Aeromonas was observed. While Salmonella enterica was inhibited to a very less extent, Vibrio cholerae, Serratia grimesii, and Enterobactor aerogenes were not affected at all (Table 1). The inhibition pattern was very similar to that described previously with crude preparation of plantaricin LR14 [9].
S. No.
Pathogenic strains
Loss of viability (%)*
Aeromonas sp.
41.77±2.91
Salmonella enterica
4.22±1.21
Streptococcus flexneri
45.88±1.92
Serratia grimesii
0.00
Vibrio cholerae
0.00
Enterococcus fecalis
15.94±0.98
Enterobactor aerogenes
0.00
Bacillus sp.
26.58±1.89
E. coli (eurogenic)
27.84±1.76
Yersinia enterocolitica
45.56±2.13
Bacillus licheniformis
24.67±1.36
Listeria monocytogenes
20.27±1.06
Micrococcus luteus
99.68±2.65
*Experiments were done in triplicate and values are mean of three independent experiments. Control without treatment with bacteriocin was considered as hundred percent.
IS AEROMONAS SPP THE ONLY ONE DONE IN TRIPLICATE?
No, all the experiments were done in triplicate. The same has been corrected accordingly.
Table 1: Percentage loss of viability of different pathogenic strains after treatment with plantaricin LR14.
The inhibition of a wide-range of bacterial strains indicated the broad host-range of plantaricin LR14. That some Gram-negative strains were also affected suggesting an interesting property of plantaricin LR14. Inhibition of Gram-negative bacteria by bacteriocins of lactic acid bacteria is a unique feature and widens its applications in therapeutics also. Recently, there are several reports available on the inhibition of Gram-negative members but the detailed mechanism need to be investigated [13,15]. While enterocin LR/6 showed bactericidal mode of action [16], the antimicrobial substances produced by L. delbrueckii strain 1043 and L. plantarum TF711 showed bacteriostatic action [17]. Bacteriocin produced by P. acidilactici HA-6111-2 acted bactericidal to stationary-phase cells of E. faecium HKLHS, and bacteriostatic to stationary-phase cells of Listeria innocua N27 [6]. Inhibition of pathogenic strains by plantaricin LR14 may find its vast applications in food, medicine, and veterinary whereas bacteriocin producing LAB could be evaluated for potential use as probiotics [14,18].
Kill-kinetics
Some of the pathogenic strains (two Gram-positive and two Gramnegative members) which were sensitive to plantaricin LR14, namely L. monocytogenes, Y. enterocolitica, B. licheniformis and urogenic E. coli were selected to study their time-bound and concentration based response in detail. With these strains, cell viability, log10 CFU/ ml was recorded at different time intervals up to 24h when treated with different concentrations of the bacteriocins. The results thus obtained are represented in Figure 1A-D. When 200 and 400 AU/ ml of bacteriocin were applied to ~7.0-8.0 log10 CFU/ml of four strains, the response was variable but inhibition pattern was similar. In all cases, no significant drop in the viability was seen at 4h, but a decrease was observable at 8h of exposure. When this was extended to 24h, the viability did not drop further significantly. Similar kind of time-bound loss in the viability of L. monocytogens has also been demonstrated by Kumar and Srivastava (2011) [19]. Moreover, the results indicated that the bactericidal effect of plantaricin LR14 is slow and concentration-dependent. Of all the four strains tested, urogenic E. coli appears to be most sensitive to plantaricin LR14. These results also suggested that the two concentrations (200 and 400 AU/ml) applied failed to inhibit the pathogenic strains completely. Moreover, 8h appear to be the time when maximum inhibition was discernible.
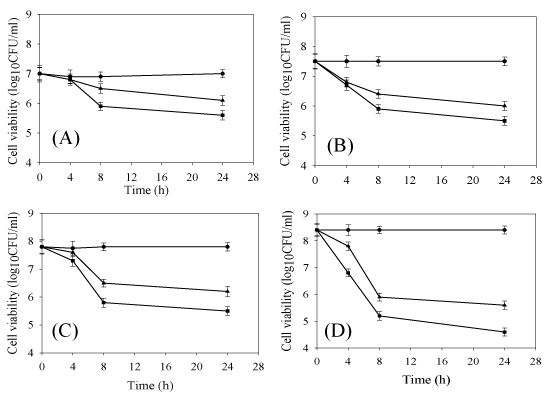
Figure 1: Effect of plantaricin LR14 on Listeria monocytogenes (A), Yersinia enterocolitica (B), Bacillus licheniformis (C) and urogenic E. coli (D) using two different concentrations of 200 (▲) and 400 (■) AU/ml at different time intervals and compared with untreated control (●).
Effect of higher concentrations of plantaricin LR14
In order to determine the complete loss of viability, higher concentrations (up to 1200 AU/ml) of plantaricin LR14 were further applied for treatment of target cells. When Y. enterocolitica was exposed further to higher concentrations (400, 800 and 1200 AU/ml), significant loss in viable count was recorded after 24h and drop was up to 2.2 and 1.5 log10 CFU/ml respectively from 7.9 log10 CFU/ml of untreated control (Figure 2). On the other hand, the same concentrations of plantaricin LR14 were able to inhibit L. monocytogenes up to 1.6 log10 CFU/ml and complete loss of viability, respectively. Bacillus licheniformis was able to grow only up to 2.2 log10 CFU/ml at 800 AU/ml and there was complete loss of viability recorded at the concentration of 1200 AU/ml while urogenic E. coli was not able to grow at these concentrations. This result is again in consistence with the above findings that E. coli is the most sensitive to plantaricin LR14. Thus, at higher concentration used (1200AU/ ml), Bacillus licheniformis and E. coli can be completely inhibited (Figure 2). Therefore, antimicrobial action of plantaricin LR14 is concentration-dependent and the effective concentration will have to be worked out for each pathogen under consideration for various biomedical and food-safety applications as also suggested for other bacteriocins [18,20],.

Figure 2: Effect of higher concentrations of plantaricin LR14 on the cell viability of the target bacteria.
Conclusions
Thus, the present investigation has identified a bacteriocinogenic protein, plantaricin LR14 with fairly broad antimicrobial-spectrum. Though, the list of bacteria tested by us is by no means an exhaustive one, it suggests that other potential pathogens can also be tested. The broad host-range of plantaricin LR14 and inhibition of Gramnegative bacteria could pave the way for its applications in foods and other clinical aspects as well. This natural alternative is envisaged to replace not only the artificial preservatives that may have many side effects, but also prove environmentally safe.
Acknowledgements
The facilities provided to the Department of Genetics, Delhi University by Department of Science and Technology, Government of India under FIST programme are thankfully acknowledged. The present investigation was financially supported by Council of Scientific and Industrial Research and Department of Biotechnology, New Delhi. SKT was supported by Indian Council of Medical Research, New Delhi, India.
References
- Nes IF, Diep DB, Håvarstein LS, Brurberg MB, Eijsink V, Holo H. Biosynthesis of bacteriocins in lactic acid bacteria. Antonie Van Leeuwenhoek. 1996; 70: 113-128.
- Abdel-Mohsein H, Yamamoto N, Otawa K, Tada C, Nakai Y. Isolation of bacteriocin-like substances producing bacteria from finished cattle-manure compost and activity evaluation against some food-borne pathogenic and spoilage bacteria. J Gen Appl Microbiol. 2010; 56: 151-161.
- Begde D, Bundale S, Mashitha P, Rudra J, Nashikkar N, Upadhyay A. Immunomodulatory efficacy of nisin--a bacterial lantibiotic peptide. J Pept Sci. 2011; 17: 438-444.
- Holo H, Nilssen O, Nes IF. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991; 173: 3879-3887.
- Muriana PM, Klaenhammer TR. Conjugal Transfer of Plasmid-Encoded Determinants for Bacteriocin Production and Immunity in Lactobacillus acidophilus 88. Appl Environ Microbiol. 1987; 53: 553-560.
- Jiménez-Díaz R, Rios-Sánchez RM, Desmazeaud M, Ruiz-Barba JL, Piard JC. Plantaricins S and T, Two New Bacteriocins Produced by Lactobacillus plantarum LPCO10 Isolated from a Green Olive Fermentation. Appl Environ Microbiol. 1993; 59: 1416-1424.
- Cintas LM, Casaus P, Fernández MF, Hernández, PE. Comparative antimicrobial activity of pediocin PA-1, enterocin L50, nisin A and lactocin S against spoilage and food-borne pathogenic bacteria. Food Microbiol. 1998; 15: 289-298.
- Bhunia AK, Johnson MC, Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988; 65: 261-268.
- Tiwari SK, Srivastava S. Characterization of a bacteriocin from Lactobacillus plantarum strain LR/14. Food Biotechnol. 2008; 22: 241-267.
- Kindoli S, Lee HA, Kim JH. Properties of Bac W42, a bacteriocin produced by Bacillus subtilis W42 isolated from Cheonggukjang. J Microbiol Biotechnol. 2012; 22: 1092-1100.
- Tiwari SK, Srivastava S. Statistical optimization of culture components for enhanced bacteriocin production by Lactobacillus plantarum LR/14. Food Biotechnol. 2008b; 22: 64-77.
- Tiwari SK, Srivastava S. Purification and characterization of plantaricin LR14: a novel bacteriocin produced by Lactobacillus plantarum LR/14. Appl Microbiol Biotechnol. 2008; 79: 759-767.
- Kumar M, Tiwari SK, Srivastava S. Purification and characterization of enterocin LR/6, a bacteriocin from Enterococcus faecium LR/6. Appl Biochem Biotechnol. 2010; 160: 40-49.
- Jimenez JJ, Borerro J, Diep DB, Gutiez L, Nes IF, Herranz C, et al. Cloning, production and functional expression of the bacteriocin sakacin A (SakA) and two SakA-derived chimeras in lactic acid bacteria (LAB) and the yeasts Pichia pastoris and Kluyveromyces lactis. J Ind Microbiol Biotechnol. 2013; 40: 977-993.
- Smaoui S, Elleuch L, Bejar W, Karray-Rebai I, Ayadi I, Jaouadi B, et al. Inhibition of fungi and gram-negative bacteria by bacteriocin BacTN635 produced by Lactobacillus plantarum sp. TN635. Appl Biochem Biotechnol. 2010; 162: 1132-1146.
- Kumar M, Srivastava S. Effect of calcium and magnesium on the antimicrobial action of enterocin LR/6 produced by Enterococcus faecium LR/6. Int J Antimicrob Agents. 2011; 37: 572-575.
- Hernández D, Cardell E, Zárate V. Antimicrobial activity of lactic acid bacteria isolated from Tenerife cheese: initial characterization of plantaricin TF711, a bacteriocin-like substance produced by Lactobacillus plantarum TF711. J Appl Microbiol. 2005; 99: 77-84.
- Montalbán-López M, Sánchez-Hidalgo M, Valdivia E, Martínez-Bueno M, Maqueda M. Are bacteriocins underexploited? Novel applications for old antimicrobials. Curr Pharm Biotechnol. 2011; 12: 1205-1220.
- Kumar M, Srivastava S. Antilisterial activity of a broad-spectrum bacteriocin, enterocin LR/6 from Enterococcus faecium LR/6. Appl Biochem Biotechnol. 2010; 162: 698-706.
- Kaur B, Balgir PP, Mittu B, Kumar B, Garg N. Biomedical applications of fermenticin HV6b isolated from Lactobacillus fermentum HV6b MTCC10770. Biomed Res Int. 2013: 168438.