
Research Article
Austin J Anal Pharm Chem. 2015; 2(5): 1051.
Development of a Spectrophotometric Method for Determination of Hydrogen Peroxide using Response Surface Methodology
Shariati-Rad M*, Irandoust M and Salarmand N
Department of Analytical Chemistry, Faculty of Chemistry, Razi University, Kermanshah, Iran
*Corresponding author: Shariati-Rad M, Department of Analytical Chemistry, Faculty of Chemistry, Razi University, Kermanshah, Iran.
Received: October 15, 2015; Accepted: November 13, 2015; Published: November 16, 2015
Abstract
A spectrophotometric method was introduced for determination of hydrogen peroxide in different samples. The method is based on the hydroxylation of phenol. The reaction takes place in the presence of Fe2+ in the sulfuric acid medium. Factors influencing the reaction were explored by response surface methodology. Inoptimal conditions, a wide linear range for calibration was obtained as 2.0×10-7-3.0×10-4mol L-1 and its detection limit was 9.5×10-8mol L-1. The product of the reaction possesses two bands with maxima located at 245 and 300 nm. The method was successfully applied for determination of hydrogen peroxide in water and rainwater samples.
Keywords: Spectrophotometric; Hydrogen peroxide; Response surface methodology; Rain water
Introduction
Hydrogen peroxide (H2O2) is one of the reactive oxygen species found in seawater as a product formed photo chemically from dissolved organic matter (DOM) [1]. Hydrogen peroxideis a key species in the reactions of the troposphere, being involved in important reactions such as the catalyzed and uncatalyzed aqueous phase oxidation of Sulphur dioxide (SO2) and the ultraviolet enhanced aqueous phase oxidation of organic species [2].
Hydrogen peroxide is widely used in the fields of foods, pharmaceuticals, dental products, textiles, environmental protection and it is also involved in advanced oxidation processes and various biochemical processes [3-6].
Determination of hydrogen peroxide is usually based on the production of colored peroxy compounds or on its oxidizing and reducing properties [7]. Based on this property, numerous methods have been developed for the determination of hydrogen peroxide. These methods can be classified into spectrophotometric [2,5,8-11], spectrofluorimetric [12-14] and electrochemical methods [15-22]. However, chromatographic methods have also been applied for determination of hydrogen peroxide [23-25].
Exploration of the literatures reveals that hydrogen peroxide has been found rather ubiquitously in a wide range of concentrations in natural waters. In ground water, the concentrations as low as 50 nmol L-1 [26] has been reported. In the surface oceans, concentration of hydrogen peroxide varies from about 10 to several hundred nmol L-1 [27,28]. In lakes, estuaries and rivers, higher concentrations up to several μmol L-1 have been reported [29,30]. In rain water, the highest concentrations ranging from several μmol L-1 to tens of μmol L-1 [30- 33] have been obtained. Therefore, in addition to the need for a rapid, simple and sensitive method for determination of hydrogen peroxide, it is necessary to develop a method with a wide dynamic linear range.
Hydrogen peroxide involves in Fenton reaction, which is very important in both laboratories and industries [5,34,35]. In the present work, the reaction of hydrogen peroxide with phenol in acidic solution and in the presence of Fe2+ provides a method for the spectrophotometric determination of hydrogen peroxide in aqueous solution.
Experimental
Apparatus
Recording of the absorption spectra in the spectral range of 200– 400 nm was performed by an Agilent 8453 UV-Vis spectrophotometer equipped with diode array detector in 1 cm path length quartz cells. Design and analysis of the experiments were carried out by the MINITAB (Minitab Inc. Release 16.0) statistical package.
Sample collection
Rainwater was collected in three container located in different area of Razi University during a rainy night. Volumes equivalent to 10 mL of each container were taken and mixed and homogenized well. Three 100 mL tap water samples were collected in different times in a day without adding any preservative. After mixing the water samples of each type and homogenizing, an appropriate volume was taken for the analysis. The selected water samples were filtered through a Whatman No. 41 filter paper.
Reagents and solutions
All of the chemicals and reagents used in this work were of analytical reagent grade. Iron chloride (FeCl2), H2O2 (35%, w/w), phenol and sulfuric acid were purchased from Merck (Darmstadt, Germany).Deionized water was used in all experiments.
A stock 0.01molL-1 standard solution of hydrogen peroxide was prepared in deionized water. Working solutions were prepared by diluting the standard stock solution to appropriate volumes with deionized water whenever required. Stock 300.0 mgL-1 standard solutions of phenol and 5.00×10-3molL-1 FeCl2 were prepared in deionized water.
Results and Discussion
Response surface methodology
By using design of experiment (DOE) based on statistical principles, researchers can extract, from a minimum number of experiments, a maximum of useful information about the system under study [36]. Among this information is the interaction between the factors. For this purpose, all factors are changed from one experiment to the next, simultaneously. The reason of performing this type of experiment is that variables can influence each other and the optimal value for one of them may be dependent on the values of the others [36]. Interaction means that the effect of a factor on the response depends on its level or on the level of the other factor(s). In response surface methodology (RSM), surface illustration of the variation of the response with the change in the level of factors is used to decide about how the levels of factors influence the response. In central composite design (CCD), as a response surface methodology, the central point for each factor in the coded form is zero and the design is symmetrical around it [37]. Factors and their considered center points in the current experiments are concentration of sulfuric acid (x1), Fe2+(x2) and phenol (x3) are 0.15 mol L-1, 2.75×10-4mol L-1 and 100.0 mg L-1, respectively. For a system with three factors (n = 3), CCD consists of 20 experiments. Values of the factors in these 20 experiments and obtained responses are shown in Table 1. Concentration of hydrogen peroxide in these experiments is 1.00×10- 4mol L-1.
Experiment no.
x1 (mol L-1)
x2 (mol L-1)
x3 (mg L-1)
Response
245 nm
300 nm
1
0.25
5.00×10-5
50.0
0.006
-0.021
2
0.15
0.00
100.0
-1.551
-1.232
3
0.00
2.75×10-4
100.0
0.772
0.236
4
0.25
5.00×10-5
150.0
0.164
0.092
5
0.15
2.75×10-4
100.0
0.806
0.466
6
0.05
5.00×10-4
50.0
1.045
0.638
7
0.25
5.00×10-4
50.0
0.918
0.567
8
0.25
5.00×10-4
150.0
1.009
0.610
9
0.15
2.75×10-4
100.0
0.913
0.537
10
0.05
5.00×10-5
150.0
0.272
0.141
11
0.32
2.75×10-4
100.0
0.665
0.358
12
0.15
2.75×10-4
100.0
0.722
0.407
13
0.05
5.00×10-5
50.0
0.151
0.081
14
0.15
2.75×10-4
100.0
0.951
0.561
15
0.15
2.75×10-4
100.0
0.820
0.476
16
0.15
6.50×10-4
100.0
1.078
0.637
17
0.15
2.75×10-4
100.0
0.753
0.430
18
0.05
5.00×10-4
150.0
1.237
0.719
19
0.15
2.75×10-4
184.0
0.818
0.486
20
0.15
2.75×10-4
16.0
0.821
0.488
Table 1: Experiments based on the central composite design with three factors (responses at 245 nm and 300 nm).
The responses are the changes of absorbances at the specified wavelengths after addition of hydrogen peroxide to the mixture of the reagents at optimal values. In experiments 1 and 2, negative values can be seen. In these cases, after addition of hydrogen peroxide, absorbances decrease. In experiment 2, this decrease is very large. This can be due to the absence of Fe2+.
Analysis of variance (ANOVA) of the experiments in Table 1, considering responses at 300 nm, has been collected in Table 2. Relatively high values of errors of the coefficients refer to the selection of a narrow range of the factors in the designed experiments. In fact, the main purpose was to optimize the factors not to find the significant factors.
Term
Coefficient
Standard error
ta
pb
Constant
0.472
0.085
5.544
0.000
x1
-0.009
0.056
-0.162
0.874
x2
0.394
0.056
6.973
0.000
x3
0.022
0.056
0.382
0.710
x1×x1
-0.020
0.055
-0.363
0.724
x2×x2
-0.230
0.055
-4.183
0.002
x3×x3
0.047
0.055
0.859
0.411
x1×x2
-0.004
0.074
-0.050
0.961
x1×x3
0.002
0.074
0.025
0.981
x2×x3
-0.006
0.074
-0.084
0.935
Regression
F
7.55
p
0.002
R2 %
87.17
a. Statistical t value.
Table 2: ANOVA table for the factors and different interaction terms.
Statistical parameters of the model (F, p and R²%) with the terms in the Table 2 indicate that the model is reliable and can be utilized for prediction purposes. Very small value of the p value of the model reveals that the variation in response is mainly due to the change in the level of factors not noise and isn’t by chance. As the value of R²% shows, the regression explains 87.17% of the variations in the response with change in factor levels. Exploring the p values of the square terms shows that the overall effect of the linear terms is statistically significant at the 95% confidencelevel (p< 0.05). Among them, concentration of Fe2+(x2) is the most significant term with p=0.000. The most significance square term is x2×x2. The interaction terms are not significant at the 95% confidence level.
In order to gain insight about the effect of each factor and also analyze the variation of the response surfaces, the three dimensional (3D) graphs for the response were plotted based on the nonlinear polynomial model as shown in Figure 1. These figures show the relationship between two factors and response at the center of the other factor. As can be seen from Figure 1a and Figure 1c, with change in the level of x2 (concentration of Fe2+), curvature occurs in the response surface. This confirms the significance of the term x2×x2 which was also concluded from p values. Response surfaces in Figure 1a and Figure 1c show that in relatively high concentrations of Fe2+ the response is higher. The lowest influence on the response can be seen for x1 (concentration of sulfuric acid).
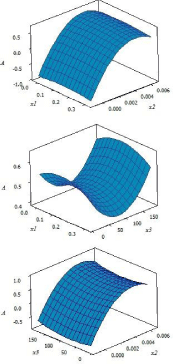
Figure 1: Variation of the response with (a) x1 and x2, (b) x1 and x3 and (c)
x2 and x3.
Spectral behavior
Spectrum of the reagent mixture (phenol, Fe2+ and sulfuric acid) and spectrum of the mixture in the presence of two concentrations of hydrogen peroxide have been shown in Figure 2. As can be seen, the reagent mixture has a main intense peak located at about 275 nm with a broad shoulder. The peak at 275 nm can be related to phenol and the shoulder to Fe2+ in the sulfuric acid medium. In the presence of hydrogen peroxide, the absorbance in the whole range (220-400 nm) increases (see Figure 2). The increase in absorbance results in the evolution of two new bands at 245 and 300 nm which are attributed to the product. The intense band at about 275 nm belongs to phenol. Therefore, this band can’t be used for the analysis.
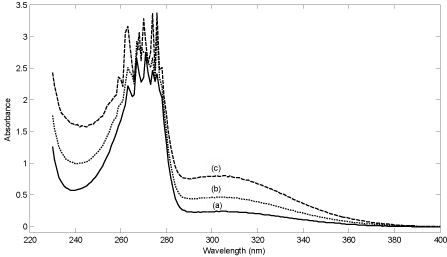
Figure 2: (a) Spectra of reagent mixture (phenol = 180 mg L-1, Fe2+ = 5.0×10-4
mol L-1 and sulfuric acid = 0.05 mol L-1), (b) reagent mixture in the presence
of hydrogen peroxide with concentration of 4.0×110-5 mol L-1 and (c) reagent
mixture in the presence of hydrogen peroxide with concentration of 1.0×10-
4mol L-1.
The mechanism of the reaction between phenol and hydrogen peroxide in the presence of Fe2+ in sulfuric acid medium can be written as [5,34,35]:
Fe2+ +H2O2→Fe3++•OH + OH− (1)
•OH + C6H5OH→ C6H4(OH)2 (2)
As stated by Zazo et al. [38], the main products of the oxidation of phenol by Fenton’s reagents (hydrogen peroxide and Fe2+) are catechol, hydroquinone and p-benzoquinone. The first two compounds have two hydroxyl groups in the benzene ring. Under the experimental conditionsof the work by Zazo et al. [38], close to 90% of phenol is converted in about 30 min, giving rise to dihydroxybenzenes upon hydroxylation of the aromaticring and catechol is the main primary oxidation product. This indicates that hydroxylation takes place predominantly in the ortho position.
Calibration
The calibration graphs for the determination of hydrogen peroxide were obtained under the optimal reaction conditions. Calibration curves were obtained by adding different concentrations of hydrogen peroxide to the solution containing Fe2+, phenol and sulfuric acid in optimal values. Absorbances at 245 nm and 300nm were used to obtain calibration curves. Statistical parameters of the calibration curves have been collected in Table 3.
Parameters
Characteristic
Calibration curve1
Calibration curve 2
λ max (nm)
245
300
Molar absorptivity (L mol-1 cm-1)
4.6×103
2.6×103
Linear range (µmolL-1)
0.20-300.00
0.20-300.00
Intercept of calibration curve
0.060
0.042
Slope of calibration curve
4477.9
2594.1
Standard error of intercept
0.020
0.015
t statistics of intercept
3.07
2.72
Standard error of slope
168.5
137.7
Standard error of regression
0.045
0.037
t statistics of slope
26.57
18.84
R2
0.9916
0.9807
Detection limit (µmol L-1)a
0.095
0.170
a. Calculated as DL = yB + 3sB, where yB is the signal of the blank (intercept of the calibration curve) and sB is the standard deviation of the blank.
Table 3: Statistical results of the calibration of hydrogen peroxide by the proposed method.
Clearly, sensitivities of the calibration curves are different in different wavelengths. This is due to the different absorptivity of the product in different wavelengths.
As can be inferred from data in Table 3, linear range of the two calibrations is wide (more than three orders of magnitude). Therefore, they can be employed to determine hydrogen peroxide in different samples with a wide range of concentrations. The high value of correlation coefficient of the regression equations which are close to unity validates the linearity of the calibration curves. Moreover, sensitivity of the method can be realized from the calculated detection limits and the lower limit of the calibration curves especially for the calibration at 245 nm.
Application of the method
In order to examine the suitability of the recommended method for determination of hydrogen peroxide in real samples, it was applied to tap and rain water samples. The results of the analyses have been collected in Table 4. The data in Table 4 show that the method is accurate and precise.
Real sample
Added concentration
Predicted concentration
RSD
Percent recovery
Tap water
0.0
9.7
4.3
98.6
50.0
59.0
4.2
Rain water
0.0
42.0
3.5
106
50.0
95.0
3.1
Table 4: Results of the analysis of the real samples by the proposed method. The results are for five determinations.
Comparison with the reported results
In Table 5, the results of the published spectrophotometric and spectrofluorimetric methods for determination of hydrogen peroxide have been collected. The criteriaused for comparison are limit of detection and dynamic linear range. As can beinferred from the dynamic linear ranges in the second column of Table 5, the proposed method have the widest dynamic linear range. Moreover, the lowest limit of the linear range of the proposed method is lower compared with the methods collected in Table 5. In terms of limit of detection, in both wavelengths; a comparable or even lower detection limits have been obtained.
Reagents
Dynamic linear range
(µmolL-1)
Limit of detection
(µmolL-1)
Remarks
Ref.
Metal nanoparticle loaded carbon nanotube modified screen printed carbon electrode
100.0-1000.0
20
Amperometry
[19]
Rhodium nanoparticle-modified screen-printed graphite electrodes
5-600
2
Electrochemical
[20]
0.1-70.0
-
Electrochemical
[21]
An ionic liquid-Fe3O4 nanoparticles-graphite composite electrode
1–25
0.5
electrochemical
[18]
l-anilinonaphthalene-8-sulfonic acid
l-50
0.3
spectrophotometric
[39]
Eriochrome black T
0.2–10.0
-------------
Spectrophotometric
[40]
2,9-dimethyl-l,l0-phenanthroline (DMP)
1.0-120.0
______
spectrophotometric
[41]
titanium(IV)in presence of H2SO4
4.0 -60.0
1.0
spectrophotometric
[42]
1,l0-Phenanthroline
2.94 – 74.0
--------------------
Spectrophotometric
[43]
4,7-diphenyL-1,l0-phenanthroline
0.882- 2.94
-------------------
Spectrophotometric
[43]
Ceric ion in acid solution
----------------
2.94
Spectrofluorometric
[7]
Amodifed p-hydroxyphenylacetic acid (PHPA)
1.47- 1.47×103
0.29
Spectrophotometric
[2]
0.5–58.75
0.4
Amperometric
[44]
Leuco Crystal Violet
0.02
Spectrophotometric
[45]
Osmium(VIII) and m-carboxyphenylfluorone
(MCPF)
0.588–11.94
------------
Spectrophotometric
[46]
Phenol,Fe2+,H2SO4
0.20-300.00
0.0946 (245nm)
This method
0.17 (300nm)
Table 5: Reported results for determination of hydrogen peroxide.
Effect of foreign ions
The interference of foreign ions commonly present in water samples was studied by adding known amounts of the foreign ions to a solution containing hydrogen peroxide with a concentration of 1.00×10-4mol L−1. The tolerance limit of a potentially interfering ion was taken as its maximum amount causing an error of ≥±5% during the determination of hydrogen peroxide in water samples. The tolerance limits for the ions studied are given in Table 6. As can be inferred from data in Table 6, in most cases the interference from the studied species is low.
Foreign species
Tolerance limit
245 nm
300 nm
SO42-
24
40
NO3-
60
10
NO2-
14
14
Cl-
30
30
Mg2+
16
24
Acetic acid
30
30
Formaldehyde
30
30
Formic acid
10
8
Glucose
16
28
Table 6: Effect of interfering ions on the determination of hydrogen peroxide (100.00 μmol L-1).
Conclusions
This paper proposes a simple spectrophotometric method for fast determination of H2O2 in water samples. Applying the method to real samples resulted in satisfactory precision and accuracy. The relatively low detection limit proved that the proposed method in question was sensitive. The proposed method has a wide dynamic linear range which is one of the advantages of the method over the reported ones for determination of hydrogen peroxide different samples.
References
- Cooper WJ, Zika RG, Petasne RG, Plane JM. Photochemical formation of hydrogen peroxide in natural waters exposed to sunlight. Environ Sci Technol. 1988; 22: 1156-1160.
- PA Tanner, AYS Wong. Spectrophotometric determination of hydrogen peroxide in rain water. Anal. Chim. Acta. 1998; 370: 279.
- Demirkol O, Cagri-Mehmetoglu A, Qiang Z, Ercal N, Adams C. Impact of food disinfection on beneficial biothiol contents in strawberry. J Agric Food Chem. 2008; 56: 10414-10421.
- Luo W, Abbas ME, Zhu L, Deng K, Tang H. Rapid quantitative determination of hydrogen peroxide by oxidation decolorization of methyl orange using a Fenton reaction system. Anal Chim Acta. 2008; 629: 1-5.
- Nogueira RF, Oliveira MC, Paterlini WC. Simple and fast spectrophotometric determination of H(2)O(2) in photo-Fenton reactions using metavanadate. Talanta. 2005; 66: 86-91.
- Zhang K, Mao L, Cai R. Stopped-flow spectrophotometric determination of hydrogen peroxide with hemoglobin as catalyst. Talanta. 2000; 51: 179-186.
- B Demirata-Öztürk, G Ozen, H Filik, I Tor, H Afsar. Spectrofluorometric Determination of Hydrogen Peroxide. J. Fluoresc. 1998; 8: 185-189.
- Wei H, Wang E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem. 2008; 80: 2250-2254.
- K Tamaoku, Y Murao, K Akiur. New water-soluble hydrogen donors for the enzytmatic spectrophotometric determination of hydrogen peroxide. Anal. Chim. Acta. 1982; 136: 121-127.
- XS Chai, QX Hou, Q Luo, JY Zhu. Rapid determination of hydrogen peroxide in the wood pulp bleaching streams by a dual-wavelength spectroscopic method. Anal. Chim. Acta. 2004; 507: 281-284.
- M Hoshino, S Kamino, M Doi, S Takada, S Mitani, R Yanagihara, et al. Spectrophotometric determination of hydrogen peroxide with osmium(VIII) and m-carboxyphenylfluorone. Spectrochim. Acta A. 2014; 117: 814-816.
- Chen H, Yu H, Zhou Y, Wang L. Fluorescent quenching method for determination of trace hydrogen peroxide in rain water. Spectrochim Acta A Mol Biomol Spectrosc. 2007; 67: 683-686.
- SSM Rodrigues, DSM Ribeiro, L Molina-Garcia, AR Medina, JAV Prior, JLM Santos. Fluorescence enhancement of CdTe MPA-capped quantum dots by glutathione for hydrogen peroxide determination. 2014; 122: 157-165.
- A Sakuragawa, T Taniai, T Okutani. Fluorometric determination of micro amounts of hydrogen peroxide with an immobilized enzyme prepared by coupling horseradish peroxidase to chitosan beads. Anal. Chim. Acta. 1998; 374: 191-200.
- Zheng X, Guo Z. Potentiometric determination of hydrogen peroxide at MnO2-doped carbon paste electrode. Talanta. 2000; 50: 1157-1162.
- K De Wael, Q Bashir, SV Vlierberghe, P Dubruel, HA Heering, A Adriaens. Electrochemical determination of hydrogen peroxide with cytochrome c peroxidase and horse heart cytochrome c entrapped in a gelatin hydrogel. Bioelectrochem. 2012; 83: 15-18.
- JM You, D Kim, SK Kim, MS Kim, HS Han, S Jeon. Novel determination of hydrogen peroxide by electrochemically reduced graphene oxide grafted with aminothiophenol–Pd nanoparticles. Sens. Actuat. B. 2013; 178: 450-457.
- CL Yu, NC Lo, H Cheng, T Tsuda, T Sakamoto, Y Han Chen, et al. An ionic liquid-Fe3O4 nanoparticles-graphite composite electrode usedfornonenzymatic electrochemical determination of hydrogen peroxide. J. Electroanal. Chem. 2014; 729: 109-115.
- P Reanpang, S Themsirimongkon, S Saipanya, O Chailapakuld, J Jakmunee. Cost-effective flow injection amperometric system with metal nanoparticle loaded carbon nanotube modified screen printed carbon electrode for sensitive determination of hydrogen peroxide. Talanta. 2015; 144: 868-874.
- Gatselou VA, Giokas DL, Vlessidis AG, Prodromidis MI. Rhodium nanoparticle-modified screen-printed graphite electrodes for the determination of hydrogen peroxide in tea extracts in the presence of oxygen. Talanta. 2015; 134: 482-487.
- Canbay E, Şahin B, Kıran M, Akyilmaz E. MWCNT-cysteamine-Nafion modified gold electrode based on myoglobin for determination of hydrogen peroxide and nitrite. Bioelectrochemistry. 2015; 101: 126-131.
- Ghaderi S, Mehrgardi MA. Prussian blue-modified nanoporous gold film electrode for amperometric determination of hydrogen peroxide. Bioelectrochemistry. 2014; 98: 64-69.
- Gimeno P, Bousquet C, Lassu N, Maggio AF, Civade C, Brenier C, et al. High-performance liquid chromatography method for the determination of hydrogen peroxide present or released in teeth bleaching kits and hair cosmetic products. J Pharm Biomed Anal. 2015; 107: 386-393.
- Hu HC, Jin HJ, Chai XS. Rapid determination of hydrogen peroxide in pulp bleaching effluents by headspace gas chromatography. J Chromatogr A. 2012; 1235: 182-184.
- M Tarvin, B McCord, K Mount, K Sherlach, ML Miller. Optimization of two methods for the analysis of hydrogen peroxide: High performance liquid chromatography with fluorescence detection and high performance liquid chromatography with electrochemical detection in direct current mode. J. Chromatrogr. A. 2010; 1217: 7564-7572.
- TR Helm, GK George, MJ Barcelona. Fluorometric determination of hydrogen peroxide in groundwater. Anal. Chem. 1987; 59: 582-586.
- RG Zika, JW Moffett, RG Petasne, WJ Cooper, ES Saltzman. Spatial and temporal variations of hydrogen peroxide in Gulf of Mexico waters. Geochim. Cosmochim. Acta. 1985; 49: 1173-1184.
- KS Johnson, SW Willason, DA Wiesenburg, SE Lohrenz, RA Amone. Hydrogen peroxide in the western Mediterranean Sea: a tracer for vertical advection. Deep-Sea Res. 1989; 36: 241-254.
- V Ye. Sinel’nikov. Hydrogen peroxide level in river water, and methods for detecting it. J. Hydrobiol. 1971; 7: 115.
- WJ Cooper, DR Lean. Hydrogen Peroxide Concentration in a Northern Lake: Photochemical Formation and Diel Variability. Environ. Sci. Technol. 1989; 23: 1425-1428.
- RG Zika, ES Saltzman, WL Chameides, DD Davis. H2O2 levels in rain¬water collected in south Florida and the Bahama Islands. J.Geophys. Res. 1982; 87: 5015-5017.
- TJ Kelly, PH Daum, SE Schwartz. Measurements of peroxides in cloud¬water and rain. J. Geophys. Res. 1985; 90: 7861-7871.
- WJ Cooper, ES Saltzman, RG Zika. The contribution of rain¬water to variability in surface ocean hydrogen peroxide. J. Geophys. Res. 1987; 92: 2970-2980.
- J Perkowski, W Józwiak, L Kos, P Stajszcyk. Application of Fenton’s reagent in detergent separation in highly concentrated water solutions. Fibres and Textiles in Eastern Europe. 2006; 14: 114-119.
- ME Abbas, W Luo, L Zhu, J Zou, H Tang. Fluorometric determination of hydrogen peroxide in milk by using a Fenton reaction system. Food Chem. 2010; 120: 327-331.
- Leardi R. Experimental design in chemistry: A tutorial. Anal Chim Acta. 2009; 652: 161-172.
- Leardi R. Experimental design in chemistry: A tutorial. Anal Chim Acta. 2009; 652: 161-172.
- Zazo JA, Casas JA, Mohedano AF, Gilarranz MA, Rodríguez JJ. Chemical pathway and kinetics of phenol oxidation by Fenton's reagent. Environ Sci Technol. 2005; 39: 9295-9302.
- Chung HK, Dasgupta PK, Marx JN. Spectrophotometric determination of H(2)O(2) with 1-anilinonaphthalene-8-sulfonic acid and 4-aminoantipyrine with hematin as catalyst. Talanta. 1993; 40: 981-988.
- Zhu M, Huang X, Liu L, Shen H. Spectrophotometric determination of hydrogen peroxide by using the cleavage of Eriochrome black T in the presence of peroxidase. Talanta. 1997; 44: 1407-1412.
- AN Baga, GR Johnson, NB Nazhat, RA Saadalla-nazhat. A simple spectrophotometric determination of hydrogen peroxide at low concentrations in aqueous solution. Anal. Chim. Acta. 1988; 204: 349-353.
- AM Almuaibed. Flow spectrophotometric method for determination of hydrogen peroxide using a cation exchanger for preconcentration. Anal. Chim. Acta. 1994; 295: 159-163.
- R Bailey, DF Boltz. Differential Spectrophotometric Determination of Hydrogen Peroxide Using 1,10-Phenanthroline and Bathophenanthroline. Anal. Chem. 1959; 31: 117-119.
- H Razmi, H Heidari. Amperometric determination of hydrogen peroxide on surface of a novel PbPCNF-modified carbon-ceramic electrode in acidic medium. J. Electroanal. Chem. 2009; 629: 101-108.
- Zhang LS, Wong GT. Spectrophotometric determination of H(2)O(2) in marine waters with leuco crystal violet. Talanta. 1994; 41: 2137-2145.
- Hoshino M, Kamino S, Doi M, Takada S, Mitani S, Yanagihara R, et al. Spectrophotometric determination of hydrogen peroxide with osmium(VIII) and m-carboxyphenylfluorone. Spectrochim Acta A Mol Biomol Spectrosc. 2014; 117: 814-816.