
Research Article
Austin J Anal Pharm Chem. 2015; 2(5): 1052.
N-(3-Substituted-benzylidene-2-oxoindolin-5-yl) acetamide Derivatives as Src Kinase Inhibitors: Synthesis, Biological Evaluation and Molecular Docking Studies
Kilic-Kurt Z¹, Bakar F² and Ölgen S³*
¹Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Ankara University, Ankara, Turkey
²Department of Biochemistry, Faculty of Pharmacy, Ankara University, Ankara, Turkey
³Department of Pharmaceutical Chemistry, School of Pharmacy, Istanbul Kemerburgaz University, Istanbul, Turkey
*Corresponding author: Ölgen S, Department of Pharmaceutical Chemistry, School of Pharmacy, Istanbul Kemerburgaz University, Bagcılar, Istanbul, 34217, Turkey
Received: November 08, 2015; Accepted: December 01, 2015; Published: December 07, 2015
Abstract
The synthesis of new N-(3-substituted-benzylidene-2-oxoindolin-5-yl) acetamide (5-14) derivatives along with their inhibitory activity against Src is reported. Among all compounds, only 10, 12 and 13 were found slightly active against Src with IC50 values of 3.55, 6.39 and 7.29 mM, respectively. Docking studies have been also performed to analyze the binding mode of compounds and the results showed that the most active compound 10 showed a hydrogen bond with Leu273 for the binding to the Src active site. It was found that replacement of ethylthiourea, benzylthiourea and methylaminosulphonic acid group with acetamido group decreases the activity of compounds.
Keywords: Indolin-2-one; Src inhibitors; Molecular docking; Cancer; Synthesis
Introduction
Since the understanding of the crucial role of Src in tumor development and metastasis, it has been a promising target for cancer theraphy over the past nearly three decades [1]. Src (c-Src) is the first discovered intensively studied the protypical member of Src Family Kinase (SFK) enzymes. Src is at the center of an immense signaling network, and it can also be activated by integrins, receptor tyrosine kinases, cytokine receptors and G protein-coupled receptors [2]. Following activation of Src, it integrates and regulates a number of cellular signaling pathways, including integrin/FAK, mitogenactivated protein kinase/extracellular signal–regulated kinase (MAPK/ERK), Janus-activated kinase (JAK)/signal transducers and activators of transcription (STAT) and phosphatidylinositol 3-kinase (PI3K)/AKT [3-5]. Through these interactions, Src controls multiple cancer cell functions, including cell cycle progression, survival, and metastasis. Increased Src activity has been observed in many human malignancies, including colon, breast, pancreas, lung and brain cancers [6-8]. Moreover, Src has a critical role in other pathological disorders, such as myocardial infarction [9], stroke, osteoporosis [10], and neurodegeneration [11].
In recent years much attention has been paid to development of Src inhibitors. Based upon activation mechanism of Src, several purine, pyrrolopyrimidine, pyridopyrimidine, naphthyridone, quinazoline, oxindoles and quinoline-based inhibitors were designed against ATP-binding site of Src [12]. In the literature, it was shown that various substituted indolin-2-one derivatives have the ability to inhibit several SFKs [13-15]. Several 5-methylaminosulphonic acid containing 3-substituted benzylidene derivatives possess potent activities against several SFK enzymes. Among them compound I and II showed good inhibitory potence against Src kinase with IC50 values of 10 nM and 70 nM, respectively. In addition, compounds III and IV inhibited Src, Yes, Lck and Fyn as follow: compound III, IC50= 0.03 μM (Src), IC50= 0.01 μM (Yes), IC50= 0.05 μM (Fyn), IC50= 0.1 μM (Lck); compound IV, IC50= 0.02 μM (Src), IC50= 0.01 μM (Yes), IC50= 0.1 μM (Lck), IC50= 0.4 μM (Fyn). We previously identified a series of 3-(substituted-benzylidene)-1,3-dihydro-indolin-2-tione derivatives (compound V and VI, Figure 1) as moderately active Src inhibitors with IC50 of 21.91 and 21.20 μM, respectively [16]. In our recent study, we reported the synthesis and Src inhibitory activity of novel 1,3,5-substituted-indolin-2-one derivatives. Among this series, some of the compounds (VII-X, Figure 1) were found as promising Src inhibitors with IC50 of 1.02, 2.06, 1.24 and 4.04 μM values [17]. In the light of the above literature reports and in continuation of our efforts to develop Src inhibitors, herein, we report the synthesis and evaluation of Src kinase inhibitory activity of a series of new N-(3- substituted-benzylidene-2-oxoindolin-5-yl)acetamide derivatives. Moreover, the structure-activity relationships and possible enzyme binding modes were also illustrated by performing docking studies.
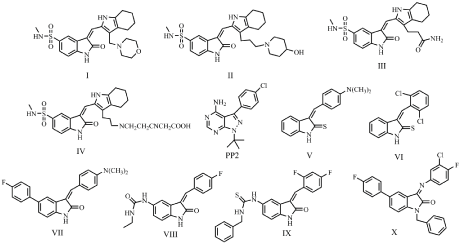
Figure 1: Indole derivatives as Src kinase inhibitors.
Materials and Methods
Chemistry
Isatine, hydrazine hydrate, ethyl acetate, hexane, sulfuric acide, methanol were purchased from Merck. 2,4-difluorobenzaldehyde, 4-fluoro-benzaldehyde, 4-chloro-benzaldehyde, N,Ndimethylamino- benzaldehyde, potassium nitrate, palladium %10, 3,4-dichloro-benzaldehyde were purchased from Aldrich. 4-Methoxybenzaldehyde and 3-fluoro-benzaldehyde were purchased from Fluka. 2,6-dichloro-benzaldehyde, 2-chloro-6-fluoro-benzaldehyde, p-tolyl-benzaldehyde were purchased Acros. Piperidine, ethanol, acetic anhydride, hydrochloric acide were purchased from Riedel. Analytical TLC was carried out on Merck 0.2 mm pre-coated silica Isatine, hydrazine hydrate, ethyl acetate, hexane, sulfuric acide, methanol were purchased from Merck. 2,4-difluorobenzaldehyde, 4-fluoro-benzaldehyde, 4-chloro-benzaldehyde, N,Ndimethylamino- benzaldehyde, potassium nitrate, palladium %10, 3,4-dichloro-benzaldehyde were purchased from Aldrich. 4-Methoxybenzaldehyde and 3-fluoro-benzaldehyde were purchased from Fluka. 2,6-dichloro-benzaldehyde, 2-chloro-6-fluoro-benzaldehyde, p-tolyl-benzaldehyde were purchased Acros. Piperidine, ethanol, acetic anhydride, hydrochloric acide were purchased from Riedel. Analytical TLC was carried out on Merck 0.2 mm pre-coated silica
Synthesis of oxindole (1)
A mixture of isatine (6.82 g, 0.04 mol) and hydrazine hydrate (28 ml) was refluxed at 140 °C for 4h. The reaction mixture was poured into ice-cold water and acidified by 6 N HCl. After standing at room temperature for 2 days, 2.58 g pure oxindole (1) was obtained. Yield 41%, mp 127 °C (lit. 127-129 °C), [18].
Synthesis of 5-nitro-oxindole (2)
Oxindole 1 (0.03 mol; 5 g) was dissolved in 28 ml of cold concentrated sulphuric acid at 0 °C. After complete dissolution of potassium nitrate (0.03 mol; 3.87 g) was added as portions. The temperature of the mixture should not exceed 5 °C. After further stirring for 30 min, the mixture was poured on to 300 g of ice. The precipitate was collected by filtration, washed with water, and dried. The crude product was purified by recrystallization from acetic acid (50%) to give 2.37 g of 5-nitro-oxindole (2). Yield: 35%, mp 240–243 °C [19].
Synthesis of 5-amino-oxindole (3)
A suspension of 5-nitro-oxindole (7.1 mmol; 1.2 g ) and 10% palladium/C (0.32 g) in methanol (50 ml) was hydrogenated for 3 h under 45 psi. Then, the reaction mixture was filtered through celite and the resulting cake was washed with methanol. The filtrate was concentrated and 0.5 g pure compound was obtained. Yield: 47%, mp 202-204°C (lit.213-214°C), [20].
Synthesis of N-(2-oxoindolin-5-yl)acetamide (4)
To 5-amino-oxindole (3, 1.75 mmol; 0.25 g) in THF (5 ml) was added acetic anhydride (1.72 mmol; 0.16 ml). After further stirring for 2h, the precipitate was filtered and dried to give 0.2 g of N-(2- oxoindolin-5-yl)acetamide (4). Yield: 61%, mp 275-277 °C [21].
General Synthesis of N-(3-substituted-benzylidene-2- oxoindolin-5-yl)acetamide derivatives (5-14)
A reaction mixture of N-(2-oxoindolin-5-yl)acetamide (4, 1 eq), the substituted-benzaldehyde (1.2 eq), and piperidine (0.1 eq) in ethanol (1-2 ml/1 mmol) was stirred at 90 °C for 3-5 h. After the reaction was completed, the mixture was cooled and the precipitate filtered and washed cold ethanole. The pure compounds were obtained with 10-80 % yield.
(E)-N-(3-(4-Chlorobenzylidene)-2-oxoindolin-5-yl) acetamide (5)
Yield: 75%, mp: 295°C. 1H NMR (DMSO-d6, 400 MHz), δ (ppm): 2.00 (s, 3H, CH3), 6.73 (d, J=8 Hz, 1H, H-7), 7.23 (dd, Jo= 8, Jm= 2 Hz, 1H, H-6), 7.49 (d, J= 8.8 Hz, 2H, H-10 and H-14), 7.61 (s, 1H, H-8), 7.88 (d, J= 2 Hz, 1H, H-4), 8.38 (d, Jo= 8.8, = 2 Hz, 2H, H-11 and H-13), 9.82 (s, 1H, NHCOH3), 10.57 (s, 1H, indole-NH). MS m/z 313.8 [M+1]+. Elemental analysis calculated (%) for C17H13ClN2O2: C: 65.29, H: 4.19, N: 8.96. Found: C: 65.46, H: 4.21, N: 9.01.
(E)-N-(3-(4-Fluorobenzylidene)-2-oxoindolin-5-yl) acetamide (6)
Yield: 49%, mp: 272-274°C. 1H (d6 400 MHz), δ (ppm): 2.03 (s, 3H, H3), 6.75 (d, J= 8 Hz, 1H, H-7), 7.25 (dd, Jo = 2 Hz, 1H, H-6), 7.31 (d, J= 8 Hz, 2H, H-10 and H-14), 7.65 (s, 1H, H-8), 7.90 (d, J= 2 Hz, 1H, H-4), 8.50 (q, 2H, H-11 and H-13), 9.83 (s, 1H, NHCOH3), 10.57 (s, 1H, indole -NH). MS m/z 297.8 [M+1]+. Elemental analysis calculated (%) for C17H13FN2O2 C: 68.91, H: 4.42, N: 9.45. Found: C: 69.13, H: 4.28, N: 9.50.
(E)-N-(3-(3-Fluorobenzylidene)-2-oxoindolin-5-yl) acetamide (7)
Yield: 44%, mp: 209-210°C. 1H (d6 400 MHz), δ (ppm): 2.03 (s, 3H, H3), 6.77 (d, J= 8 Hz, 1H, H-7), 7.27 (dd, Jo= 8, = 2 Hz, 1H, H-6), 7.30-7.58 (m, 2H, H-12 and H-13), 7.66 (s, 1H, H-8), 7.93 (d, J= 1.6 Hz, 1H, H-4), 8.01 (d, J= 8 Hz, 1H, H-14), 8.54 (d, J= 8 Hz, 1H, H-10), 9.86 (s, 1H, NHCOH3), 10.64 (s, 1H, indole-NH). MS m/z 296.8 [M+1]+. Elemental analysis calculated (%) for C17H13FN2O2: C: 68.91, H: 4.42, N: 9.45. Found C: 68.79, H: 4.21, N: 9.58.
(E)-N-(3-(2,4-Difluorobenzylidene)-2-oxoindolin-5-yl) acetamide (8)
Yield: 46%, mp: 248°C (decomp). 1H (d6 400 MHz), δ (ppm): 2.00 (s, 3H, H3), 6.80 (d, J= 8 Hz, 1H, H-7), 7.26 (dd, Jo= 8.8, Jm= 2 Hz, 1H, H-6), 7.42-7.48 (m, 3H, H-4, H-10 and H-11), 7.68 (s, 1H, H-8), 7.81 (q, 1H, H-13), 9.76 (s, 1H, NHCOH3), 10.57 (s, 1H, indole-NH). MS m/z 215.7 [M+1]+. Elemental analysis calculated (%) for C17H12F2N2O2: C: 64.97, H: 3.85, N: 8.91. Found: C: 65.35, H: 4.19, N: 8.97.
(E)-N-(3-(3,4-Dichlorobenzylidene)-2-oxoindolin-5-yl) acetamide (9)
Yield: 76%, mp: 304-305°C. 1H (d6 400 MHz), δ (ppm): 2.00 (s, 3H, H3), 6.73 (d, J= 8 Hz, 1H, H-7), 7.22 (dd, Jo Jm= 2 Hz, 1H, H-6), 7.62 (s, 1H, H-8), 7.69 (d, J= 8.4 Hz, 1H, H-13), 7.91 (d, J= 2 Hz, 1H, H-4), 8.23 (d, J= 8.2 Hz, 1H, H-14), 8.80 (d, J= 2 Hz, 1H, H-10), 9.82 (s, 1H, NHCOH3), 10.61 (s, 1H, indole-NH). MS m/z 347.8 [M]+. Elemental analysis calculated (%) for C17H12Cl2N2O2: C: 58.81, H: 3.48, N: 8.07. Found: C: 58.96, H: 3.44, N: 8.21.
(Z)-N-(3-(4-(Dimethylamino)benzylidene)-2-oxoindolin-5- yl)acetamide (10)
Yield: 55%, mp: 278-280°C. 1H (d6 400 MHz), δ (ppm): 1.96 (s, 3H, H3), 3.01 (s, 6H, N(H3)2), 6.74 (d, J= 8 Hz, 1H, H-7), 6.78 (d, J= 8 Hz, 2H, H-11 and H-13) 7.37 (dd, Jo Jm= 2 Hz, 1H, H-6), 7.46 (s, 1H, H-8), 7.64 (d, J= 8.8 Hz, 2H, H-10 and H-14), 8.11 (d, J= 1.6 Hz, 1H, H-4), 9.74 (s, 1H, NHCOH3), 10.31 (s, 1H, indole-NH). MS m/z 322.8 [M+1]+. Elemental analysis calculated (%) for C19H19N3O2: C: 71.01, H: 5.96, N: 13.08. Found: C: 70.89, H: 5.96, N: 12.96.
(E:Z=1:1)-N-(3-(4-Methoxybenzylidene)-2-oxoindolin-5-yl) acetamide (11)
Yield: 71%, mp: 240-242°C. E-isomer: 1H (d6 400 MHz), δ (ppm): 1.99 (s, 3H, H3), 3.82 (s, 3H, OH3), 6.70 (d, J= 8 Hz, 1H, H-7), 7.04 (d, J= 8 Hz, 2H, H-11 and H-13) 7.20 (dd, Jo Jm= 2 Hz, 1H, H-6), 7.54 (s, 1H, H-8), 7.69 (d, J= 8 Hz, 2H, H-10 and H-14), 7.83 (d, J= 2 Hz, 1H, H-4), 9.77 (s, 1H, NHCOH3), 10.46 (s, 1H, indole-NH). Z-isomer: 1.95 (s, 3H, H3), 3.80 (s, 3H, OH3), 6.76 (d, J= 8 Hz, 1H, H-7), 7.00 (d, J= 8 Hz, 2H, H-11 and H-13) 7.39 (dd, Jo Jm= 2 Hz, 1H, H-6), 7.52 (s, 1H, H-8), 8.02 (d, J= 2 Hz, 1H, H-4), 8.45 (d, J= 8 Hz, 2H, H-10 and H-14), 9.73 (s, 1H, NHCOH3), 10.41 (s, 1H, indole -NH). MS m/z 309.8 [M+1]+. Elemental analysis calculated (%) for C18H16N2O3: C:70.12, H: 5.23, N: 9.09. Found: C: 70.52, H: 5.26, N: 9.01.
(E)-N-(3-(4-Methylbenzylidene)-2-oxoindolin-5-yl) acetamide (12)
Yield: 34%, mp: 265-267°C. 1H (d6 400 MHz), δ (ppm): 2.02 (s, 3H, NHCOH3), 2.37 (s, 3H, H3), 6.74 (d, J= 8 Hz, 1H, H-7), 7.24-7.28 (m, 3H, H-6, H-11 and H-13), 7.58 (s, 1H, H-8), 7.88 (d, J= 1.6 Hz, 1H, H-4), 8.30 (d, J= 8.0 Hz, 2H, H-10 and H-14), 9.81 (s, 1H, NHCOH3), 10.1 (s, 1H, indole-NH). MS m/z 293.7 [M+1]+. Elemental analysis calculated (%) for C18H16N2O2: C: 73.95, H: 5.52, N: 9.58. Found: C: 73.55, H: 5.66, N: 9.46.
(E)-N-(3-(2-Chloro-6-fluorobenzylidene)-2-oxoindolin-5-yl) acetamide (13)
Yield: 72%, mp: 259°C. 1H (d6 400 MHz), δ (ppm): 1.89 (s, 3H, H3), 6.76 (d, J= 8 Hz, 1H, H-7), 7.26 (t, 1H, H-12), 7.30 (s, 1H, H-8), 7.36 (dd, Jo Jm= 2 Hz, 1H, H-6), 7.40 (s, 1H, H-4), 7.49 (d, J= 7.6 Hz, 1H, H-11), 7.56 (m, 1H, H-13), 9.70 (s, 1H, NHCOH3), 10.59 (s, 1H, indole-NH). MS m/z 331.9 [M+1]+. Elemental analysis calculated (%) for C17H12ClFN2O2: C: 61.73, H: 3.66, N: 8.47. Found: C: 61.33, H: 3.98, N: 8.40.
(E)-N-(3-(2,6-Dichlorobenzylidene)-2-oxoindolin-5-yl) acetamide (14)
Yield: 69%, mp: 176-178°C. 1H (d6 400 MHz), δ (ppm): 1.87 (s, 3H, H3), 6.75 (d, J= 8 Hz, 1H, H-7), 7.01 (d, J= 2 Hz, 1H, H-4), 7.34 (dd, Jo Jm= 2 Hz, 1H, H-6), 7.35 (s, 1H, H-8), 7.50 (t, 1H, H-12), 7.60 (d, 2H, J= 8 Hz, H-11 and H-13), 9.66 (s, 1H, NHCOH3), 10.58 (s, 1H, indole-NH). MS m/z 347.6 [M]+. Elemental analysis calculated (%) for C17H12Cl2N2O2: C: 58.81, H: 3.48, N: 8.07. Found: C: 58.82, H: 3.31, N: 8.06.
Src kinase assay
The effect of test compounds on protein tyrosine kinase was evaluated by using Universal Tyrosine Kinase Assay Kit (Takara, MK410, Japan) according to the manufacturer’s instructions. This assay is based on monitoring the transfer of g-phosphate residue from ATP to peptide substrates. The phosphorylation of tyrosine was started with addition of ATP-2Na and the plate immobilized with peptid substrate was incubated with test reagents at 37°C for 30 min. The phosphorylation level of substrate was probed with HRP-conjugated anti-phosphotyrosine (PY20) antibody. The test compounds were applied at 0.01, 0.1, 1, 10 and 100 mM concentrations and the calibrtion curve was constructed by monitoring the diminished activity of Src in at following concentrations: 0.88, 0.44, 0.22, 0.11, 0.06, 0.03, and 0.015 U/mL. The alteration on Src tyrosine kinase activity was calculated by comparing the activity in the presence of test compounds within the total activity of blank (DMSO). The IC50 value was determined by calculating the concentration of each test compound to achieve 50% inhibition of Src tyrosine kinase activity.
Molecular docking study
All the docking calculations were performed using Autodock Vina program. The crystal structure of Src kinase (PDB code 3GEQ) was extracted from the protein data bank (PDB) and it was firstly modified by removing water molecules and adding polar hydrogens. The docking area was defined by a box, centered on the native ligand PP2 (Figure 2). Grid points of 30x30x30 with 1.0 Å spacing were calculated using Autodock Vina default optimization parameters. Exhaustiveness was set to 30. For the validation of the dock method, the native ligand (PP2) was docked into its binding site. The RMSD of the docked PP2 was 0.104 Å as it appeared to be superimposed almost exactly on the native ligand. Moreover, the obtained binding free energy (ΔGb) was quite low being -8.8 kcal/mol. 2D structures of aforementioned compounds were established by using ChemBioDrawUltra 11.0, then they were energitically minimized with HyperChem8.0.7 using Semi Emperical Hamiltonian AM1 and saved in mol2 format with ChemBio3DUltra 11.0. The rigid root and rotatable bonds of compounds were defined by Autodock Tools (ADT, version 1.5.6). The resulting files were saved as pdbqt files. The docking results from each calculation were clustered on the basis of root-mean square deviation (RMSD) and were ranked according to the binding free energy. The structure with relative lower binding free energy was chosen for the optimum docking conformation.
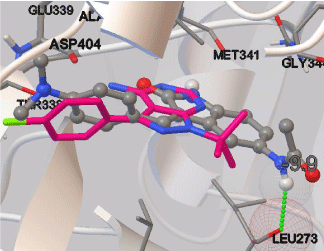
Figure 2: Comparison of PP2 (pink, stick model) and compound 10 (gray, ball
and stick model) binding properties in the active site of Src. H-bond between
compound 10 and Leu271 is represented as green dashed lines.
Results and Discussion
Chemistry
The target compounds (5–14) were reported in Scheme 1. Oxindole (1) was obtained by a Wolff–Kishner reduction of isatin in 50% yield [18]. 5-Nitro oxindole (2) was prepared by stirring of oxindole (1) with potassium nitrate in concentrated sulfuric acid at 0–5°C for 30min [19]. 5-Aminoindolin-2-one (3) was generated from nitro compound (2) by catalytic hydrogenation in moderate yield [22]. Reaction of compound 3 with acetic anhydride afforded the compound 4. Finally, the target compounds N-(3-substitutedbenzylidene- 2-oxoindolin-5-yl)acetamide (5–14) were prepared by condensation of compound 4 with substituted benzaldehydes in ethanol at reflux [23].
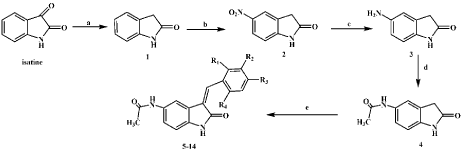
scheme 1: Synthesis of N-(3-substituted-benzylidene-2-oxoindolin-5-yl)
acetamide derivatives. Reagents and conditions: (a) Hydrazine hydrate, 140
°C, 4h, ice water, pH 2, rt, 2 days; (b) KNO3, H2SO4, 0-5 °C (c) Pd/C 10 %, H2,
MeOH, 45 psi, 3h, rt, (d) acetic anhydride, THF, 3h, rt (e) various substitutedbenzaldehydes,
piperidine, EtOH, reflux.
The all of target compounds (5-14) were obtained as the mixture of E- and Z-isomer due to the exocyclic double bond at 3-position of indole ring. Due to the rapid interconversion in solution at room temperature, the mixture of isomers could not be separated by column chromatography. The Z-isomer was assigned to the major isomer for compound 10, while other compounds were obtained as major E-isomer. The 1H chemical shifts of all compounds have been reported here to prove the major isomers. For only compound 11, E/Z ratio of isomers could be determined for as 1:1 using the corresponding chemical shifts and integrals in the 1H spectra. Some pure single isomers were obtained either Z or E isomer by precipitation and crystallized with ethanol. In the Z-isomer, H-10 and H-14 of compound 11 display a down-field shift (8.45 ppm) than E-isomer (7.69 ppm) due to the deshielding effect of the carbonyl group at the 2-position of indole ring. The signals from H-4 of Z-isomers of compound 10 (8.11 ppm) and 11 (8.02 ppm) were markedly shifted downfield relative to the H-4 signal of other E-isomers of target compounds (7.01-7.93 ppm) due to the shielding effect of H-10 and H-14 of benzylidene substituent at the 3-position of indole ring [24, 25].
Biological evaluation
The inhibitory activity of compounds (5-14) against Src kinase were evaluated by universal tyrosine kinase assay that measures the changes in the enzymatic activity of Src kinase by virtue of following the alterations in the phosphorylation level of the immobilized substrate that was used for this analysis [26]. The activities of the these compounds were analyzed at 0.01, 0.1, 1, 10, and 100mM concentrations and IC50 values were calculated. None of compounds showed strong inhibitory potencies against Src kinase. Only compounds 10, 12 and 13 were found slightly active against Src kinase with IC50 value of 3.55, 6.39 and 7.29 mM, respectively. The insertion of methyl group instead of dimethylamino in the para position of benzylidene moiety of the best active compound 10, leading to compound 12, brought approximately 2-fold decrease of Src inhibitory activity. In addition, the replacement of the dimethylamino in compound 10 with a chloro (5), fluoro (6) and methoxy (11) resulted in complete loss of activity. Compound have p-dimethylaminobenzylidene substitution at third position 10 was well tolerated comparison to compounds 12 (IC50= 6.39 mM) and 13 (IC50= 7.29 mM). This indicates that more hydrophilic compounds would help improve inhibitory activity.
Comparison with slightly active 2-chloro-6-fluorobenzylidene derivative 13, the compound bearing 2,6-dichlorobenzylidene moiety 14, did not inhibit the Src kinase enzyme. It was also reported that compounds having the polar and flexible ethylurea and benzylthiourea substituents at the 5-position of indole, significantly improved the inhibitory properties of Src kinase [17]. In addition 5-methylaminosulphonic acid containing indol-2-on derivatives possess strong activity against Src [27]. Replacing ethylthiourea, benzylthiourea and methylaminosulphonic acid group with acetamido group considerably diminished the inhibitory potence of these novel compounds. These results show that type of substituents at 5-position is very important for Src kinase inhibition.
Molecular docking
To explore the interactions of compounds with Src kinase, we docked the active compounds 10, 12 and 13 into the Src using Autodock vina. The protein structures of the Src was downloaded from PDB (PDB code: 3GEQ). The best active compound 10 (ΔGb: -9.9 kcal/mol) located in somewhat similar position to the PP2 with overlapping p-dimethylaminobenzylidene at 3-position of indole ring and p-chlorophenyl group of PP2. Moreover, acetamido group at 5-position of indole ring of compound 10 was directed toward the solvent accessible region as tertbutyl group of PP2 and form hydrogen bond with carbonyl of Leu273 (angle N-H···O = 138.6, distance = 2.1 Å, Figure 2). Among the docked compounds (10, 12 and 13), compound 12 revealed the poor binding affinity with the binding free energy of ΔGb: -9.7 kcal/mol and showed no hydrogen bonding interaction with Src kinase. Despite of the exhibiting one hydrogen bond interaction between indole carbonyl and OH of Thr338 with strong binding affinity (ΔGb: -10.1 kcal/mol), compound 13 demonstrated the weakest inhibitory activity against Src. The weak Src inhibitory activity results of compounds could be explained with lack of hydrogen bonding interaction with critical amino acids as Met 341 and Glu 339 for the binding to the Src active site.
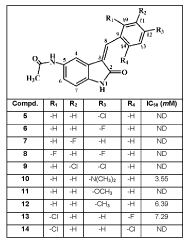
table 1: Src kinase inhibitory activity of N-(3-substituted-benzylidene-2-
oxoindolin-5-yl)acetamide derivatives.
Conclusion
In this study, we designed and synthesized novel N-(3-substitutedbenzylidene- 2-oxoindolin-5-yl)acetamide (5-14) derivatives as Src kinase inhibitors and evaluated relationships between biological activity and binding properties of compounds by Autodock vina. Some of the compounds (10, 12 and 13) exhibited slight inhibition of Src. The best inhibitor activity was obtained by compound 10 with IC50 value of 3.55 mM. According to the docking results, compound 10 formed hydrogen bond between the acetamide NH and carbonyl of Leu273, which is a different H-bond interaction than PP2. Differences in binding properties of compound 10 into the Src catalytic site might contribute explaining the weaker inhibitory activity than PP2 against Src kinase. In conclusion, it may be necessary to design some compounds with exactly the same binding properties with PP2 to obtain better activity results.
Acknowledgements
This project is received a funding support from Ankara University (BAP 09B3336002) Scientific Research Funds.
References
- Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012; 33: 122-128.
- Chojnacka K, Mruk DD. The Src non-receptor tyrosine kinase paradigm: New insights into mammalian Sertoli cell biology. Mol Cell Endocrinol. 2015; 415: 133-142.
- Ahluwalia MS, de Groot J, Liu WM, Gladson CL. Targeting SRC in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett. 2010; 298: 139-149.
- Sawyer T, Boyce B, Dalgarno D, Iuliucci J. Src inhibitors: genomics to therapeutics. Expert Opin Investig Drugs. 2001; 10: 1327-1344.
- Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004; 23: 7906-7909.
- Manetti F, Locatelli GA, Maga G, Schenone S, Modugno M, Forli S, et al. A combination of docking/dynamics simulations and pharmacophoric modeling to discover new dual c-Src/Abl kinase inhibitors. J Med Chem. 2006; 49: 3278-3286.
- Fallah-Tafti A, Foroumadi A, Tiwari R, Nasrolahi Shirazi A, Hangauer DG, Bu Y, et al. Thiazolyl N-benzyl-substituted acetamide derivatives: Synthesis, Src kinase inhibitory and anticancer activities. Eur J Med Chem. 2011; 46: 4853–4858.
- Kim EM, Mueller K, Gartner E, Boerner J. Dasatinib is synergistic with cetuximab and cisplatin in triple-negative breast cancer cells. J Surg Res. 2013; 185: 231-239.
- Dawn B, Takano H, Tang XL, Kodani E, Banerjee S, Rezazadeh A, et al. Role of Src protein tyrosine kinases in late preconditioning against myocardial infarction. Am J Physiol Heart Circ Physiol. 2002; 283: H549-556.
- Susva M, Missbach M, Green J. Src inhibitors: drugs for the treatment of osteoporosis, cancer or both? Trends Pharmacol Sci. 2000; 21: 489-495.
- Rouer E. [Neuronal isoforms of Src, Fyn and Lck tyrosine kinases: A specific role for p56lckN in neuron protection]. C R Biol. 2010; 333: 1-10.
- Tintori C, Magnani M, Schenone S, Botta M. Docking, 3D-QSAR studies and in silico ADME prediction on c-Src tyrosine kinase inhibitors. Eur J Med Chem. 2009; 44: 990-1000.
- Guan H, Laird AD, Blake RA, Tang C, Liang C. Design and synthesis of aminopropyl tetrahydroindole-based indolin-2-ones as selective and potent inhibitors of src and yes tyrosine kinase. Bioorg Med Chem. 2004; 14: 187–190.
- Milkiewicz KL, Marsilje TH, Woodworth RP, Bifulco N, Hangauer MJ, Hangauer DG. The design, synthesis and activity of non-ATP competitive inhibitors of pp60c-src tyrosine kinase. part 2: Hydroxyindole derivatives. Bioorg Med Chem Lett. 2000; 10: 483–486.
- Schmidt S, Preu L, Lemcke T, Totzke F, Schächtele C, Kubbutat MH, et al. Dual IGF-1R/SRC inhibitors based on a N'-aroyl-2-(1H-indol-3-yl)-2-oxoacetohydrazide structure. Eur J Med Chem. 2011; 46: 2759-2769.
- Olgen S, Akaho E, Nebioglu D. Synthesis and anti-tyrosine kinase activity of 3-(substituted-benzylidene)-1, 3-dihydro-indolin derivatives: investigation of their role against p60c-Src receptor tyrosine kinase with the application of receptor docking studies. Farmaco. 2005; 60: 497-506.
- Kilic-Kurt Z, Bakar F, Ölgen S. Synthesis, Biological, and Computational Evaluation of Novel 1,3,5-Substituted Indolin-2-one Derivatives as Inhibitors of Src Tyrosine Kinase. Arch Pharm (Weinheim). 2015; 348: 715-729.
- Carlo FJ. Synthesis of oxindole. J Am Chem Soc. 1944; 66: 1420.
- Kniess T, Kuchar M, Wuest F. Facile synthesis of various nitro-substituted derivatives of semaxinib (SU5416). Synth Commun. 2008; 38: 3017–3022.
- Kisteneva MS. Trombopoietin activity modulating compounds and methods. Zh. Obshchei Khim. 1956; 26: 2019–2025.
- Pauls HW, Forrest BT, Laufer R. Indazolyl, benzimidazolyl, benzotriazolyl substituted indolinone derivatives as kinase inhibitors useful in the treatment of cancer. 2009, WO 2009/079767 A1.
- Arnaiz D, Bryant J, Chou Y. Indolinone derivatives and their use in treating disease-states such as cancer. US 2005/0090541 A1.
- Sun L, Tran N, Tang F, App H, Hirth P, McMahon G, Tang C. Synthesis and biological evaluations of 3-substituted indolin-2-ones: a novel class of tyrosine kinase inhibitors that exhibit selectivity toward particular receptor tyrosine kinases. J Med Chem. 1998; 41: 2588–2603.
- Mologni L, Rostagno R, Brussolo S, Knowles PP, Kjaer S, Murray-Rust J, et al. Synthesis, structure-activity relationship and crystallographic studies of 3-substituted indolin-2-one RET inhibitors. Bioorg Med Chem. 2010; 18: 1482–1496.
- Hung CY, Hsu MH, Huang LJ, Hwang CS, Lee O, Wu CY, et al. Synthesis of 1-substituted 3-pyridinylmethylidenylindolin-2-ones and 1-substituted 3-quinolinylmethylidenylindolin-2-ones as the enhancers of ATRA-induced differentiation in HL-60 cells. Bioorg Med Chem. 2008; 16: 4222-4232.
- Taylor VC, Buckley CD, Douglas M, Cody AJ, Simmons DL, Freeman SD. The myeloid-specific sialic acid-binding receptor, CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2. J Biol Chem. 1999; 274: 11505-11512.
- Guan H, Laird AD, Blake RA, Tang C, Liang C. Design and synthesis of aminopropyl tetrahydroindole-based indolin-2-ones as selective and potent inhibitors of Src and Yes tyrosine kinase. Bioorg Med Chem Lett. 2004; 14: 187-190.