
Research Article
Austin J Anal Pharm Chem. 2015; 2(6): 1055.
Synthesis of New Chiral Phase Transfer Catalysts and their Application in the Asymmetric Darzens Reaction
Zhao Y¹, Xu P², Zhang X², Chen S², Yu Q², Wang Z² and Dai Z²*
¹Jiangsu Tianhe Pharmaceutical Company, Along the Yangtze River Economy District of Jiangdu, Yangzhou City, China
²Department of Pharmaceutical Chemistry, School of Pharmacy, China Pharmaceutical University, China
*Corresponding author: Zhenya Dai, Department of Pharmaceutical Chemistry, School of Pharmacy, China Pharmaceutical University, P. R. China.
Received: November 23, 2015; Accepted: December 17, 2015; Published: December 21, 2015
Abstract
Herein a serial of asymmetric Darzens reactions catalyzed by the novel chiral phase transfer catalysts derived from cinchona alkaloids were reported with moderate to high diastereoselectivity and with moderate enantioselectivity.
Keywords: Chiral phase transfer catalysts; Cinchona alkaloid; Darzens reaction; Diastereoselectivity; Enantioselectivity
Materials and Methods
Typical procedure of the synthesis of chiral catalyst 4a to 4d
(1S,7R,10S,E)-1-(quinolin-4-yl)-9-vinyl-1,3,6,8,9,10,11,11aoctahydro- 7,10-ethanopyrido[2,1-c][1,4]oxazocin-7-ium(4a): Cinchonidine (0.294g, 1mmol) was dissolved in THF (5ml), and sodium hydride (0.048g, 2mmol) was added. The reaction mixture was stirred and heated to 80oC and refluxed for ¹h, then (E) -1,4 – dibromo-2 – utane (0.321g, 1.5mmol) is added. The reaction mixture was further refluxed for 12 hours and the reaction was monitored by TLC. After the completion of the reaction, the solvent was evaporated and the residue was purified with silica gel (chloroform: methanol = 30:1) to give the product (0.24g, 70% yield).
¹H NMR(500 MHz, CDCl3): 8.9188-8.9043 (m, 1H) 8.2234- 8.1656 (m, 2H) 7.7959-7.6351 (m, 2H) 7.4242-7.4097 (m, 1H) 6.4735- 6.4328 (m, 1H) 6.1463-6.0199 (m,1H) 5.8805 (s, 1H) 5.7594-5.6443 (m, 2H) 5.0114-4, 9213 (m, 3H) 4.8283-4.7940 (m, 1H) 3.4942-3.4170 (m, 1H) 3.2820-3.1826 (m, 2H) 2.8837-2.7382 (m, 2H) 2.3971 (s, 1H) 1.9077-1.8730 (m, 3H), 1.6735-1.5299 (m, 2H) 13C NMR (500 MHz, CDCl3): 150.0278, 147.9949, 146.0408, 142.1767, 133.1039, 129.8867, 129.0374, 126.5786, 125.8792, 123.8068, 119.5861, 114.1071, 111.9291, 109.5361, 107.8249, 82.5908, 81.2770, 60.2081, 55.8686, 41.6474, 27.2226, 24.6763
ES-MS: 347.2 (M; [α]D 22 = +95.5°(c = 0.2 in CH2Cl2).
Elemental analysis: Calculated: C: 79.5%, H: 7.8%, N: 8.1%.
Found: C: 79.39%, H: 7.89%, N: 7.95%.
(1R,7R,10S,E)-1-(6-methoxyquinolin-4-yl)-9-vinyl- 1,3,6,8,9,10,11,11a-octahydro-7,10-ethanopyrido[2,1-c][1,4] oxazocin-7-ium(4b): Quinine (0.324g, 1mmol) was dissolved in THF (5ml), and sodium hydride (0.048g, 2mmol) was added. The reaction mixture was stirred and heated to 800C and refluxed for 1h, then (E) -1,4 – dibromo-2 – utane (0.321g, 1.5mmol) is added. The reaction mixture was further refluxed for 12 hours and the reaction was monitored by TLC. After the completion of the reaction, the solvent was evaporated and the residue was purified with silica gel (chloroform: methanol = 30:1) to give the product (0.238g, 63% yield).
¹H NMR (500 MHz, CDCl3): 8.7290-8.7204 (m,1H) 8.0506- 8.0238 (m,1H) 7.3945-7.3349 (m, 2H) 7.2528-7.2218 (m, 1H) 6.4336-6.4090 (m, 1H) 6.0792-6.0454 (m, 1H) 5.7119-5.6122 (m, 2H) 5.5391-5.5336 (m, 1H) 4.9527-4.8838 (m, 2H) 4.7799-4.7595 (m, 1H) 3.9461-3.9340 (m, 3H) 3.3115 (s, 1H) 3.1652-3.0853 (m, 2H) 2.7290- 2.6179 (m, 2H) 2.2770 (s, 1H) 2.1537 (s, 2H) 1.8751-1.7688 (m, 4H) 1.5377-1.5175 (m, 3H) 1.2454-1.2100 (m, 1H) 13C NMR (500 MHz, CDCl3): 157.8505, 148.6980, 147.3576, 143.9983, 141.5296, 139.6108, 138.8185, 132.7581, 129.2598, 126.1965, 121.9891, 119.2893, 102.0956, 78.5066, 76.8134, 65.8451, 58.7737, 56.7155, 54.0146, 42.7227, 37.3716, 26.7743, 24.7174, 20.4527, 18.0087 ES-MS: 377.2 (M); [α]D 22 = +100°(c = 0.2 in CH2Cl2). Elemental analysis: Calculated: C: 76.4%, H: 7.7%, N: 7.4% Found: C: 76.28%, H: 7.92%, N: 7.58%.
(1S,7R,10S,E)-1-(6-methoxyquinolin-4-yl)-9-vinyl- 1,3,6,8,9,10,11,11a-octahydro-7,10-ethanopyrido[2,1-c][1,4] oxazocin-7-ium(4c): Quinidine (0.324g, 1mmol) was dissolved in THF (5ml), and sodium hydride (0.048g, 2mmol) was added. The reaction mixture was stirred and heated to 800C and refluxed for 1h, then (E) -1,4 – dibromo-2 – utane (0.321g, 1.5mmol) is added. The reaction mixture was further refluxed for 12 hours and the reaction was monitored by TLC. After the completion of the reaction, the solvent was evaporated and the residue was purified with silica gel (chloroform: methanol = 30:1) to give the product (0.2g, 53.1% yield).
¹H NMR (500 MHz, CDCl3): 8.7204-8.7193 (m,1H) 8.0601-8.0294 (m, 1H) 7.4040-7.3413 (m, 2H) 7.2626-7.2166 (m,1H) 6.4454-6.4046 (m, 1H) 6.1093-6.0400 (m, 2H) 5.6780-5.5472 (m, 2H) 5.1599-5.0716 (m, 2H) 4.9482-4.8920 (m, 1H) 4.7891-4.7547 (m, 1H) 3.9442-3.9328 (m, 3H) 3.1868-3.1019 (m, 2H) 2.9826-2.7679 (m, 4H) 2.2706-2.2463 (m,1H) 1.7724 (s, 1H) 1.5069-1.4824 (m, 3H) 1.2527-1.1816 (m, 2H) 0.9454-0.8975 (m, 1H) 13C NMR (500 MHz, CDCl3): 157.1687, 150.1687, 147.3799, 145.8176, 144.0027, 140.8254, 133.1505, 131.2325, 129.6808, 126.9962, 121.2880, 119.2112, 102.2112, 82.2055, 80.6039, 60.2081, 55.4593, 49.9428, 49.0488, 48.3823, 39.6256, 27.7150, 25.9084, 24.6867 ES-MS: 377.2 (M); [α]D 22 = -14°(c = 0.2 in CH2Cl2). Elemental analysis: Calculated: C: 76.4%, H: 7.7%, N: 7.4% Found: C: 76.6%, H: 7.45%, N: 7.57%.
(1R,7R,10S,E)-1-(quinolin-4-yl)-9-vinyl-1,3,6,8,9,10,11,11aoctahydro- 7,10-ethanopyrido[2,1-c][1,4]oxazocin-7-ium(4d): Cinchonine (0.294g, 1mmol) was dissolved in THF (5ml), and sodium hydride (0.048g, 2mmol) was added. The reaction mixture was stirred and heated to 800C and refluxed for 1h, then (E) -1,4 – dibromo-2 – utane (0.321g, 1.5mmol) is added. The reaction mixture was further refluxed for 12 hours and the reaction was monitored by TLC. After the completion of the reaction, the solvent was evaporated and the residue was purified with silica gel (chloroform: methanol = 30:1) to give the product (0.197g, 56.9% yield).
¹H NMR (500 MHz, CDCl3): 8.9185-8.9043 (m, 1H) 8.1907- 8.1148 (m, 2H) 7.7660-7.6210 (m, 2H) 7.4260-7.4120 (m, 1H) 6.4651- 6.4240 (m, 1H) 6.1454-6.0176 (m, 2H) 5.9080-5.8020 (m, 1H) 5.7185- 5.6410 (m, 1H) 5.1782-5.0697 (m, 2H) 4.9745-4.9180 (m,1H) 4.8213- 4.7874 (m, 1H) 3.2700-3.1762 (m, 3H) 3.0660-2.9939 (m, 2H) 2.8654 (s, 1H) 2.3483-2.1630 (m, 3H) 1.8380 (s, 2H) 0.9761-0.8701 (m, 2H) 13C NMR(500 MHz, CDCl3): 149.9942, 147.9244, 145.8366, 140.6015, 133.0530, 129.8156, 129.0673, 126.6098, 125.8520, 123.8260, 119.3837, 118.8478, 82.0510, 80.6292, 68.2785, 59.9805,49.0344, 48.1538, 27.6099, 25.6839, 24.5560, 23.5513 ES-MS: 347.2(M); [α]D 22 = +6°(c = 0.2 in CH2Cl2) Elemental analysis: Calculated: C: 79.5%, H: 7.8%, N: 8.1% Found: C: 79.63%, H: 7.31%, N: 8.35%.
Typical procedure of the asymmetric darzens reactions
To a mixture of benzaldehyde (0.106g, 1mmol), chloroacetonitrile (0.091g, 1.2mmol) and THF (5ml), 4a (0.035g, 0.1mmol) was added and stirred for 20minutes. Solid KOH (0.067g, 1.2mmol) was added and continued stirring for 16 hours. The mixture was filtered and purified by TLC (PE: EA = 50:1) to give the cis-product (0.067 g) and trans-product (0.03 g) as colorless oil.
Cis-product 1H NMR (500 MHz, CDCl3): 7.408~7.388(3H, m), 7.282~7.263 (2H, m), 4.278~4.275 (1H, m), 3.410~3.405(1H, m) [α] D 22 =41°(major product).
Trans-product 1H NMR (500 MHz, CDCl3): 7.245~7.260 (5H, m), 4.248~4.237 (1H, m), 3.778~3.766 (1H, m)
Results and Discussions
The development of asymmetric phase transfer catalysis has become more and more significant in both economic and environment fields [1, 2, 3]. Until recently, there have been three main generations of these catalysts derived from cinchona alkaloids (Figure 1). The first generation: R=H, Ar=Phenyl; the second generation: R=Allyl, Ar=Phenyl; and the third generation: R=Alkyl, Ar=Anthracyl. The first generation of catalysts were developed by Dolling’s group in 1984 [4, 5], which were successfully applied in the asymmetric alkylation of glycine Schiff base by O’Donnell’s group with good enantioselectivity [6, 7]. The third generation of the catalysts was developed by E.J. Corey’s group [8]. Recently Waser et al. reviewed the asymmetric reactions catalyzed by the bifunctional quaternary ammonium catalysts [9], and Maruoka et al. also reviewed the asymmetric phase transfer catalysis with chiral quaternary ammonium catalysts derived from cinchona alkaloids and chiral C2-type quaternary ammonium catalysts [10].
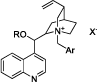
Figure 1: Three main generations of cinchona alkaloid based catalysts.
Until recently, only few chiral phase transfer catalysts have been reported to be applied in the asymmetric Darzens reaction. Deng et al. reported that the second generation of the catalysts derived from cinchona alkaloids could catalyze the asymmetric Darzens reaction with high yield and good enantioselectivity [11]. While Shioiri’s group reported diastereoselective Darzens reaction catalyzed by tetrahexylammonium bromide [12]. And macromolecular phase transfer catalysts were reported by Wang’s group and were applied in diastereoselective Darzens reaction (Figure 2) [13]. Jonczyk’s group and Murugan’s group also reported the asymmetric Darzens reaction with different kinds of chiral phase transfer catalysts [14, 15].
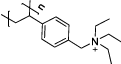
Figure 2: The catalyst synthesized by Wang’s group.
Till now, our group has reported four novel chiral phase transfer catalysts derived from cinchona alkaloids with eight-member cycle structure (Figure 3). The asymmetric alkylation reactions of glycine derivatives catalyzed by these catalysts were also investigated with high yields and moderate to excellent ee values (39.5-99.7%) [16]. In continuation of our studies on the asymmetric phase transfer catalysis, herein we report the asymmetric Darzens reaction with the novel chiral phase transfer catalysts 4a to 4d.
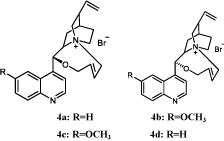
Figure 3: Four novel cinchona alkaloids based catalysts [16].
We began our investigation with non-chiral phase transfer catalyst, we tried TEBAC (triethyl benzyl ammonium chloride) and TBAB (tetrabutyl ammonium bromide) in the Darzens reaction between benzaldehyde and chloroacetonitrile in THF and we found only TBAB could catalyze the Darzens reaction, then we applied the reaction to different aldehydes and chloroacetonitrile in THF (Figure 4) and the results were listed in Table 1.
Entry
R
Catalyst
Time(h)
Yielda
cis:trans
1
H
TBAB
23
70%
1.2:1
2
p-Cl
TBAB
24
13%
1.4:1
3
p-Br
TBAB
12
30%
1.4:1
4
p-Me
TBAB
12
20%
0.9:1
5
m-Me
TBAB
12
31%
0.8:1
a. Isolated yields including cis-product and trans-product.
Table 1: The Darzens reaction between aldehydes and chloroacetonitrile under non-chiral phase transfer catalyst.
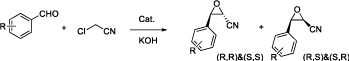
Figure 4: The reaction with different aldehydes and chloroacetonitrile in THF.
As was shown in Table 1, the rates between cis-product and transproduct were nearly 1:1. Only poor diastereoselectivity of the normal non-chiral phase transfer catalyst of TBAB was achieved and further work should be done to enhance the diastereoselectivity.
Having realized the non-chiral Darzens reaction between aldehydes and chloroacetonitrile, we turned our attention to its asymmetric version. In continuation of our studies on the asymmetric phase transfer catalysis and Darzens reaction, we tried to investigate the novel chiral phase transfer catalysts 4a to 4d developed by our group in the asymmetric Darzens reaction. Firstly, we chose the Darzens reaction between benzaldehyde and chloroacetonitrile as the model reaction and different reaction conditions were investigated and the results were listed in Table 2.
Entry
Catalyst
Time(h)
Solvent
Base
Yielda
Cis:trans
Ee of majorb
1
4a
16
THF
KOH
67%
2.1:1
70%
2
4b
16
THF
KOH
60%
2.1:1
61%
3
4c
16
THF
KOH
56%
1.5:1
45%
4
4d
16
THF
KOH
59%
1.3:1
53%
5
4a
--
toluene
KOH
--
--
--
6
4a
16
DMSO
KOH
76%
1.4:1
50%
7
4a
--
toluene
KOH
(50% in water)
--
--
--
8
4a
20
THF
NaOH
50%
1.5:1
64%
9
4a
18
THF
CsOH
53%
1.4:1
58%
a. Isolated yields including cis-product and trans-product.
b. Enantiopurity was determined by HPLC analysis using chiral colume (DAICEL Chiral cel OD-H) with hexanes/ i-PrOH as a solvent.
Table 2: The asymmetric Darzens reaction between benzaldehyde and chloroacetonitrile under different reaction conditions.
In Table 2, we found that 4a was the best catalyst and could give the best result both in cis/trans value and ee value, catalyst 4b, 4c and 4d gave relatively lower cis/tans value and lower ee values. Of all the solvents we investigated, THF gave the best yield, cis/trans value and ee value, the more dipolar solvent gave out a better yield but very low cis/trans rate and enantioselectivity (entry 6), The less polar solvent toluene gave no product at all no matter with the solid or aqueous solution of KOH as the base (entry 7 and 8). Of all the bases we investigated, solid KOH gave the best yield, the cis/trans rate and the best enantioselectivity. So the optimal reaction condition was with 4a as the catalyst, with THF as the solvent, and with solid KOH as the base (entry 1). Under the optimal reaction condition, the Darzens reaction between different aldehydes and chloroacetonitrile were investigates, and the results were collected in Table 3.
Entry
R
Yielda
Time(h)
cis:trans
ee of majorb
1
H
67%
16
2.2:1
70%
2
p-Cl
63%
9
3.8:1
35%
3
p-Br
66%
8
4.1:1
50%
4
p-Me
68%
8
5.8:1
17%
5
m-Me
55%
8
6.9:1
30%
a. Isolated yields including cis-product and trans-product.
b. Enantiopurity was determined by HPLC analysis using chiral colume (DAICEL Chiralcel OD-H) with hexanes/ i-PrOH as a solvent.
Table 3: The asymmetric Darzens reaction between different aldehydes and chloroacetonitrile.
As was shown in Table 3, the catalyst 4a could bring much higher yield in the mass. The reaction catalyzed by 4a was much faster than common non-chiral phase transfer catalysts such as TBAB and higher diastereoselectivity were also achieved. The highest rate (cis: trans) was achieved as 6.9:1 with 3-methylbenzaldehyde as the substrate (entry 5). For the reactions catalyzed by 4a, low to moderate ee values were also achieved, and the highest ee value was achieved to be 70% with benzaldehyde as the substrate (entry 1).
Conclusion
In all, we successfully applied the newly-designed chiral phase transfer catalysts 4a to 4d in the asymmetric Darzens reactions and satisfying and interesting results were achieved. Further work is under way to understand the mechanism and improve the diastereoselectivity and the enantioselectivity of the reaction.
Acknowledgment
We were thankful to the National Natural Science Foundation of China for their financial support (No. 21102180).
References
- Maruoka K. Practical Aspects of Recent Asymmetric Phase-Transfer Catalysis. Org. Proc. Res. Dev. 2008; 12: 679-697.
- Ebrahim S, Wills M. Synthetic applications of polymeric α-amino acids. Tetrahedron: Asymmetry. 1997; 8: 3163.
- Ooi T, Maruoka K. Asymmetric organocatalysis of structurally well-defined chiral quaternary ammonium fluorides. Acc Chem Res. 2004; 37: 526-533.
- Dolling UH, Davis P, Grabowski EJJ. Efficient catalytic asymmetric alkylations. 1. Enantioselective synthesis of (+)-indacrinone via chiral phase-transfer catalysis. J. Am. Chem. Soc. 1984; 106: 446.
- Hughes DL, Dolling UH, Ryan KM, Schoenewaldt EF, Grabowski EJJ. Efficient catalytic asymmetric alkylations. 3. A kinetic and mechanistic study of the enantioselective phase-transfer methylation of 6,7-dichloro-5-methoxy-2-phenyl-1-indanone. J. Org. Chem. 1987; 52: 4745.
- O’Donnell MJ, Bennett WD, Wu SJ. The stereoselective synthesis of .alpha.-amino acids by phase-transfer catalysis. Am. Chem. Soc. 1989; 111: 2353.
- Lipkowitz KB, Cavanaugh MW, Baker B, O’Donnell MJ. Theoretical studies in molecular recognition: asymmetric induction of benzophenone imine ester enolates by the benzylcinchoninium ion. J. Org. Chem. 1991; 56: 5181.
- Corey EJ, Xu F, Noe MC. A Rational Approach to Catalytic Enantioselective Enolate Alkylation Using a Structurally Rigidified and Defined Chiral Quaternary Ammonium Salt under Phase Transfer Conditions. J. Am. Chem. Soc. 1997; 119: 12414-12415.
- Novacek J, Waser M. Bifunctional Chiral Quaternary Ammonium Salt Catalysts: A Rapidly Emerging Class of Powerful Asymmetric Catalysts. Eur. J. Org. Chem. 2013; 4: 637-648.
- Hashimoto T, Maruoka K. Recent development and application of chiral phase-transfer catalysts. Chem Rev. 2007; 107: 5656-5682.
- Liu Y, Provencher BA, Bartleson KJ, Deng L. Highly Enantioselective Asymmetric Darzens Reactions with a Phase Transfer Catalyst. Chem Sci. 2011; 2: 1301-1304.
- Arai S, Suzuki Y, Tokumaru K, Shioiri T. Diastereoselective Darzens reactions of α-chloroesters, amides and nitriles with aromatic aldehydes under phase-transfer catalyzed conditions. Tetrahedron lett. 2002; 43: 833-836.
- Wang ZT, et al. Efficient Darzens condensation reactions of aromatic aldehydes catalyzed by polystyrene-supported phase-transfer catalyst. Jour. Mole. Cat. A: Chem. 2004; 218: 157-160.
- Jonczyk A, Zomerfeld T. Convenient synthesis of t-butyl Z-3-substituted glycidates under conditions of phase-transfer catalysis. Tetrahedron Lett. 2003; 44: 2359-2361.
- Murugan E, Siva A. Effective Darzen's condensation of 4-nonanolide with 1,6-dibromohexan-2-one using new bead-shaped insoluble multi-site (six-site) phase transfer catalyst and low concentration of aqueous NaOH. Jour. Mole. Cat. A: Chem. 2007; 277: 81-92.
- Wang Z, Huang D, Xu P, Dong X, Wang X, Dai Z. The asymmetric alkylation reaction of glycine derivatives catalyzed by the novel chiral phase transfer catalysts. Tetrahedron Lett. 2015; 56: 1067-1071.