
Research Article
Austin J Anal Pharm Chem. 2015;2(2): 1038.
Simultaneous Analysis of Honokiol and Magnolol in Rat Serum by HPLC with Fluorescence Detection after Solidphase Extraction for Pharmacokinetic Studies
Yasuhiko Higashi*
Department of Analytical Chemistry, Faculty of Pharmaceutical Sciences, Hokuriku University, Japan
*Corresponding author: Yasuhiko Higashi, Department of Analytical Chemistry, Faculty of Pharmaceutical Sciences, Hokuriku University, Kanagawa-machi, Japan.
Received: April 03, 2015; Accepted: April 22, 2015; Published: April 24, 2015
Abstract
The author and co-workers previously reported an HPLC method coupled with fluorescence detection (FL) for determination of honokiol and magnolol, and showed that Hange-koboku-to dried extract granules (1.00 g) contained honokiol (3.30 mg) and magnolol (5.40 mg). In this study, the author modified the previous method to make it suitable for simultaneous HPLC-FL analysis of honokiol and magnolol in rat serum. Solid-phase extraction with a Bond Elut-C18 cartridge was carried out for pretreatment of rat serum. 1,1-Bis(4- hydroxyphenyl)cyclohexane was utilized as an internal standard. The mobile phase was prepared by the addition of methanol (750 mL) to a solution of trifluoroacetic acid (0.1 v/v%) in 250 mL of water. The regression equations for honokiol and magnolol in rat serum showed good linearity in the range of 0.1 to 2.5 Μg/mL with lower limits of detection (signal-to-noise ratio of 3) of 0.011 and 0.014 Μg/mL, respectively. The coefficient of variation of the assay and the extraction ratios of honokiol and magnolol by the pretreatment were satisfactory. Magnolol was determined in rat serum after a single p.o. administration of the granules (18 mg/kg as honokiol and 30 mg/kg as magnolol) to rats and its pharmacokinetic parameters were estimated. The level of honokiol in the serum was less than the lower limit of quantification.
Keywords: HPLC; Fluorescence detection; Magnolol; Honokiol; Solidphase extraction; Pharmacokinetic study
Abbreviations
HPLC: High-performance Liquid Chromatography; FL: Fluorescence Detection; UV: Ultraviolet Absorption Detector; MS: Mass Spectrometry; CZE: Capillary Zone Electrophoresis; ECD: Electrochemical Detection; LIF: Laser-induced Fluorescence; IS: Internal Standard; p.o.: Per Orally; AUC: Area Under the Serum Concentration-time Curve; Cmax: Peak Serum Concentration; Tmax: Time to Reach Cmax
Introduction
The bark of Magnoliae Cortex has been used in traditional Chinese medicine for the treatment of thrombotic stroke, typhoid fever, and headache [1]. Two constituents of Magnoliae Cortex, honokiol and magnolol, were reported to produce sedation, ataxia, muscle relaxation and loss of the righting reflex in rats [2], and they also improved learning and memory impairment [3]. Honokiol activates extracellular signal-regulated kinases to promote neurite outgrowth [4], while magnolol has anti-inflammatory and analgesic effects, and ameliorates diabetic nephropathy [5,6].
In connection with pharmacokinetic studies of honokiol and magnolol and quality evaluation of Magnoliae Cortex, several methods are available for determination of honokiol and/or magnolol, including HPLC-ultraviolet absorption detector (UV) [7-11], HPLCmass spectrometry (MS) [12,13], capillary zone electrophoresis (CZE) with UV, electrochemical detection (ECD) or laser-induced fluorescence (LIF) detection [14-16], and high-capacity high-speed counter-current chromatography [17]. Further, a recently reported ΜHPLC-ECD method exhibited very high sensitivity (0.13 pg for honokiol and magnolol) for determining honokiol and magnolol contents in Magnolia Bark [18]. An HPLC method with fluorescence detection (FL), using honokiol as an internal standard (IS), has been described for magnolol determination, and it was applied for a pharmacokinetic study of magnolol in rats [19]. Pretreatment of plasma samples was performed by precipitation with acetonitrile [19]. However, injection of such a sample leads to degradation of the analytical column. In addition, the tissue distribution and bioavailability of magnolol were investigated in Sprague-Dawley rats by HPLC-UV with the lower limit of detection of 0.06 Μg/mL [20]. But, this procedure required liquid-liquid extraction of serum samples with ethyl acetate [20], which is unsuitable for routine application. We considered that pretreatment using solid-phase extraction would be advantageous.
Hange-koboku-to dried extract granules (3.75 g) consist of a mixture of a half extract (1.25 g) from five medical herbs (Japanese Pharmacopoeia requirements: Pinelliae Tuber, 3.0 g; Polia, 2.5 g; Magnoliae Cortex, 1.5 g; Perillae Herba, 1.0 g; Zingiberis Rhizoma, 0.5 g) and three additives (2.5 g, magnesium stearate, lactose hydrate, sucrose fatty acid ester). Recently, the author and co-workers previously developed HPLC-FL method to show that Hange-kobokuto dried extract granules (1.00 g) contain honokiol (3.30 mg) and magnolol (5.40 mg) [21]. In the present study, the author developed a modification of that method for simultaneous HPLC-FL analysis of honokiol and magnolol in rat serum, using solid-phase extraction for pretreatment of rat serum. 1,1-Bis(4-hydroxyphenyl)cyclohexane as an IS was used, since it resemble honokiol and magnolol in chemical structure and it was reported to be well separated from peaks of honokiol and magnolol [21]. The developed method was used for pharmacokinetic studies in rats after a single p.o. administration of Hange-koboku-to dried extract granules.
Material and Methods
Reagents
Honokiol and magnolol were purchased from Nacalai tesque (Kyoto, Japan). 1,1-Bis(4-hydroxyphenyl)cyclohexane was purchased from Tokyo Chemical Industry Co. (Tokyo, Japan). Their chemical structures are shown in Figure 1. Hange-koboku-to dried extract granules (Lot No. BD4131, Tsumura & Co., Tokyo) were obtained from a pharmaceutical market. Methanol and general reagents were obtained from Wako Pure Chemical Industries (Osaka, Japan). A Milli-Q water purification system (Millipore Corp., Bedford, MA, U.S.A.) was used to obtain water for HPLC use.
Figure 1: Molecular structures of honokiol, magnolol, and IS.
Equipment
The HPLC system comprised a model L-6200 pump (Hitachi), a Rheodyne injection valve (Cotati, CA, U.S.A.) with a 100-ΜL loop and a model RF-10A fluorometer (Shimadzu, Kyoto, Japan). The fluorometer was operated at an excitation wavelength of 275 and an emission wavelength of 315 nm up to 6.7 min, then switched to excitation and emission wavelengths of 304 and 340, respectively, until 10.1 min. After 10.1 min, the emission wavelength was changed to 405 nm. The columns (Nacalai tesque) were 150 mm × 4.6 mm i.d. C18-MS-II (Nacalai tesque) with 5 Μm particles. Quantification of the peaks was performed with a Chromatopac Model C-R3A integrator (Shimadzu). The mobile phase was prepared by the addition of methanol (750 mL) to a solution of trifluoroacetic acid (0.1 v/v %) in 250 mL of water. The samples were eluted from the column at room temperature at a flow rate of 1.0 mL/min.
Calibration curves for honokiol and magnolol in rat serum and solid-phase extraction
Standard honokiol and magnolol solutions (each 50 Μg/mL) were prepared in methanol and stored at -18oC. Equal volumes of the two solutions were mixed and diluted with water to obtain a series of working solutions (0, 0.1, 0.25, 0.5, 1, and 2.5 Μg/mL). Aliquots of 100 ΜL of blank rat serum were spiked with 100 ΜL of standard mixture (0, 0.1, 0.25, 0.5, 1, and 2.5 Μg/mL), 100 ΜL of 1,1-bis(4-hydroxyphenyl) cyclohexane as an IS (2 Μg/mL), and 20 ΜL of 85% o-phosphoric acid. The samples were vortexed vigorously for 30 s and loaded onto Bond Elut-C18 cartridges (VARIAN, 100 mg, 1 mL) conditioned with 1 mL of methanol and 1 mL of water. After addition of 1 mL of 5% methanol, compounds of interest were eluted with 1 mL of methanol. After evaporation under vacuum with a concentrator (TC-8, TAITEC Corp., Japan), residues were reconstituted by addition of 300 ΜL of mobile phase, and 100 ΜL samples were directly injected onto the HPLC system.
Extraction ratio of honokiol and magnolol
The two standard solutions (0.1 and 1 Μg/mL, 100 ΜL) and IS solution (2 Μg/mL, 100 ΜL) were mixed with water (100 ΜL, control) or rat serum (100 ΜL). After pretreatment of rat serum as described above, extraction ratios were calculated as percentage ratios of honokiol or magnolol peak areas to the IS peak area in serum sample with respect to the ratios in the control.
Animal study
Male Wistar rats (9-10 weeks, 255-272 g of weight volume) were obtained from Sankyo Laboratory Animals (Toyama, Japan) and treated in accordance with the guidelines of the Institutional Animal Care and Use Committee of Hokuriku University. Hange-kobokuto dried extract granules (1.5 g/head, i.e., 18 mg/kg as honokiol and 30 mg/kg as magnolol) were p.o. administered to rats after having been suspended in water (3 mL). Rats were fasted for 12 h prior to the administration, while water was freely available. Under light anesthesia with diethyl ether, blood samples (0.3 mL) were withdrawn from the jugular vein at the designated time intervals (0.5, 1, 2, 4, 6, 8, and 12 h) via a separate venous puncture. Blood samples were allowed to clot, then centrifuged (3,000 × g, 10 min) to obtain the serum. Drug-free pooled serum was similarly obtained from rats.
Pharmacokinetic and Statistical Analysis
The area under the serum concentration-time curve from zero to 8 h (AUC0→8) was calculated using the linear trapezoidal rule. The peak serum concentration (Cmax) and the time to reach Cmax (Tmax) were determined from the actual data obtained after a single p.o. administration. Data are expressed as the mean ± S.D, (n=5).
Results and Discussion
Figure 2 shows typical chromatograms of a rat serum sample spiked with standard honokiol and magnolol (each 0.5 Μg/mL) and IS (2 Μg/mL) (A) and a rat serum sample at 2 h after a single p.o. administration of the granules to rats (B). The retention times of IS, honokiol, and magnolol were 5.6, 7.2, and 10.7 min, respectively.
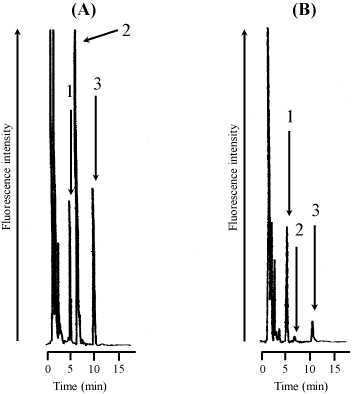
Figure 2: Typical chromatograms of rat serum spiked with standard (A) and
rat serum after a single p.o. administration of Hange-koboku-to dried extract
granules to rats (B).Chromatograms: (A), rat serum spiked with 2 Μg/mL of IS
and 0.5 Μg/mL of honokiol and magnolol; (B), rat serum sample at 2 h after
administration spiked with IS. Peaks:1, IS; 2, honokiol; 3, magnolol. Hangekoboku-
to dried extract granules were administered to rats (1.5 g/head, i.e.,
18 mg/kg as honokiol and 30 mg/kg and magnolol).
Standard curves were constructed by plotting integrated peak areas vs. honokiol and magnolol concentration. Linear relationships were obtained for honokiol (y = 7.333x + 0.040, in the concentration range from 0.1 to 2.5 Μg/mL, r2 = 0.9940) and for magnolol (y = 2.953x + 0.019, in the concentration range from 0.1 to 2.5 Μg/mL, r2 = 0.9930).
The lower limits of quantification for honokiol and magnolol were 0.037 Μg/mL (absolute amount of 1.2 ng) and 0.047 Μg/mL (absolute amount of 1.5 ng), respectively (signal-to-noise ratio of 10:1). The linearity from quantification limit to 0.1Μg/mL is as follows. Linear relationships were obtained for honokiol (y = 7.640x + 0.008, in the concentration range from 0.037 to 0.1 Μg/mL, r2 = 0.9893) and for magnolol (y = 3.135x + 0.005, in the concentration range from 0.047 to 0.1 Μg/mL, r2 = 0.9890). The lower limits of detection for honokiol and magnolol were 0.011 Μg/mL (absolute amount of 0.37 ng) and 0.014 Μg/mL (absolute amount of 0.44 ng), respectively (signal-tonoise ratio of 3:1). Table 1 summarizes the lower limits of detection and quantification of methods previously reported for honokiol and magnolol assay. The ΜHPLC-ECD method of Kotani et al. is one of the most sensitive, with detection limits for honokiol and magnolol of 0.13 pg [18]. The sensitivity of the method presented here is slightly poorer than that of our previous method [21], because of minor interference in blank rat serum and slight loss of analyte during pretreatment of rat serum with solid-phase extraction. While the detection limits of honokiol and magnolol in the present assay were 4.3- to 31-fold improved compared with several results [7,10,16,20], it was 55- to 140-fold inferior previous report [13].
Analytical methods
Quantification limit (mg/mL)
Detection limit (mg/mRL)
eferences
Honokiol
Magnolol
Honokiol
Magnolol
HPLC-FL (Absolute amount)
0.037 (1.2 ng)
0.047 (1.5 ng)
0.011 (0.37 ng)
0.014 (0.43 ng)
Present method
HPLC-FL (Absolute amount)
0.021 (1.1 ng)
0.028 (1.4 ng)
0.0047 (0.24 ng)
0.0061 (0.32 ng)
[21]
HPLC-FL
Not described
Not described
[19]
mHPLC-ECD (Absolute amount)
(0.67 pg)
(0.67 pg)
(0.13 pg)
(0.13 pg)
[18]
CZE-LIF
Not described
Not described
[15]
CZE-UV
Not described
Not described
[15]
CE-ECD
0.266
0.266
0.101
0.136
[16]
HPLC-MS
0.0025
0.0025
Not described
[12]
HPLC-MS
0.0005
0.0002
0.0002
0.0001
[13]
HPLC-UV
Not described
0.13
Not described
0.06
[20]
HPLC-UV
0.013
0.025
Not described
[11]
HPLC-UV
0.32
0.88
0.16
0.44
[10]
HPLC-UV
0.81
1.03
0.25
0.23
[7]
Table 1: Method comparisons for simultaneous determination of honokiol and magnolol in rat serum.
Judging from these data, it is considered that present assay will be relatively superior in terms of sensitivity.
The solid-phase extraction ratios of six solutions containing honokiol and magnolol (each 0.1 and 1Μg/mL in rat serum) were calculated by comparison with standard solutions (Table 2). The mean ratios at 0.1 and 1 Μg/mL of honokiol were 96.1 and 101.5%, respectively. The mean ratios at 0.1 and 1 Μg/mL of magnolol were 95.2 and 98.5, respectively. The values of relative standard deviation (R.S.D.) were less than 9.2%. Previous methods have utilized the denatured protein precipitation by acetonitrile for pretreatment of biological samples instead of solid-phase extraction [19,20]. These results suggest that the tested Bond Elut-C18 cartridge is simple and appropriate for solid-phase extraction of honokiol and magnolol from rat serum.
Concentration in rat serum
Compounds
0.1 mg/mL
1 mg/mL
96.1±8.5
Honokiol
Mean±S.D. (%, n=6)
101.5±7.8
8.8
R.S.D. (%)
7.7
95.2±8.8
Magnolol
Mean±S.D. (%, n=6)
98.5±8.4
9.2
R.S.D. (%)
8.5
Table 2: Extraction ratios of honokiol and magnolol in rat serum after pretreatment with Bond Elut-C18 cartridges.
Precision and accuracy for intra-day and inter-day assays of honokiol and magnolol are shown in Table 3. In the intra- and interday assays, the range of standard deviation of the mean for honokiol and magnolol was within 2.7 to 10.1%. The recoveries of honokiol and magnolol were within 88.1 to 109.0%. The assay exhibited satisfactory precision and accuracy.
Compounds
Concentration (mg/mL)
Found (mg/mL), Mean±S.D. (n=6)
C.V. (%)
Recovery values (%)
Honokiol
Intra-day assay
0.1
0.0910±0.0070
7.7
91.0
1.0
1.04±0.05
4.8
104.0
2.5
2.57±0.07
2.7
102.8
Inter-day assay
0.1
0.109±0.011
10.1
109.0
1.0
1.02±0.07
6.9
102.0
2.5
2.44±0.07
2.9
97.6
Magnolol
Intra-day assay
0.1
0.0881±0.0078
8.9
88.1
1.0
1.03±0.03
2.9
103.0
2.5
2.72±0.15
5.5
108.8
Inter-day assay
0.1
0.102±0.010
9.8
102.0
1.0
0.975±0.082
8.4
97.5
2.5
2.59±0.19
7.3
103.6
Table 3: Intra- and inter-day assay reproducibility of honokiol and magnolol in rat serum.
The present method was used to analyze serum samples after a single p.o. administration of Hange-koboku-to dried extract granules to rats (1.5 g/head, i.e., 18 mg/kg as honokiol and 30 mg/kg as magnolol, n=5). As shown in Figure 3, the serum concentration vs. time profile of magnolol was constructed for up to 8 h. The serum level of magnolol was less than lower limit of quantification at 0.5 and 12 h after administration. Therefore, the serum level of magnolol was estimated from 1 to 8 h after administration. The values of AUC0→8, Cmax, and Tmax were 0.686 ± 0.113 Μg×h/mL, 0.119 ± 0.014 Μg/mL, and 1.60 ± 0.49 h, respectively (mean ± S.D., n=5). Lin et al. reported the disposition kinetics of magnolol in rats orally given a single dose of magnolol (50 mg/kg), obtaining the value of AUC0→8 of 228.5 nmol×min/mL (1.01 Μg×h/mL) [20]. The value of AUC0→8 from our data (a single dose of 30 mg/kg of magnolol) was 0.686 Μg×h/mL. The dose ratio is nearly the same as the ratio of AUC values, supporting the validity of our method. On the other hand, the honokiol concentration from 0 to 12 h after the administration was below the lower limit of quantification. Böhmdorfer et al. reported very high hepatic extraction ratio and clearance of honokiol (0.99 ± 0.01 and 35.8 ± 0.04 mL/min, respectively), and very low availability (0.007 ± 0.001, ratio of excreted amount of honokiol to administered amount of honokiol in rat perfused liver) in rat perfused liver [22], indicating that the intestinal absorption of honokiol is poor in rats (i.e., there is a very large first-pass effect). The finding that serum honokiol concentration after oral administration was less than the quantification limit is consistent with their result in rats.
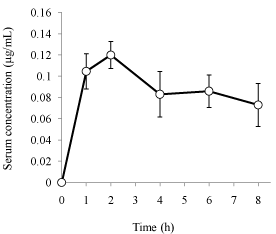
Figure 3: Serum concentration-time curve of magnolol after a single p.o.
administration of Hange-koboku-to dried extract granules to rats. Hangekoboku-
to dried extract granules were administered to rats (1.5 g/head,
i.e., 18 and 30 mg/kg as honokiol and magnolol, respectively). Each point
represents the mean ± S.D. (n=5). Honokiol levels in rat serum from 1 to 8 h
after the administration were less than the lower limit of quantification.
Conclusion
The author has modified previously developed HPLC-FL method to make it suitable for simultaneous determination of honokiol and magnolol in rat plasma after oral administration of Hange-koboku-to dried extract granules. A simple pretreatment of rat serum by means of solid-phase extraction with a Bond Elut-C18 cartridge was effective. While the extraction ratio is satisfactory and the sensitivity is only moderate in comparison with previous methods, present method is suitable for pharmacokinetic studies of magnolol after administration of Hange-koboku-to dried extract granules to rats. However, honokiol concentrations were below the lower limit of quantification in this study. Further work is needed to improve the sensitivity of honokiol detection in order to clarify the honokiol disposition kinetics.
References
- Jiangsu New Medicine College. In Dictionary of Chinese Materia Medica, Shanghai Scientific and Technological Publishers, Shanghai, 1985; 1628-1630.
- Watanabe K, Watanabe H, Goto Y, Yamaguchi M, Yamamoto N, Hagino K. Pharmacological properties of magnolol and honokiol extracted from Magnolia officinalis: central depressant effects. Planta Med. 1983; 49: 103-108.
- Matsui N, Takahashi K, Takeichi M, Kuroshita T, Noguchi K, Yamazaki K, et al. Magnolol and honokiol prevent learning and memory impairment and cholinergic deficit in SAMP8 mice. Brain Res. 2009; 1305: 108-117.
- Zhai H, Nakade K, Oda M, Mitsumoto Y, Akagi M, Sakurai J, et al. Honokiol-induced neurite outgrowth promotion depends on activation of extracellular signal-regulated kinases (ERK1/2). Eur J Pharmacol. 2005; 516: 112-117.
- Lee MM, Huang HM, Hsieh MT, Chen CS, Yeh FT, Kuo JS. Anti-inflammatory and neuroprotective effects of magnolol in chemical hypoxia in rat cultured cortical cells in hypoglycemic media. Chin J Physiol. 2000; 43: 61-67.
- Sohn EJ, Kim CS, Kim YS, Jung DH, Jang DS, Lee YM, et al. Effects of magnolol (5,5'-diallyl-2,2'-dihydroxybiphenyl) on diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. Life Sci. 2007; 80: 468-475.
- Tang W, Wan M, Zhu Z, Chen G, Huang X. Simultaneous determination of eight major bioactive compounds in Dachengqi Tang (DT) by high-performance liquid chromatography. Chin Med. 2008; 3: 5.
- Li K, Yuan J, Su W. Determination of liquiritin, naringin, hesperidin, thymol, imperatorin, honokiol, isoimperatorin, and magnolol in the traditional Chinese medicinal preparation Huoxiang-Zhengqi liquid using high-performance liquid chromatography. Yakugaku Zasshi 2006; 126: 1185-1190.
- Chan SS, Zhao M, Lao L, Fong HH, Che CT. Magnolol and honokiol account for the anti-spasmodic effect of Magnolia officinalis in isolated guinea pig ileum. Planta Med. 2008; 74: 381-384.
- Zhang H, Chen S, Qin F, Huang X, Ren P, Gu X. Simultaneous determination of 12 chemical constituents in the traditional Chinese medicinal prescription Xiao-Yao-San-Jia-Wei by HPLC coupled with photodiode array detection. J Pharm Biomed Anal. 2008; 48: 1462-1466.
- Wu X, Chen X, Hu Z. High-performance liquid chromatographic method for simultaneous determination of honokiol and magnolol in rat plasma. Talanta. 2003; 59: 115-121.
- Wu YT, Lin LC, Tsai TH. Simultaneous determination of honokiol and magnolol in Magnolia officinalis by liquid chromatography with tandem mass spectrometric detection. Biomed Chromatogr. 2006; 20: 1076-1081.
- Xu F, Liu Y, Zhang Z, Song R, Dong H, Tian Y. Rapid simultaneous quantification of five active constituents in rat plasma by high-performance liquid chromatography/tandem mass spectrometry after oral administration of Da-Cheng-Qi decoction. J Pharm Biomed Anal. 2008; 47: 586-595.
- Yao X, Xu X, Yang P, Chen G. Carbon nanotube/poly(methyl methacrylate) composite electrode for capillary electrophoretic measurement of honokiol and magnolol in Cortex Magnoliae Officinalis. Electrophoresis. 2006; 27: 3233-3242.
- Chen CL, Chang PL, Lee SS, Peng FC, Kuo CH, Chang HT. Analysis of magnolol and honokiol in biological fluids by capillary zone electrophoresis. J Chromatogr A. 2007; 1142: 240-244.
- Chen G, Xu X, Zhu Y, Zhang L, Yang P. Determination of honokiol and magnolol in cortex Magnoliae Officinalis by capillary electrophoresis with electrochemical detection. J Pharm Biomed Anal. 2006; 41: 1479-1484.
- Chen L, Zhang Q, Yang G, Fan L, Tang J, Garrard I, et al. Rapid purification and scale-up of honokiol and magnolol using high-capacity high-speed counter-current chromatography. J Chromatogr A. 2007; 1142: 115-122.
- Kotani A, Kojima S, Hakamata H, Jin D, Kusu F. Determination of honokiol and magnolol by micro HPLC with electrochemical detection and its application to the distribution analysis in branches and leaves of Magnolia obovata. Chem Pharm Bull (Tokyo). 2005; 53: 319-322.
- Tsai TH, Chou CJ, Chen CF. Glucuronidation of magnolol assessed using HPLC/fluorescence. Planta Med. 1995; 61: 491-492.
- Lin SP, Tsai SY, Lee Chao PD, Chen YC, Hou YC. Pharmacokinetics, bioavailability, and tissue distribution of magnolol following single and repeated dosing of magnolol to rats. Planta Med. 2011; 77: 1800-1805.
- Higashi Y, Liu J, Fujii Y. High-performance liquid chromatography coupled with fluorescence detection for simultaneous determination of honokiol and magnolol in Hange-koboku-to dried extract granules. J Liq Chromatogr & Rel Technol. 2012; 35: 321-330.
- Böhmdorfer M, Maier-Salamon A, Taferner B, Reznicek G, Thalhammer T, Hering S, et al. In vitro metabolism and disposition of honokiol in rat and human livers. J Pharm Sci. 2011; 100: 3506-3516.