
Special Article - Surface Chemistry
Austin J Anal Pharm Chem. 2015;2(3): 1040.
Electrochemical Behaviour of Selected β-Amino Alcohols and Amino Acids
Serifi O1,2*, Tsopelas F1, Ochsenkühn- Petropoulou M1, Detsi A2
1Laboratory of Inorganic and Analytical Chemistry, School of Chemical Engineering, National Technical University of Athens, Greece
2Laboratory of Organic Chemistry, School of Chemical Engineering, National Technical University of Athens, Greece
*Corresponding author: Serifi O, Laboratory of Inorganic and Analytical Chemistry, School of Chemical Engineering, National Technical University of Athens, Zografou Campus, Greece.
Received: March 29, 2015; Accepted: April 27, 2015; Published: April 30, 2015
Abstract
In the present work, the electrochemical behaviour of a series of 17 commercially available compounds was investigated at a glassy carbon working electrode under different conditions. The effect of specific and non–specific solute–solvent interactions affecting the oxidation potentials of the investigated compounds was confirmed by employing Modified Linear Solvation Energy Relationship (LSER) analysis. The role of ionization to the electrochemical oxidation of the investigated β–amino alcohols and their derivatives was also established, using oxidation potentials obtained at the pH values of 4.0, 7.4, 9.0 and 11.0. In all cases, a linear decrease of oxidation potential by increasing the pH was observed. Chronoamperometric signal of 300, 500 and 800 mV and radical scavenging activity towards DPPH of the compounds were further employed as additional measurements for the evaluation of their antioxidant profile. The obtained electroanalytical data imply that the β–amino alcohols, amino acids and a biogenic amine studied in this work possess moderate to strong antioxidant properties.
Keywords: β– Amino alcohols; Amino acids; Antioxidant activity; Voltammetry; Oxidation potentials; DPPH; Radical scavenging
Introduction
?ntioxidant activity of novel bioactive compounds is of paramount importance in medicinal chemistry. Indeed, unbalance between pro–oxidants and antioxidants in profit of pro–oxidants causes elevated oxidative stress. Pro–oxidants are able to readily damage biological molecules [1]. In addition, a pro–oxidant can be an oxidant of pathological importance [2]. Even though pro–oxidants are considered as harmful molecules [2], they can play a beneficial role as signal transduction messenger cells to mediate signal transduction. In addition, they have the physiological function in the non–phagocytic cells to mediate signal transduction of some growth factors and cytokines to achieve certain cellular function, named as “redox signaling”. Pro–oxidants are also considered as powerful oxidative stress biomarkers and they have been shown in many cases as cancer chemo–preventive agents [3, 4].
The β-amino alcohol moiety is a common structural component in a vast group of naturally occurring and synthetic molecules possessing a wide range of biological activities [5]. Especially, β-amino alcohols have been widely reported to act as chemical indicators stimulating the plant self-defence mechanism against oxidative stress, while they can act as pro oxidants under certain conditions [6-13]. Oxidative stress has been implicated in the pathogenesis of several human diseases, such as atherosclerosis, diabetes mellitus, chronic inflammation, neurodegenerative disorders and certain types of cancer [14].
Antioxidant activity of bioactive species can be studied by means of electrochemical parameters. Electroanalytical data can be correlated with those derived from antioxidant protocols [15], because redox behaviour of an antioxidant at an electrode is related to its behaviour in chemical redox reaction with a radical [16]. In particular, oxidation potentials provide an estimation of the energy required for a compound to donate an electron. Indeed, the more negative oxidization potential is, the more easily the compound will donate an electron and the higher its expected antioxidant activity [17, 18]. The determination of the oxidation potential and, generally, the investigation of the electrochemical behaviour of a compound can easily be performed by applying cyclic voltammetry. The latter has been considered in recent years as a conventional methodology for evaluating the antioxidant capacity of human and horse plasma, animal tissues, edible plants, wines and different types of tea and coffees [15, 16, 19]. Another electroanalytical technique for the evaluation of antioxidant profile of bioactive species is chronoamperometry, whose results can be correlated with antioxidant protocols such as the evaluation of the scavenging ability against stable free radicals like 1,1- diphenyl-2-dipicrylhydrazyl (DPPH) [20, 21]. Electroanalytical studies of β-amino alcohols and amino acids [22] can possibly contribute to the development of a useful measure for the evaluation of their biological properties such as antioxidant capacity.
In the present work, the electrochemical behaviour of a series of commercially available amino-compounds and their derivatives was investigated under different conditions. In order to study the parameters affecting the oxidation potential of the investigated compounds in physiological conditions, Modified Linear Solvation Energy Relationships (LSER) analysis was employed. The effect of pH on the oxidation potentials was also established. The chronoamperometric signal of the compounds under investigation at oxidation potentials of 300, 500 and 800 mV was also determined. The antioxidant activity of selected compounds was evaluated in vitro by measuring their ability to scavenge the stable free radical 1,1- diphenyl-2-dipicrylhydrazyl (DPPH).
Experimental
Instrumentation
Instrumentation The electrochemical investigation of the β-amino alcohols and their derivatives was performed using the polarograph 797 VA Computrace (Metrohm). As working electrode, a glassy carbon (GC) was employed. The reference electrode was an Ag/AgCl one, filled with 3M KCl (≥99.5% p.a., Merck) in High Purity Water (HPW) supplied by an EASYpure II (Model D 7381, Barnsted International) water purification system, and the auxiliary a Pt electrode. Working electrode was polished at the beginning of each measurement with alumina powder (0.3 mm) for 3min, using a polishing cloth, and it was rinsed with deionized water and ethanol. Furthermore, in the end of each working day, it was sonicated firstly for 5 min in distilled water and, secondly, for another 5 min in acetone. The condition of the electrode was frequently checked using a 0.1M solution of Fe(CN)6 4-. The determination of the free-radical scavenging capacity was evaluated with the stable radical 2,2-diphenyl-1-picrylhydrazyl (DPPH•). A spectrophotometer (Helios Unicam, USA) was used to measure the absorbance at 517 nm where the delocalisation gives rise to the deep violet colour, characterised by an absorption band in methanol solution.
Reagents
All reagents were of analytical grade. L-DOPA (≥98%, Sigma- Aldrich), dopamine hydrochloride (99%, Alfa Aesar), L-tyrosine hydrochloride (99%, Alfa Aesar), tyrosol (≥ 99.5%, FlukaChemika), tyramine (97%, Acros Organics), serinol (≥ 98%, Sigma-Aldrich), L-serine (≥ 98%, Sigma-Aldrich), Boc-tyrosine (≥ 98%, Alfa Aesar), ethanolamine (≥ 98%, Sigma-Aldrich), 2-amino-2-methyl-1- propanol (≥ 90%, Sigma-Aldrich), 2-amino-1-phenylethanol (≥ 98%, Sigma-Aldrich), 2-amino-3- phenyl-1- propanol (≥ 98%, Sigma-Aldrich), L-phenylalanine (≥ 98%, Sigma-Aldrich), L-cysteine (≥ 99%, FlukaChemika), 2-(methylamino)ethanol (≥ 98%, Sigma- Aldrich), (S)-(+)-2-amino-1-propanol (≥ 98%, Sigma-Aldrich) and norepinephrine (≥ 98%, Sigma-Aldrich) were the compounds under investigation. Their structures are presented in Table 1. L(+) ascorbic acid (≥99.7%, Carlo-Erba) was used as the reference compound.
: Structural formula of the investigated compounds.
Buffer solutions were prepared by dissolution of the appropriate amounts of CH3COOH (Merck), CH3COONa ( =99% Sigma Aldrich), NaH2PO4 ? H2O ( ≥98% Sigma Aldrich), Na2HPO4 (Acros Organics), HCl (37% Fluca), Na2CO3 (Acros Organics), NaHCO3 (Acros Organics) in HPW. DPPH (1, 1-diphenyl, 2-picrylhydrazyl) was obtained by Sigma-Aldrich and methanol used for the assay was of analytical grade (Fisher Scientific).
Electrochemical studies
All studies were carried out in room temperature (22±1°C). The electrochemical investigation, involving cyclic voltammetry and chronoamperometry was performed by spiking micro-amounts of the compounds under investigation in different buffers in a 10 mL Metrohm polarographic cell. The final concentration of the investigated species in the polarographic cell was about 1.0 mM. As buffers, the following solutions were tested:
(A) CH3COOH / CH3COONa, pH=4.0
(B) ?aH2PO4 / Na2HPO4, pH=7.4
(C) Na2HPO4 adjusted at pH=9.0 with HCl
(D) Na2CO3 / NaHCO3, pH=11.0
Cyclic voltammograms were recorded between -400 and 1200 mV with a scanning rate of 20 mVs-1, while for chronoamperometric measurements, the potentials of 300, 500 and 800 mV were selected. All measurements were carried out at least in triplicate, and the mean value was considered.
Solvation properties
Solvation parameters were taken from the module Absolv, included in ADME Boxes version 3.0 software (Pharma Algorithms) and they are presented in Table S1, in supplementary material. These parameters involve the hydrogen-bond acidity (A), the hydrogenbond basicity (B), the dipolarity/ polarizability (S), the excess molar refraction (E) and the McGowan molecular volume (V).
Compound
A
B
S
E
V
1
1.56
1.44
1.77
1.33
1.43
2
0.98
1.08
1.35
1.15
1.22
3
1.28
1.29
1.60
1.18
1.37
4
0.81
0.67
1.12
1.01
1.12
5
0.71
0.94
1.17
1.01
1.16
6
0.74
1.29
0.96
0.64
0.75
7
1.03
1.30
1.15
0.60
0.76
8
1.28
1.33
1.93
1.22
2.15
9
0.46
0.94
0.72
0.42
0.55
10
0.46
0.98
0.66
0.40
0.83
11
0.46
1.19
1.10
1.03
1.16
12
0.46
1.06
1.17
1.00
1.30
13
0.78
1.02
1.39
0.95
1.31
14
0.78
1.05
1.12
0.83
0.87
15
0.38
0.84
0.58
0.37
0.69
16
0.46
0.97
0.71
0.43
0.69
17
1.23
1.61
1.49
1.40
1.27
Table S1: The Abraham descriptors of the investigated compounds.
Statistical analysis
Linear regression analysis was performed using the Statistica- Axa 7.0 software package (StatSoft, Tulsa, OK, USA). The following statistic data were provided for the obtained equations: N is the number of data points, s is the standard deviation, R is the correlation coefficient, R2 is the adjusted square of the correlation coefficient and F is the Fisher test, calculated for a significance level of 0.05.
Antioxidant protocol – DPPH assay
The DPPH scavenging assay was performed according to Brand- Williams et al. (1995) [23, 24]. Briefly, 0.1 mL of appropriately diluted samples was added to 3.9 mL of DPPH• (3.0 mg/100 ml) in methanol in the spectrophotometric cuvette. After stirring vigorously for 30 s, the absorbance at the wavelength of 517 nm was recorded at 20 and 60 minutes. The changes in absorbance were measured at 25 °C. The percent radical scavenging was calculated as follows:
% Scavenging= 100 *(A0 – At)/ A0
Where A0 is the initial DPPH absorbance and At is the absorbance at 20 min or 60 min of reaction. IC50 (the half maximal inhibitory concentration of the substance that produces 50% scavenging) was extrapolated from the plot of the percent radical scavenging versus the substance concentration, obtained for the different substrates.
Results and Discussion
Electrochemical study at physiological conditions (pH 7.4)
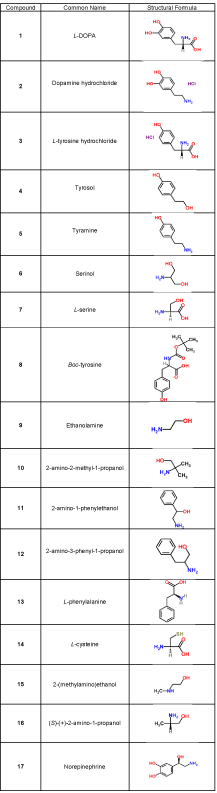
The electrochemical behaviour of the investigated compounds was studied by cyclic voltammetry using a GC electrode, which has been the working electrode of choice in analogous studies [17, 21, 25]. The electrochemical investigation was firstly performed in phosphate buffer at pH=7.4 in order to mimic the physiological pH conditions. All compounds gave one well-defined anodic peak with no cathodic steps, which implies an irreversible electrochemical process. This can be explained on the basis that the oxidation process is probably followed by a chemical reaction that rapidly removes the generated product [26, 27]. A representative cyclic voltammogram for serinol at pH 7.4 is illustrated in Figure 1. The first cycle curves of cyclic voltammogram were used to determine the anode peak voltages (Eap) of the investigated compounds, as well as their half-peak voltages (E1/2), considered as the voltage at which the current equals the half of the maximum anode peak current. In Table 2, Eap and E1/2 values are presented. Among the investigated amino acids, L-DOPA (1) and L-serine (7) showed the lowest oxidation potentials (0.25 and 0.26V, respectively) whereas among the studied amino alcohols the lowest Eap were found for serinol (6) and 2-amino-2-methyl-1-propanol (10) (0.19 and 0.28V, respectively). These Eap values are comparable with the one found for the reference compound, ascorbic acid (Eap=0.21V), which is a well-known antioxidant thus; they could be considered as an indication for significant antioxidant activity. Oxidation potentials between 0.32 and 0.50 V were found for the amino acids Boc-tyrosine (8), and L-cysteine (14), and the amino-alcohols dopamine (2), 2-amino-1-phenylethanol (11), 2-(methylamino)ethanol (15), (S)- (+)-2-amino-1-propanol (16) and norepinephrine (17). These Eap values imply intermediate antioxidant properties. The rest of the studied compounds showed Eap higher than 0.50V therefore they are expected to be weak antioxidants. The ΔE= Eap-E1/2values, presented also in Table 2 are around 70 mV which corresponds the removal of one electron during the first step of the electrochemical oxidation [21].
Compound
pH=4
pH=7.4
pH=9
pH=11
Eap (V)
E1/2 (V)
ΔE (mV)
Eap (V)
E1/2 (V)
?? (mV)
Eap (V)
E1/2 (V)
?? (mV)
Eap (V)
E1/2 (V)
?? (mV)
1
0.47 ± 0.02
0.41 ± 0.03
60 ± 5
0.25 ± 0.03
0.18± 0.02
70 ± 5
0.15 ± 0.04
0.09 ± 0.02
60 ± 5
0.07 ± 0.03
0.005 ± 0.03
65 ± 5
2
0.51 ± 0.02
0.445 ± 0.02
65 ± 5
0.35 ± 0.02
0.275 ± 0.04
75 ± 5
0.27 ± 0.03
0.2 ± 0.02
70 ± 5
0.22 ± 0.02
0.145 ± 0.03
75 ± 5
3
0.71 ± 0.03
0.645 ± 0.02
65 ± 5
0.51 ± 0.03
0.44 ± 0.04
70 ± 5
0.42 ± 0.03
0.355 ± 0.03
65 ± 5
0.35 ± 0.02
0.275 ± 0.03
75 ± 5
4
0.65 ± 0.04
0.59 ± 0.04
60 ± 5
0.52 ± 0.03
0.445 ± 0.03
75 ± 5
0.46 ± 0.03
0.4 ± 0.03
60 ± 5
0.43 ± 0.02
0.35 ± 0.03
80 ± 5
5
0.70 ± 0.04
0.625 ± 0.02
75 ± 5
0.56 ± 0.02
0.48 ± 0.02
80 ± 5
0.50 ± 0.02
0.425 ± 0.03
75 ± 5
0.44 ± 0.03
0.37 ± 0.02
70 ± 5
6
0.77 ± 0.04
0.705 ± 0.03
65 ± 5
0.19 ± 0.02
0.115 ± 0.03
75 ± 5
-0.05 ± 0.03
0.025 ± 0.02
75 ± 5
-0.36 ± 0.03
-0.29 ± 0.02
70 ± 5
7
0.63 ± 0.02
0.57± 0.02
60 ± 5
0.26 ± 0.04
0.185 ± 0.03
75 ± 5
0.08 ± 0.02
0.01 ± 0.04
70 ± 5
-0.19 ± 0.02
-0.11 ± 0.04
80 ± 5
8
0.70 ± 0.03
0.64 ± 0.03
60 ± 5
0.44 ± 0.02
0.37 ± 0.02
70 ± 5
0.34 ± 0.04
0.27 ± 0.02
70 ± 5
0.13 ± 0.02
0.46 ± 0.04
80 ± 5
9
1.11 ± 0.03
1.025 ± 0.02
75 ± 5
0.6 ± 0.03
0.52 ± 0.02
80 ± 5
0.32 ± 0.04
0.25 ± 0.02
70 ± 5
0.11 ± 0.04
0.03 ± 0.02
80 ± 5
10
0.86 ± 0.02
0.79 ± 0.02
70 ± 5
0.28 ± 0.03
0.2 ± 0.03
80 ± 5
0.16 ± 0.02
0.085 ± 0.04
75 ± 5
-0.11 ± 0.03
-0.035 ± 0.02
75 ± 5
11
0.77 ± 0.04
0.7 ± 0.02
70 ± 5
0.5 ± 0.02
0.425 ± 0.02
75 ± 5
0.38 ± 0.02
0.315 ± 0.04
65 ± 5
0.29 ± 0.03
0.22 ± 0.03
70 ± 5
12
0.9 ± 0.03
0.825 ± 0.03
75 ± 5
0.58 ± 0.03
0.505 ± 0.02
75 ± 5
0.43 ± 0.03
0.36 ± 0.03
70 ± 5
0.31 ± 0.02
0.23 ± 0.02
80 ± 5
13
1.2
± 0.03
1.12± 0.03
80 ± 5
0.72 ± 0.02
0.645 ± 0.04
75 ± 5
0.5 ± 0.03
0.425 ± 0.03
75 ± 5
0.33 ± 0.02
0.255 ± 0.03
75 ± 5
14
0.82 ± 0.02
0.755 ± 0.04
65 ± 5
0.38 ± 0.04
0.305 ± 0.02
75 ± 5
0.17 ± 0.03
0.1 ± 0.03
70 ± 5
-0.01 ± 0.02
0.06 ± 0.03
70 ± 5
15
0.74
± 0.04
0.67 ± 0.02
70 ± 5
0.45 ± 0.02
0.37 ± 0.03
80 ± 5
0.33 ± 0.03
0.26 ± 0.02
70 ± 5
0.23 ± 0.03
0.15 ± 0.02
80 ± 5
16
0.74 ± 0.02
0.665 ± 0.02
75 ± 5
0.42 ± 0.03
0.345 ± 0.03
75 ± 5
0.27 ± 0.02
0.205 ± 0.02
65 ± 5
0.18 ± 0.03
0.105 ± 0.04
75 ± 5
17
0.75 ± 0.02
0.69± 0.02
60 ± 5
0.32 ± 0.02
0.25 ± 0.02
70 ± 5
0.15 ± 0.02
0.085 ± 0.02
65 ± 5
-0.01 ± 0.03
0.06 ± 0.02
70 ± 5
Ascorbic acid
0.29 ± 0.02
0.22 ± 0.02
70 ± 5
0.21 ± 0.02
0.15 ± 0.02
65 ± 5
0.15 ± 0.02
0.13 ± 0.02
70 ± 5
0.19 ± 0.02
0.13 ± 0.02
65 ± 5
Table 2: Anodic peak voltages (Eap), half-peak potentials (E1/2) and their differences ΔE= Eap-E1/2 of the compounds under investigation obtained at a glassy carbon electrode under different pH values.
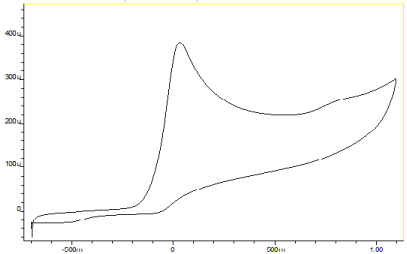
Figure 1: Cyclic voltammograms at a glassy carbon electrode of Serinol at
pH 7.4. Sweep rate: 20mV·s-1.
Modified linear solvation energy relationships (LSER) analysis
The Abraham’s Linear Solvation Energy Relationships (LSER) are widely utilized to characterize many biological and physicochemical processes, governed by forces, such as hydrophobic interactions, steric and electronic effects and hydrogen bond formation [28]. In the case of electro active species, selective solvation of both initial compounds and their intermediates is likely to occur, including non-specific solute-solvent interaction (stabilization of a charge or a dipole by virtue of its dielectric effect) and specific solute-solvent interaction, such as hydrogen bonding and electron pair donor or acceptor mechanism. The general equation proposed by Abraham et al. is presented in Eq. (1) [29, 30].
S.P. = a· A + b· B + s· S + e· E + v· V + c (1)
Where SP is a dependent solute property in a given system, such as the Eap values.
In the case of electrochemical oxidation, the coefficients a, b, s, e and v reveal the differences between the phases of the solution in the electrolytic cell and the electrode. For LSER analysis the Eap values of the 17 investigated compounds obtained at pH= 7.4 were used. The obtained relationship is presented in Eq. (2):
Eap = (0.628 ± 0.134) - (0.545 ± 0.204)· A - (0.360 ± 0.149) ·B +(0.738 ± 0.369) · S -(0.035± 0.165) · E - (0.166 ± 0.195) · V (2)
(N=17, R= 0.816, R²= 0.666, s= 0.098, F= 4.39)
As shown, the coefficients of the parameters E and V are not statistically significant (t<2.0). Therefore, regression analysis was repeated taking into account only the statistically significant parameters of A, B and S. The obtained relationship is presented in Eq. (3):
Eap = (0.621 ± 0.126) - (0.431 ± 0.147)· A - (0.332 ± 0.138) ·B +(0.457 ± 0.127) · S (3)
(N=17, R= 0.802, R²= 0.644, s= 0.093, F= 7.823)
As shown, oxidation potential data greatly correlate with solvatochromic parameters, implying that both specific and nonspecific solute-solvent interactions influence the reactivity of the studied compounds towards the working electrode. More to the point, the statistically significant coefficient of A reveals the influence of hydrogen bond donor interactions of the reactants with the solvent. This observation is in agreement with the role of amino groups in the first step of oxidation on a GC electrode, as shown in Scheme 1 [31].
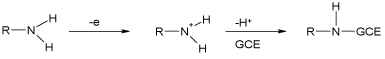
Scheme 1: Proposed mechanism of oxidation of amino-group on a GC
electrode [31].
Moreover, the significance of coefficient of B may reflect the solvation of the polar intermediates of amino alcohols through hydrogen bond acceptor interactions. However, electrochemical reactivity of amino alcohols is also influenced by non-specific interactions, which are more evident in the case of the reactants’ intermediates (positive sign of S). It should be noted that analogous observations were found for a series of oxicams, reported in a previous work by some of us [20].
Effect of ionization
It is well known that the electrochemical oxidation is affected greatly by the pH value in the case of ionizable compounds. In the case of amino alcohols, their oxidation can be performed either at the amino-group, or at the hydroxyl group. Under basic or neutral conditions oxidation usually starts at the nitrogen atom [32], as confirmed by the solvatochromic analysis. However, change of pH can lead to alteration of ionization of the amino group, from neutral at basic pH values to fully protonated state at acidic conditions.
In order to gain insight into the effect of ionization to the oxidation potentials of the compounds under study, the electrochemical investigation was also performed at the pH values of 4.0, 9.0 and 11.0. The Eap, E1/2 and ΔE= Eap-E1/2 values of the investigated species are presented in Table 2. As shown, a considerable decrease of their oxidation potential by increasing the pH was observed. It is interesting that a linear correlation Eap=f(pH) followed by good statistics was found for the investigated compounds. Results of the linear regression Eap/pH are shown in Table 3. Slopes may reflect chemical structures. Indeed, compounds with phenolic group, such as L-DOPA and dopamine exhibited values around 0.05 V/pH, while species with aliphatic hydroxyl group, such as serinol and ethanolamine as well as amino acids, such as phenylalanine and L-cysteine, showed values over than 0.10 V/pH. In all cases, the compounds studied gave one well-defined anodic peak, implying an irreversible process as observed at pH=7.4. It should be mentioned that ascorbic acid pka value is 4.20 therefore at pH 6.2 is fully ionised, so further increase of pH does not affect its ionisation and the standard error in Eap corresponding value in pH 9 and pH 11 may be similar. Moreover, Tyrosol shows the lowest correlation (Eap=f(pH)) apparently due to its structure which lacks an amino group and has a different oxidation mechanism.
Compound
Coefficients
Statistical parameters
Eap
a
R2
s
F
1
0.692 ± 0.034
0.061 ± 0.003
0.990
0.021
196.2
2
0.671 ± 0.031
0.043 ± 0.002
0.985
0.019
128.9
3
0.909 ± 0.033
0.058 ± 0.005
0.988
0.020
171.1
4
0.770 ± 0.032
0.042 ± 0.003
0.972
0.020
69.0
5
0.845 ± 0.016
0.045 ± 0.003
0.995
0.010
385.2
6
1.405 ± 0.029
0.173 ± 0.010
0.999
0.018
2103.4
7
1.106 ± 0.036
0.114 ± 0.012
0.997
0.023
639.2
8
1.028 ± 0.043
0.092 ± 0.006
0.992
0.026
238.1
9
1.679 ± 0.068
0.140 ± 0.012
0.994
0.042
309.9
10
1.373 ± 0.105
0.119 ± 0.005
0.983
0.065
116.4
11
1.035 ± 0.048
0.076 ± 0.006
0.986
0.030
146.0
12
1.229 ± 0.047
0.090 ± 0.004
0.991
0.029
223.5
13
1.682 ± 0.078
0.136 ± 0.009
0.989
0.048
180.0
14
1.327 ± 0.067
0.136 ± 0.012
0.992
0.042
236.1
15
0.991 ± 0.041
0.069 ± 0.005
0.990
0.025
206.5
16
1.048 ± 0.068
0.095± 0.006
0.980
0.042
99.0
17
1.166 ± 0.067
0.100 ± 0.004
0.989
0.041
185.3
Table 3: Statistical analysis of the correlation Eap= E(pH=0)- a·pH for the 17 compounds under investigation.
Chronoamperometric investigation
There is evidence that a correlation between the chronoamperometric signal and reactivity towards reactive oxygen species and free radicals can be achieved in certain conditions [20, 21, 33]. In our previous investigation concerning a series of chalcone analogues [21], principal component analysis revealed the association of chronoamperometric signal with the ability of the compounds to scavenge H2O2 evaluated using the luminol chemiluminescence assay
In the present work, chronoamperograms were recorded at the potentials of 300 mV, 500 mV and 800 mV, characterizing compounds with high, intermediate and weak antioxidant activity, respectively [34]. Measurements were performed at pH=7.4 with respect to the physiological conditions. Chronoamperometric signals for the studied compounds and the well known strong antioxidant ascorbic acid at the potentials of 300, 500 and 800 mV are presented in Table 4. As expected, L-DOPA (1), serinol (6), L-serine (7), 2-amino- 2-methyl-1-propanol (10), norepinephrine (17) and ascorbic acid, which had shown Eap in the range of 210-320mV, gave measurable signals at the glassy carbon electrode at all the three potentials that were used. Compounds 2, 8, 11, 14, 15 and 16 gave measurable signals at the glassy carbon electrode at 500mV whereas compounds 3, 4, 5, 9, 12 and 14 gave chronoamperometric signals only at 800mV.
Compound
Chronoamperometric signal (μ?)
300mV
500mV
800mV
1
7.2 ±0.5
7.7 ± 0.2
7.9 ± 0.3
2
-
4.7 ± 0.2
5.2 ± 0.3
3
-
-
2.1 ± 0.2
4
-
-
2.3 ± 0.3
5
-
-
2.0 ± 0.2
6
6.0 ± 0.5
6.6 ± 0.3
6.7 ± 0.2
7
3.7 ± 0.3
4.3 ± 0.6
4.5 ± 0.2
8
-
4.4 ± 0.3
5.2 ± 0.3
9
-
-
1.1 ± 0.2
10
4.0 ± 0.3
4.6 ± 0.2
4.7 ± 0.2
11
-
3.7 ± 0.3
4.2 ± 0.5
12
-
-
2.7 ± 0.3
13
-
-
3.9 ± 0.5
14
-
2.9 ± 0.2
3.6 ± 0.3
15
-
6.0 ± 0.4
6.3 ± 0.2
16
-
5.3 ±0.5
6.0 ± 0.2
17
4.6 ± 0.2
4.7 ± 0.2
5.0 ± 0.4
Ascorbic acid
2.1 ± 0.3
2.6 ± 0.4
2.8 ± 0.4
Table 4: Amperometric signal (Ip) of the tested compounds measured with chronoamperometry at 300, 500 and 800 mV at pH 7.4 using glassy carbon electrode as working electrode.
DPPH radical scavenging ability
In order to estimate the antioxidant activity of the studied compounds, their ability to scavenge the stable free radical DPPH was evaluated (Table 5). When the deep violet solution of DPPH radical was mixed with a solution of strong antioxidant it turned yellow and the degree of discoloration indicates the free radical scavenging potentials of the compound by their hydrogen donating ability. Compounds 1, 2, 4, 5, 6, 7, 8 and 10 were selected for this experiment. In the DPPH radical scavenging assay the decrease in DPPH absorbance was measured as a function of time (20 min and 60 min). For each compound, the concentration (mM) of the substance that produces 50% scavenging (IC50) was measured.
Compound
IC(50) (mM)
20 min
60 min
1
1.14
1.04
2
0.34
0.32
4
843.4
542.7
5
771.4
425.9
6
>1000
>1000
7
>1000
>1000
8
>1000
>1000
10
>1000
>1000
Table 5: DPPH radical scavenging ability of compounds 1, 2, 4, 5, 6, 7, 8 and 10.
The results of the DPPH assay show that L-DOPA (1) and dopamine (2) exhibit strong antioxidant activity (IC50 1.04 and 0.32 mM, at 60min, respectively). These findings are in accordance with the Eap measurements which implied that these compounds should exhibit important antioxidant activity. Dopamine (2) exhibits the highest antioxidant activity among the compounds tested in this work. On the other hand, tyrosol (4) and tyramine (5), with Eap 520 and 510mV, respectively, show a significantly lower DPPH scavenging ability (IC50 542.7 and 425.9mM, at 60min, respectively). It seems that in the case of these compounds there is a good correlation between the measured Eap and their antioxidant activity. It is worth noting that all these compounds possess one or two phenolic hydroxyl groups, a structural feature that promotes interaction with the DPPH radical. In the case of the amino alcohols serinol (6) and 2-amino-2-methyl- 1-propanol (10), as well as the amino acid L-serine (7), although their Eap indicated a good antioxidant activity, the compounds failed to interact with the DPPH radical. This difference could be attributed to the mechanism of action of the DPPH assay, which involves mainly proton transfer. Neither of these compounds possesses a labile proton capable of giving this reaction at the conditions of the assay. Boctyrosine (8) (Eap=440mV) did not show any interaction with DPPH.
Conclusions
Modified Linear Solvation Energy Relationship analysis at physiological conditions showed that both specific and non-specific solute-solvent interactions influence the reactivity of compounds towards glassy carbon electrode. Specific interactions involve influence of hydrogen bond donor interactions of the reactants with the solvent and solvation of the polar intermediates of amino alcohols through hydrogen bond acceptor interactions. The role of ionization to the electrochemical oxidation of the investigated β-amino alcohols and selected amino acids and amines was also established. A linear decrease of their oxidation potential by increasing the pH was observed. The obtained electro analytical data imply that some of the β-amino alcohols and amino acids studied in this work possess moderate to strong and intermediate antioxidant properties. The DPPH radical scavenging ability of selected compounds showed that, in the case of compounds possessing a catechol system, electrochemical data are in accordance with the antioxidant activity evaluated by the DPPH assay. As antioxidant activity is a multifactorial process, evaluation of the antioxidant activity of the compounds using other assays could shed more light to the correlation between electrochemical behavior and antioxidant ability.
References
- Shackelford RE, Kaufmann WK, Paules RS. Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med. 2000; 28: 1387-1404.
- Morel Y, Barouki R. Repression of gene expression by oxidative stress. Biochem J. 1999; 342 Pt 3: 481-496.
- Fyrst H, Oskouian B, Bandhuvula P, Gong Y, Byun HS, Bittman R, et al. Natural sphingadienes inhibit Akt-dependent signaling and prevent intestinal tumorigenesis. Cancer Res. 2009; 69: 9457-9464.
- Kumar A, Pandurangan AK, Lu F, Fyrst H, Zhang M, Byun HS, et al. Chemopreventive sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3β pathway in colon cancer. Carcinogenesis. 2012; 33: 1726-1735.
- Chen N., W. Jia and J. Xu. A Versatile Synthesis of Various Substituted Taurines from Vicinal Amino Alcohols and Aziridines. Eur. J. of Org. Chem. 2009; 33: 5841-5846.
- Mascher R., S. Fischer, W. Scheiding, A. Neagoe and H. Bergmann. Exogenous 2-aminoethanol can diminish paraquat induced oxidative stress in barley (Hordeum vulgare L.). Plant growth regulat. 2005; 45: 103-112.
- Horváth I, van Hasselt PR. Inhibition of chilling-induced photooxidative damage to leaves of Cucumis sativus L. by treatment with amino alcohols. Planta. 1985; 164: 83-88.
- Geoghegan, K. F and J. G. Stroh. Site-directed conjugation of nonpeptide groups to peptides and proteins via periodate oxidation of a 2-amino alcohol. Application to modification at N-terminal serine. Bioconjugate Chem. 1992; 3: 138-146.
- Valverde M. G., R. Pedrosa and M. Vicente. A Novel and Efficient Oxidation of 1,2-Amino Alcohols to Dialkylamides Synlett. 2002; 12: 2092-2094.
- Gosain R., A. M. Norrish and M. E. Wood. Free-radical functionalisation of ß-amino alcohols via 1,5-hydrogen atom abstraction in 1,3-oxazolidines. Tetrahedron Lett.1999; 40: 6673-6676.
- Lutz R. E., R. H Jordan and W. L. Truett. Factors Interfering with the Oppenauer Oxidation of Amino Alcohols. J. Am. Chem. Soc. 1950; 72: 4085-4089.
- Arsu N. Use of 2-(N-methyl-N-phenylamino)-1-phenylethanol as synergist in UV-curing applications. J. Photoch. Photobio. A. 2002; 153: 129-133.
- Scheers EM, Forsby A, Dierickx PJ. Cytotoxicity of amino alcohols to rat hepatoma-derived Fa32 cells. Altern Lab Anim. 2002; 30: 309-312.
- Magalhães LM, Segundo MA, Reis S, Lima JL. Methodological aspects about in vitro evaluation of antioxidant properties. Anal Chim Acta. 2008; 613: 1-19.
- Yakovleva K. E., S. A. Kurzeev, E. V. Stepanova, T. V. Fedorava, B. A. Kuznetsov and O. V. Koroleva. Cyclic voltammetry study of plasma antioxidant capacity-Comparison with the DPPH and TAS spectrophotometric methods. Appl. Biochem. 2007; Microbiol. 43: 661-668.
- Martinez S., L. Valek, J. Resetic and D. Ferenec-Ruzic. Cyclic voltammetry study of plasma antioxidant capacity- Comparison with the DPPH and TAS spectrophotometric methods. J. Electroanal. 2006; Chem. 588: 68-73.
- Mannino S., O. Brenna, S. Buratti, M.S. Cosio. A new method for the evaluation of the “antioxidant power” of wines. Electroanal. 1998; 10: 908-912.
- Tsopelas F, A. Tsantili- Kakoulidou and M. Ochsenkühn-Petropoulou. Lipophilicity, biomimetic retention profile and antioxidant activity of selenium species, Microchem. J. 2013; 110: 711-718.
- Zielinska D, Szawara-Nowak D, Zielinski H. Comparison of spectrophotometric and electrochemical methods for the evaluation of the antioxidant capacity of buckwheat products after hydrothermal treatment. J Agric Food Chem. 2007; 55: 6124-6131.
- Tsopelas F., M. Ochsenkühn-Petropoulou, N. Zikos, E. Spyropoulou, I. Andreadou and A. Tsantili-Kakoulidou. Electrochemical study of some non-steroidal anti-inflammatory drugs: solvent effect and antioxidant activity, J. Solid State Electr. 2011; 15: 1099-1108.
- Serifi O., F. Tsopelas, A. M. Kypreou, M. Ochsenk?hn-Petropoulou, P. Kefalas and A. Detsi. Antioxidant behaviour of 2'-hydroxy-chalcones: a study of their electrochemical properties. J. Phys. Org. Chem. 2013; 26: 226-231.
- Masek A., E. Chrzescijanska, M. Zaborski. Estimation of the Antioxidative Properties of Amino Acids – an Electrochemical Approach. Int. J. Electrochem. Sci. 2014; 9: 7904-7915.
- Brand-Williams W., M. E. Cuvelier and C. Berset. Use of a free radical method to evaluate antioxidant activity. Food Science and Technology. 1995; 28: 25–30.
- Alexandrakis Z., K. Kyriakopoulou, G. Katsaros, M. Krokida and P. Taoukis. Selection of Process Conditions for High Pressure Pasteurization of Sea Buckthorn Juice Retaining High Antioxidant Activity. Food and Bioprocess Technology. 2014; 7: 3226-3234.
- Heinze, J. Cyclic Voltammetry-"Electrochemical Spectroscopy. Angew. Chem. Int. Ed. 1984; 23: 831-847.
- Simić A, Manojlović D, Segan D, Todorović M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules. 2007; 12: 2327-2340.
- Bard, A. J. and L. R. Faylkner in Electrochemical methods, fundamentals and applications. 2001. New York, USA: Wiley.
- Zhao Y.H., J. Le, M.H. Abraham, A. Hersey, P.J. Eddershaw, C.N. Luscombe, et al. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure-activity relationship (QSAR) with the Abraham descriptors. J. Pharm. Sci. 2001; 90: 749-784.
- Abraham MH, Chadha HS, Mitchell RC. Hydrogen bonding. 33. Factors that influence the distribution of solutes between blood and brain. J Pharm Sci. 1994; 83: 1257-1268.
- Abraham M. H., H.S. Chadha, R.A.E. Leitao, R.C. Mitchell, W.J. Lambert, R. Kaliszan, et al. Determination of solute lipophilicity, as log P(octanol) and logP(alkane) using poly(styrene–divinylbenzene) and immobilised artificial membrane stationary phases in reversed-phase high-performance liquid chromatography. J. Chromatogr. A. 1997; 766: 35-47.
- Deinhammer R.S., M. Ho, J.W. Anderegg and M.D. Porter. Electrochemical oxidation of amine-containing compounds: a route to the surface modification of glassy carbon electrodes. Langmuir. 1994; 10: 1306-1313.
- Hammerich, O. and H. Lund. Organic Electrochemistry. 2000. New York, USA: Mercel Dekker.
- Zikos N., A. Karaliota and M. Liouni. Chronoamperometry as a tool for the evaluation of antioxidant properties of red wines. J. Anal. Chem. 2011; 66: 859-864.
- Blasco A. J., Rogerio M. C., Gonzalez M. C. and Escarpa A. “Electrochemical Index” as a screening method to determine“total polyphenolics” in foods: A proposal Anal. Chim. Acta. 2005; 539: 237-244.