
Research Article
Austin J Anal Pharm Chem. 2015; 2(5): 1054.
GC-MS and HPLC Methods for Determination of Estriol Hormone in Pharmaceutical Preparations
Bilal Yilmaz*
Department of Analytical Chemistry, Faculty of Pharmacy, Ataturk University, 25240, Erzurum, Turkey
*Corresponding author: Dr. Bilal Yilmaz, Department of Analytical Chemistry, Faculty of Pharmacy, Ataturk University, 25240, Erzurum, Turkey.
Received: November 16, 2015; Accepted: December 09, 2015; Published: December 11, 2015
Abstract
This paper describes two rapid, sensitive and specific methods for determination of estriol hormone in pharmaceutical preparations by gas chromatography (GC) with mass spectrometry (MS) and high-performance liquid chromatography (HPLC) with fluorescence detection. The calibration curves were linear for estriol (r > 0.99) at the concentration range of 12.5- 500 ng/ml and 10-400 ng/ml for GC-MS and HPLC, respectively. The relative standard deviation (RSD) for intra- and inter-day precision was less than or equal to 4.72 and 6.25%, respectively. The developed methods were applied to a pharmaceutical preparation (Gynoflor) as tablet form which does not require any preliminary separation or treatment of the samples. No interference was found from tablet excipients at the selected assay conditions. Also, the results obtained from the methods were statistically compared and no significant difference was found.
Keywords: Estriol; GC-MS; HPLC; Tablet; Validation
Introduction
Estrogens are naturally occuring hormones from androgen precursors in the ovarian follicles of premenopausal women under the influence of the pituitary. They are given for replacement therapy in deficiency states for menopausal, postmenopausal disorders and contraception. They are administered orally, subcutaneously via an implant, locally as vaginal cream or tablets, intramuscularly or transdermally via a skin patch. Also, they are also used in the management of breast cancer in menopausal and postmenopausal women and in the management of prostate cancer [1].
Estriol, (1,3,5,(10)-estratriene-3,16α, 17β-triol), is by far the most abundant estrogen present in pregnant mammals. Oral estriol tablets have been used with good results, primarily for the treatment of local urogenital complaints in postmenopausal women for over 40 years. One of its product characteristics is that it does not stimulate the endometrium. Therefore, it can be used uninterrupted without the cyclical addition of a progestogen to protect the endometrium. Although all available data confirm the endometrial safety of oral estriol tablets, it is considered relevant to update the existing longterm data on this topic [2,3].
Several methods have been reported for determination of estriol including HPLC [4], immunoassay [5] and GC-MS [6] in biological samples. Since most chromatographic methods have been developed for environmental samples, immunoassay is the preferred techniques for biological samples because of its specificity and sensitivity. Radioimmunoassay (RIA) [7], enzyme immunoassay (EIA) [8] and fluorescence immunoassay (FIA) [9] have been widely applied in screening and determination of estriol. RIA allows rapid and sensitive screening of large number of samples. However, the major disadvantage of this conventional technique is that it requires radioisotopes and produces radioactive waste. Although EIA and FIA are inexpensive, they are labor-intensive and not sensitive enough for determination of estriol.
To our knowledge, there is no GC-MS method for determination of estriol in pharmaceutical preparations in literature. Therefore, we report GC-MS and HPLC methods for determination of estriol in pharmaceutical preparations. The proposed methods in this study are accurate, sensitive, and precise and can be easily applied to Gynoflor tablet as pharmaceutical preparation. The results obtained by the methods were statistically compared and there was no significant difference between two methods.
Materials and Methods
Chemicals
Estriol was obtained from Hıfzıssıhha Laboratory (Erzurum, Turkey). Acetonitrile (HPLC grade) was purchased from Fluka (Buchs, Switzerland), and other chemicals and solvents used were of analytical grade. N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Gynoflor tablet containing 0.03 mg of estriol was obtained from pharmacy (Erzurum, Turkey).
GC-MS conditions
Chromatographic analysis was carried out on an Agilent 6890N gas chromatography system equipped with 5973 series mass selective detector, 7673 series auto sampler and chemstation (Agilent Technologies, Palo Alto, CA). HP-5 MS column with 0.25μm film thickness (30m × 0.25mm I.D., USA) was used for separation. Split less injection was used and the carrier gas was helium at a flow rate of 2 ml/min. The MS detector parameters were transfer line temperature 280°C, solvent delay 3 min and electron energy 70 eV.
HPLC conditions
A Perkin Elmer series 200 HPLC system equipped with programmable fluorescence detector and Total Chromatography Data System software was used (Perkin Elmer Life and Science, Shelton, CT, USA). Separation was achieved using an Ace C18 column (5μm, 4.6×250mm i.d.) with a guard column (4mm × 3mm i.d., Phenomenex) packed with the same material at a flow rate of 1 ml/ min. The injection volume was 20μl. The eluent was monitored by fluorescence detection at 280nm (excitation) and 310nm (emission).
Preparation of stock and standard solutions
The stock solution of estriol was prepared with acetonitrile to a concentration of 1000 ng/ml and stored at -200C under refrigeration. Standard solutions were prepared as 12.5-500ng/ml for GC-MS and 10-400ng/ml for HPLC. The quality control (QC) solutions were prepared by adding aliquots of standard working solution of estriol to final concentrations of 75, 250 and 450ng/ml for GC-MS and 75, 250 and 350ng/ml for HPLC.
Procedure for pharmaceutical preparation
The average tablet mass was calculated from the mass of tablets of Gynoflor (0.03 mg estriol tablet, which was composed of estriol and some excipients). They were then finely ground, homogenized and portion of the powder was weighed accurately, transferred into a 10 ml brown measuring flask and diluted to scale with acetonitrile. The mixture was sonicated for at least 10 min to aid dissolution and then filtered through a Whatman 42 paper. An appropriate volume of filtrate was diluted further with acetonitrile so that the concentration of estriol in the final solution was within the working range and then analyzed by GC-MS and HPLC.
Data analysis
All statistical calculations were performed with the Statistical Product and Service Solutions (SPSS) for Windows, version 10.0. Correlations were considered statistically significant if calculated P values were 0.05 or less.
Results and Discussion
Method development and optimization
The method development for the assay of estriol was based on its chemical properties. The column and acquisition parameters were chosen to be a starting point for the method development. Estriol is a polar molecule. Therefore, the capillary column coated with 5% phenyl and 95% dimethylpolysiloxane is a good choice for separation of estriol. GC-MS method sensitivity is not enough for the determination of estriol. For this reason, MSTFA was chosen as a chromagenic derivatization reagent.
MSTFA is an effective trimethylsilyl donor. MSTFA reacts to replace labile hydrogens on a wide range of polar compounds with a -Si(CH3)3 group and is used to prepare volatile and thermally stable derivatives for GC-MS [10]. The hydroxy (-OH) groups, which render the compounds non-volatile and polar, were converted to the corresponding silyl (-O-TMS) groups, thereby rendering them volatile and non-polar. The effects of time and temperature on the reaction were investigated. To 50μl of 1000ng/ml estriol solution and 50μl of MSTFA solution was added and reacted at room temperature, 50°C and 75°C for 5 min, 10min and 20min. The resulting samples were quantitated by GC-MS system. After standing for 10min at room temperature, maximum peak areas were quantitated.
Different temperature programs were investigated for GC-MS method. The injection port and detector temperatures were set to 250 and 290ºC, respectively. The end of this investigation, the temperature program of GC-MS was as follows: initial temperature was 140°C, held for 1.0min, and then increased to 310°C at a rate of 40°C/min for 2.5min. The injector volume was 1μl in split less mode.
During HPLC method development, it was focused on the optimization of column detection, sample preparation and chromatographic separation. Reversed-phase column (C18) can be used for the separation of non-ionic as well as ion forming non-polar to medium polar substances while normal phase chromatography can be used for the separation of non-ionic and/or non-polar substances. Majority of the ionizable pharmaceutical compounds can be very well separated on C18 [11]. Thus, estriol can be satisfactorily separated by reversed phase chromatography.
Several tests were performed for optimizing the components of mobile phase in order to achieve good chromatographic peak shape and resolution. The test results showed that the solvent system of water could improve the peak shapes of estriol. Good separation of target compounds and short run time were obtained using a mobile phase system of water containing 0.1% trifluoroacetic acid (TFA)- methanol (40:60, v/v). The retention time of estriol (3.60 min) was quite short than that studied by Tagawa et al. [4]. On the other hand, the mobile phase in the proposed method methanol-water instead of buffered systems is used in previously reported HPLC method [4]. Therefore, flushing of the column after analysis is not required.
Method validation
System Suitability: A system suitability test of the HPLC system was performed before each validation run. Five replicate injections of a system suitability/calibration standard and one injection of a check standard were made. Area relative standard deviation, tailing factor and efficiency for the five suitability injections were determined. The check standard was quantified against the average of the five suitability injections. For all sample analyses, the tailing factor was ≤ 1.12, efficiency ≥ 2045 and %RSD ≤1.83%.
Specificity: The specificity of the two methods was investigated by observing interferences between estriol and the excipients. For GC-MS, electron impact mode with selected ion monitoring (SIM) was used for quantitative analysis (m/z 504 for estriol). The mass spectrum after derivatization of estriol with MSTFA is shown in Figure 1.
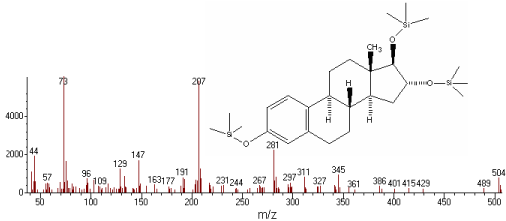
Figure 1: MS spectra after derivatization of estriol with MSTFA.
The retention time of estriol-tri-TMS for GC-MS was approximately 6.82min (Figure 2). Also, HPLC analysis was performed in less than 5min (Figure 3).
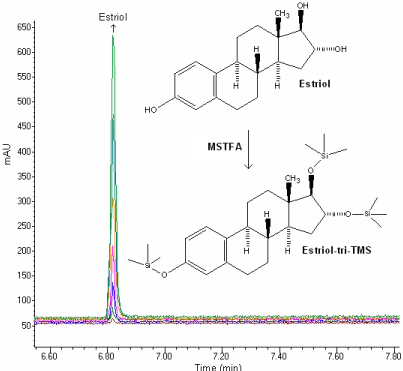
Figure 2: GC-MS chromatogram of estriol (12.5, 25, 50, 100, 250, 400 and
500 ng/ml).
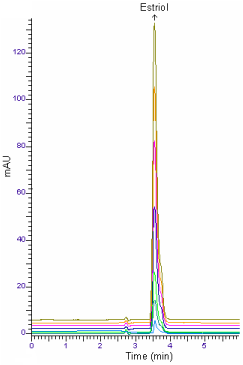
Figure 3: HPLC chromatogram of estriol (10, 25, 50, 100, 200, 300 and 400
ng/ml).
Linearity: For GC-MS and HPLC measurements, the solutions were prepared by dilution of the stock solution of estriol to reach a concentration range of 12.5-500 ng/ml (12.5, 25, 50, 100, 250, 400 and 500 ng/ml) and 10-400 ng/ml (10, 25, 50, 100, 200, 300 and 400 ng/ml), respectively. Calibration curves were constructed for estriol standard by plotting the concentration of compound versus peak area response. The calibration curves were evaluated by its correlation coefficients. The correlation coefficients (r) of all the calibration curves were consistently greater than 0.99. The linear regression equations were calculated by the least squares method using Microsoft Excel® program and summarized in Table 1.
Methods
Range
(ng/ml)
LRa
Sa
Sb
R
LOD
(ng/ml)
LOQ
(ng/ml)
GC-MS
12.5-500
y=22.82x + 4.364
2.874
0.356
0.9971
4.0
12.5
HPLC
10-400
y=36.19x - 6.963
4.210
0.198
0.9982
3.0
10
aBased on three calibration curves, LR: linear regression, Sa: standard deviation of intercept of regression line,
Sb: Standard deviation of slope of regression line; R: Coefficient of correlation; y: peak area; x: estriol concentration.
LOD: Limit of detection; LOQ: limit of quantification.
Table 1: Linearity of estriol by GC-MS and HPLC methods.
Precision and accuracy: Assay precision was determined by repeatability (intra-day) and intermediate precision (inter-day). Repeatability during the same day and intermediate precision on different days (3 days) were evaluated with six replicates of QC samples. The accuracy of this analytic method was assessed as the percentage relative error. The intra-day RSD was ≤4.72% and the inter-day RSD was ≤6.25% for QC samples. The intra- and inter-day relative error for accuracy was ≤3.52%. Results are shown in Table 2.
Method
Added
(ng/ml)
Intra-day
Inter-day
GC-MS
Found ± SD
Accuracy
Precision
RSD%a
Found ± SD
Accuracy
Precision
RSD%a
75.0
76.7 ± 3.346
2.27
4.36
76.9 ± 3.120
2.53
4.06
250
246.7 ± 7.482
-1.32
3.03
243.3 ± 9.047
-2.68
3.72
450
451.3 ± 21.303
0.29
4.72
465.4 ± 24.156
3.42
5.12
HPLC
75.0
73.2 ± 2.852
-2.40
3.89
77.1 ± 3.624
2.80
4.70
250
241.8 ± 6.126
-3.52
2.53
258.4 ± 12.425
3.36
4.81
350
345.1 ± 15.126
-1.40
4.38
358.4 ± 22.417
2.40
6.25
SD: Standard deviation of six replicate determinations, RSD: relative standard deviation
a Average of six replicate determinations
Accuracy: (%relative error) (found-added)/addedx100
Table 2: Precision and accuracy of estriol by GC-MS and HPLC methods.
Limits of Detection (LOD) and Quantitation (LOQ): The LOD is the lowest concentration of the analyte detected by the method. The LOQ is the minimum quantifiable concentration. The signal-tonoise ratio of 3:1 and 10:1 were taken as LOD and LOQ, respectively. In the present study the LOQ values of estriol were 12.5 and 10ng/ ml for GC-MS and HPLC, respectively. These values are also listed in Table 1.
Stability
Stability studies indicated that the samples were stable when kept at room temperature, +40C and -200C refrigeration temperature for 24h (short-term) and 72h (long-term). There was no significant change in the analysis over a period of 72 hours. The mean RSD between peak areas for the samples stored under refrigeration (4±1 °C), at room temperature (25±1 °C) and refrigeration (-20 ± 1 °C) were found to be 5.241%, 5.42% and 6.43%, respectively, suggesting that the drug solution can be stored without any degradation over the studied time interval (Table 3).
Stability (%)
Room temperature stability
Refrigeratory stability, +4°C
Frozen stability, - 20°C
(Recovery % ± RSD)
(Recovery % ± RSD)
(Recovery % ± RSD)
Method
Added
(ng/ml)
24 h
72 h
24 h
72 h
24 h
72 h
GC-MS
75.0
99.2 ± 3.11
102.1 ± 4.29
98.9 ± 2.84
101.4 ± 4.41
97.6 ± 3.08
102.4 ± 4.46
250
101.2 ± 2.86
98.5 ± 3.52
97.4 ± 2.97
99.4 ± 5.27
102.7 ± 3.45
98.4 ± 4.70
450
98.6 ± 1.94
99.6 ± 3.46
101.5 ± 3.18
102.3 ± 4.74
99.7 ± 3.79
102.2 ± 4.68
HPLC
75.0
98.2 ± 3.25
101.4 ± 4.42
101.2 ± 4.11
98.2 ± 4.46
97.2 ± 4.76
99.2 ± 5.43
250
991.2 ± 2.94
102.3 ± 3.84
98.6 ± 3.42
101.9 ± 5.42
97.9 ± 3.95
102.1 ± 6.43
350
101.2 ± 3.84
98.4 ± 5.24
102.2 ± 5.49
98.4 ± 4.18
101.7 ± 3.76
103.1 ± 5.28
RSD: Relative standard deviation of six replicate determinations.
Table 3: Stability of estriol in solution.
Recovery
To determine the accuracy of the GC-MS and HPLC methods and to study the interference of formulation additives, the recovery was checked as three different concentration levels (50, 150, 350 ng/ ml) and analytical recovery experiments were performed by adding known amount of pure drugs to pre-analyzed samples of 50ng/ml commercial dosage form Gynoflor. The percent analytical recovery values were calculated by comparing concentration obtained from the spiked samples with actual added concentrations. These values are also listed in Table 4.
Method
Pharmaceutical preparation
Added
(ng/ml)
Intra-day
Inter-day
GC-MS
Found ± SD
Recovery
(%)
RSDa
(%)
Found ± SD
Recovery
(%)
RSDa
(%)
Gynoflor tablet
(50 ng/ml)
50.0
49.1 ± 1.31
98.2
2.69
51.6 ± 2.28
103.2
4.42
150
148 ± 2.76
98.7
1.84
147 ± 3.41
98.0
2.32
350
354 ± 8.84
101.1
2.50
359 ± 12.46
102.6
3.47
HPLC
Gynoflor tablet
(50 ng/ml)
50.0
48.9 ± 2.01
97.8
4.11
51.2 ± 2.12
102.4
4.14
150
145 ± 2.98
96.7
2.06
146 ± 2.84
97.3
1.94
350
356 ±11.09
101.7
3.12
356 ± 10.12
101.7
2.84
SD: Standard deviation of six replicate determinations, RSD: relative standard deviation.
aAverage of six replicate determinations.
Table 4: Recovery of estriol in pharmaceutical preparation by GC-MS and HPLC methods.
Comparison of the methods
Today, GC-MS and HPLC methods are important and widely used as analytical techniques of quantitative and qualitative analysis. As compared to HPLC, high-resolution capillary GC-MS has inherently high resolving power and high sensitivity with excellent precision and accuracy [12].
A survey of literature reveals that no GC-MS and HPLC methods for determination of estriol in pharmaceutical preparations. The present work describes the validation parameters stated either by USP 26 [13] or by the ICH guideline [14] to achieve GC-MS and HPLC methods for determination of estriol. The proposed methods are very effective for the assay of estriol in tablets (Figures 4 and 5).
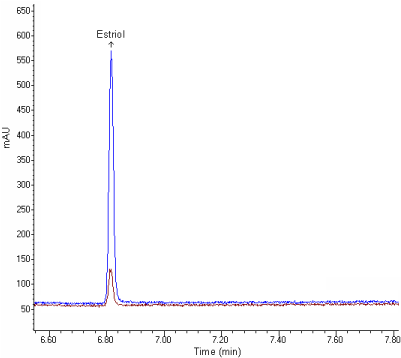
Figure 4: GC-MS chromatogram of Gynoflor tablet solution containing 50,
400 ng/ml of estriol.
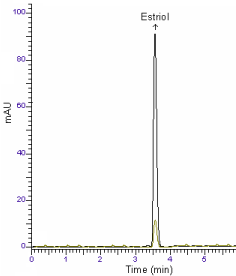
Figure 5: HPLC chromatogram of Gynoflor tablet solution containing 25, 200
ng/ml of estriol.
The validity of the proposed methods was presented by recovery studies using the standard addition method. For this purpose, a known amount of reference drug was spiked to formulated tablets and the nominal value of drug was estimated by the proposed methods. Each level was repeated six times. The results were reproducible with low SD and RSD. No interference from the common excipients was observed. According to the statistical comparison (Student t-test) of the results there is no significant difference between GC-MS and HPLC methods (Table 5).
Commercial preparation
Method
n
Found SD
(mg)
Recovery
(%)
RSDa
(%)
Confidence interval
t- test
Gynoflor tablet
(0.030 mg)
GC-MS
6
0.029 ± 0.0012
96.7
4.13
0.028-0.032
tt=1.78
tc=1.43
HPLC
6
0.031 ± 0.0016
103.3
5.16
0.029-0.033
n: number of determination, SD: standard deviation of six replicate determinations, RSDa: relative standard deviation.
tc: Calculated t-value, tt: tabulated t-value, Ho hypothesis: no statistically significant difference exists between two methods.
tt > tc: Ho hypothesis is accepted (P > 0.05).
Table 5: Determination of estriol in pharmaceutical preparation.
Conclusion
In this work, two new chromatographic methods have been developed and validated for routine determination of estriol hormone in pharmaceutical preparations. Linearity range, precision, accuracy, LOD and LOQ are suitable for the quantification of estriol in pharmaceutical preparations. The chromatographic run time of 7 min allows the analysis of a large number of samples in a short period of time. Therefore, the methods are also suitable for analysis of sample during accelerated stability studies, routine analysis of formulations and raw materials.
References
- Cesarino I, Cincotto FH, Machado SAS. A synergistic combination of reduced graphene oxide and antimony nanoparticles for estriol hormone detection. Sensors and Act B. 2015; 210: 453-459.
- Bradley LA, Canick JA, Palomaki GE, Haddow JE. Undetectable maternal serum unconjugated estriol levels in the second trimester: Risk of perinatal complications associated with placental sulfatase deficiency. Am J Obstet Gynecol. 1997; 176: 531-535.
- Reyes-Romero MA. The physiological role of estriol during human fetal development is to act as antioxidant at lipophilic milieus of the central nervous system. Med Hypotheses. 2001; 56: 107-109.
- Tagawa N, Tsuruta H, Fujinami A, Kobayashi Y. Simultaneous determination of estriol and estriol 3-sulfate in serum by column-switching semi-micro high-performance liquid chromatography with ultraviolet and electrochemical detection. J Chromatogr B. 1999; 723: 39-45.
- Podestà A, Smith CJ, Villani C, Montagnoli G. Shared reaction in solid-phase immunoassay for estriol determination. Steroids. 1996; 61: 622-626.
- Caban M, Lis E, Kumirska J, Stepnowski P. Determination of pharmaceutical residues in drinking water in Poland using a new SPE-GC-MS(SIM) method based on Speedisk extraction disks and DIMETRIS derivatization. Sci Total Environ. 2015; 538: 402-411.
- Schiøler V1, Thode J . Six direct radioimmunoassays of estradiol evaluated. Clin Chem. 1988; 34: 949-952.
- Worthman CM, Stallings JF, Hofman LF. Sensitive salivary estradiol assay for monitoring ovarian function. Clin Chem. 1990; 36: 1769-1773.
- Hanning A, Lindberg P, Westberg J, Roeraade J. Laser-induced fluorescence detection by liquid core waveguiding applied to DNA sequencing by capillary electrophoresis. Anal Chem. 2000; 72: 3423-3430.
- Yilmaz B, Arslan S. Determination of atenolol plasma concentration after derivatization with N-Methyl-N-(trimethylsilyl) trifluoroacetamide. Chromatographia. 2009; 70: 1399-1404.
- (First ed.) In: P.D. Sethi, Editor, High Performance Liqid Chromatography-Quantitative Analysis of Pharmaceutical Formulations. CBS Publishers & Distributors, Mumbai. 2001; 3-212.
- Yilmaz B, Arslan S, Akba V. Gas chromatography-mass spectrometry method for determination of metoprolol in the patients with hypertension. Talanta. 2009; 80: 346-351.
- USP 26/NF21, The United States Pharmacopeia, 26th Rev, and the National Formulary, 21st ed, United States Pharmacopeial Convention, Inc. (Eds.), Rockville, 2003.
- International Conference on Harmonisation (ICH) of Technical requirements for Registration of Pharmaceuticals for Human Use: Harmonised Tripartite Guideline on Validation of Analytical Procedures: Methodology, Recommended for Adoption at Step 4 of the ICH Process on November by the ICH Steering Committee, Published by IFPMA, Switzerland, 1996.