
Research Article
Austin J Anal Pharm Chem. 2016; 3(2): 1063.
Formulation and Evaluation of Unidirection Bucco- Adhesive Tablet of Sumatriptan Succinate for Migraine Disorder
Champaneri AM*, Soniwala MM and Chavda JR
Department of Pharmaceutics, B. K. Mody Government Pharmacy College, Polytechnique Campus, Near Aji Dam, India
*Corresponding author: Ajay M Champaneri, Department of Pharmaceutics, B. K. Mody Government Pharmacy College, Polytechnique Campus, Near Aji Dam, India
Received: March 24, 2016; Accepted: May 03, 2016; Published: May 05, 2016
Abstract
The objective of this research work was to formulate and evaluate PEO WSR 301 bucco-adhesive tablet in combination with Carbopol 934p for controlled release of Sumatriptan Succinate. To bypass high hepatic first pass metabolism, unidirection bucco-adhesive tablet is selected dosage form for the experimental work. Initially preliminary trials were carried out for the selection of excipients and their relative quantity for incorporation in the dosage form. From the results, Polyethylene oxide-PEO WSR 301 (mucoadhesive polymer) and Carbopol 934p (control release) were selected as a suitable excipients for experimentation. Composition of the mucoadhesive tablet was optimized using 3² full factorial design where amount of PEO WSR 301 (X1) and amount of Carbopol 934p (X2) were taken as independent variables and mucoadhesive strength, Drug release at 6 hour and % swelling index taken as response variables. The formulations of design batches were characterized for post compression parameters like weight variation, hardness, thickness, friability, Drug content, swelling index, ex-vivo Mucoadhesive strength, and surface pH, drug release at 6 hr., ex-vivo residence time, and curve fitting analysis. The optimized formulation was obtained using Minitab software based on desirability value. Characterization of optimized batch was carried out by, ex-vivo permeation study.
From the results of meting point, DSC and FTIR study the drug and its compatibility with other excipients was confirmed. λmax of Sumatriptan Succinate was found to be 227 nm and linearity was 0.9995. Mucoadhesive strength and swelling index were in range of 0.25-0.43 N and 45-50% respectively. Drug release at 6hr. was in the range of 87-95%. The Bucco-adhesive of Sumatriptan succinate provides good concept to bypass the extensive hepatic first-pass metabolism. Formulated tablet using PEO-WSR 301 and Carbopol 934p showed good mucoadhesion, release profile, swelling index and permeation behavior. The Sumatriptan Succinate unidirection bucco-adhesive tablet is a promising approach for the effective treatment of disease as it provides control drug release in 6 hr.
Keyword: Unidirection buccal tablet; Sumatriptan succinate; Migraine; PEO WSR 301; Carbopol 934p
Introduction
Sumatriptan succinate is 1-[3-(2-dimethylaminoethyl)-1Hindol- 5-yl]-N-methyl-methane sulfonamide succinate [1,2]. It is a 5-HT1 receptor agonist used in the treatment of migraine. Migraine is a condition that affects approximately 10% of the adult population worldwide, yielding approximately 600 million people with about 28 million in the USA alone. In addition to headache, migraine can be associated with a variety of other symptoms, including diarrhea, cold extremities, facial pallor, nausea, vomiting, and sensitivity to external stimuli such as light, sound, or odor. Such migraines typically last for up to 24h, but can range from 4 to 72h and patients often experience migraine attacks one to two times per month. The oral formulation offers convenience and ease of use but produces unreliable blood levels and inconsistent response. Recurrence (rebound) occurs with these formulations. This common problem with recurrence is likely due to persistence of the original event with a time course exceeding the duration of action from the currently available formulations. This is particularly so because sumatriptan has a serum elimination half-life of only 2h and most of the active drug is eliminated within 4–6 h in the majority of patients. Thus, an optimal product would seek to provide the advantages of rapid, systemic administration of sumatriptan succinate. Buccal drug delivery system has the potential to fill an unmet need in migraine care by providing direct access to the systemic circulation through the internal jugular vein bypassing the first pass metabolism leading to high bioavailability. Other advantages are noninvasive administration, rapid-onset of action, convenient and easily accessible site, self-administrable, low enzymatic activity, suitability for drugs or excipients that mildly and reversibly damages or irritates the mucosa, painless administration, easy drug withdrawal, cheap and have superior patient compliance. In this work, it is designed to develop 9h bucco-adhesive tablets of sumatriptan succinate with the following objectives to avoid hepatic first pass metabolism, to reduce the frequency of administration, overcome the side effects, simplify the treatment regimen, and to obtain greater therapeutic efficacy to improve patient compliance.
Material and Method
Material
Sumatriptan succinate raw material was received as gift sample from Sun Pharmaceutical Limited, Ahmedabad, PEO WSR 301 from Lubrizol, Carbopol 934 p from ACS chemical, Ahmedabad, Lactose Monohydrate from Loba chemicals, Thane, Ethyl Cellulose from Loba chemicals, Mumbai, Magnesium Stearate from ASES, Jodhpur and Talc from Vikas pharma, Mumbai.
Method
Method of core tablet: The Sumatriptan succinate unidirection Bucco-adhesive tablet were prepared by direct compression method, the composition of various formulation was mentioned in Table 1 [3]. All the ingredient was individually passed through sieve no 60. The required quantities of mucoadhesive polymer were mix properly with other excipients. Prepared the core tablet in the tablet compression machine mini press-I (Karnavati, Ahmedabad). After prepared core tablet then using ethyl cellulose (backing layer) for the unidirectional design. Composition of the mucoadhesive tablet was optimized using 3² full factorial design where amount of PEO WSR 301 (X1) and amount of Carbopol 934p (X2) were taken as independent variables and mucoadhesive strength, Drug release at 6 hour and % swelling index taken as response variables. The formulations of design batches were characterized for post compression parameters like weight variation, hardness, thickness, friability, Drug content, swelling index, ex-vivo Mucoadhesive strength, surface pH, drug release at 6hr., ex-vivo residence time, and curve fitting analysis. The optimized formulation was obtained using Minitab software based on desirability value. Characterization of optimized batch was carried out by, ex-vivo permeation study.
Method of core-in-cup tablet: Total weight of the core tablet was 80mg, prepared by direct compression in 8 mm punch (excipient same as shown above). Resulted round shape flat core tablet is recompressed in 10mm round shape flat punch after adding ethyl cellulose (70mg) mixture at free three side around the tablet.
Identification of drug
Melting point method: Melting point of drug was determined by Capillary Method. Fine powder of Sumatriptan succinate was filled in glass capillary tube (previously sealed at one end), tube was placed in melting point apparatus and the temperature at which powder melted was noted.
IR Spectroscopy: FTIR studies were carried out to identify the drug [4]. Fourier Transform Infrared Spectroscopy studies were carried out by sample with dried potassium bromide and acquiring IR spectrum in the range of 400-4000 cm-1. The FT-IR spectrum of the obtained sample of the drug was compared with the standard FT-IR spectra of the pure drug.
DSC Study: Thermal behavior of drug was examined using thermal analyzer [4]. All accurately weighed samples (about 1mg) were placed in sealed aluminum pans before heating under nitrogen flow (20mL/min) at a scanning rate of 10 °C min-1 from 50 to 300 °C. An empty aluminum pan was used as reference.
Compatibility studies
FTIR spectroscopy: Compatibility must be established between the active ingredients and other excipients to produce a stable, efficacious, attractive and safe product. FTIR spectra of Sumatriptan succinate with different polymers and other excipients were recorded between 400 to 4000 cm–1 using FTIR spectrometer (Shimadzu).
Evaluation parameter
Pre Compression parameter of core powder blend
Bulk density: A bulk density is defined as the mass of powder divided by the volume [5,6,7]. A bulk density largely depends on the particle shape, as particles becomes more spherical in shape, bulk density is increase. In addition as granules size increase, bulk density decrease. Powder weighing 10 g was placed into 100 ml measuring cylinder. Volume occupied by the powder was noted without disturbing the cylinder and bulk density was calculated in gm/ml by the following equation.
Bulk density = Weight of powder / Bulk volume
Tapped density: Tapped density was achieved by mechanically tapping a measuring cylinder containing 20 tablet powders. After observing the initial volume, the cylinder was mechanically tapped and volume reading was taken until further volume changes were observed. The mechanical tapping was achieved by raising the cylinder and allowing it to drop under own weight a specific distance. Device that rotates the cylinder during tapping may be preferred to minimize any possible separation of the mass during tapping down. Cylinder dropping distance: 14 ± 2 mm at a normal rate of 300 drops /min. The final volume was recorded and the tap density was calculated by the following equation.
Tapped Density = Weight of powder / Tapped volume
Hausner’s ratio: The Hausner’s ratio is a number that is correlated to the flow ability of a powder or granular material.
Hausner’s Ratio = Tapped Density/Bulk density
Compressibility index (Carr’s index) [7]: Compressibility index of the drug was determined using the following formula
Carr’s Index (%) = [(Tapped Density-Bulk Density) x100]/ Tapped Density
Angle of repose: Angle of repose was determined by measuring the height, radius of the heap of the powder blend. A cut system funnel was fixed to a stand and bottom of the funnel was fixed at a height of 2cm from the plane. Powder blend was placed in funnel and allowed to flow freely and measured the height and radius of the heap.
tanθ=h/r
Where, h = height of heap
r = radius of heap
Post compression parameters of core tablet
Thickness: The thickness of buccal tablet was determined using digital micrometer screw gauge. Ten individual tablets from each batch were used and the average thickness was calculated.
Hardness: Hardness test was done for five tablets from each batch using hardness tester and average values was calculated. The hardness was measured in terms of kg/cm2.
Friability test: Friability is the measure of tablet strength. Roche type friabilator was used for testing the friability using the following procedure. Twenty tablets were weighed accurately and placed in the tumbling apparatus that revolves at 25rpm dropping the tablets through a distance of six inches with each revolution. After 4min, the tablets were weighed and the percentage loss was determined.
% Friability loss = [Initial weight − Final weight/Initial weight] X 100
Weight variation test: The USP-XXIX weight variation test was carried out by weighing 20 tablets individually, calculating the average weight, comparing the individual tablet weight to average weight. The tablet meet USP-XXIX test if no tablet differs by more than two times of percentage deviation USP-XXIX Standards for Weight Variation Test.
Content uniformity: Ten tablets from each batch was taken, crushed and mixed. From the mixture 5mg of drug equivalent of mixture was extracted thoroughly with 100mL of pH 6.8 phosphate buffer. The amount of drug present in each extract was determined using UV spectrophotometer at 271 nm. This procedure was repeated thrice and this average was calculated.
Ex-vivo mucoadhesion studies: The ex-vivo mucoadhesive strength was performed after application of the buccal tablet on freshly cut goat buccal mucosa. The fresh goat buccal mucosa was tied on the glass slide, and a mucoadhesive core side of each tablet was wetted with phosphate buffer pH 6.8 and adhered to the sheep buccal mucosa by applying a light force with a fingertip for 30 seconds. The modified physical balance was adjusted by keeping glass beaker on another side. Water was added by burette and weight of water needed to detach the tablet from goat buccal mucosa was recorded for the measure of mucoadhesive strength in grams [8,9] (Figure 1).
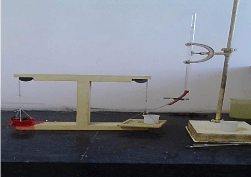
Figure 1: Assembly for mucoadhesion strength study.
Force of adhesion (N) = [Mucoadhesive strength] / 1000 X 9.81
In-vitro drug release studies [10,11]: The dissolution test was carried out using USP dissolution testing apparatus II. The test was performed at a paddle speed of 50rpm using 500ml of phosphate buffer, pH 6.8, as the dissolution medium at 37±0.50°C. The tablet was stuck on the paddle from the side of backing layer using cyanoacrylate adhesive to mimic unidirectional drug release. An aliquot of 10ml of the sample solution was withdrawn at the interval of 15, 30, 60, 120, 180, 240, 300, 360 min and the absorbance was measured at identical wavelength [12-14] (Figure 2).

Figure 2: Assembly for In-vitro drug release studies.
In vitro swelling rate: After weighing the tablet (W1), it was immersed in pH 6.8 phosphate buffer solution maintained at 37°C. The weight at the end of 360 min was reported (W2). The swelling index was determined from the formula:
% Swelling Index = [(W2-W1)/ W1] ×100
Where, W1 = initial weight
W2= final weight
Ex-vivo permeation studies [15,16]: Diffusion studies were carried out to evaluate the permeability of drug across the porcine buccal mucosal membrane using glass surface franz diffusion cell. Porcine buccal mucosa was obtained from a local slaughter house and was used within 2h of slaughter. The tissue was stored in 0.2 molar phosphate buffer (PBS), pH 7.4, solution upon collection. The epithelium was separated from underlying connective tissues with surgical scissors and clamped in between donor and receiver chambers of the diffusion cells for permeation studies. Receptor compartment contained 21ml of pH 7.4 phosphate buffer, while donor compartment was filled with 3 ml simulated saliva of pH 6.8. The tablet was placed on the mucosal surface in donor compartment, and 2ml aliquots was removed at suitable intervals from the receptor compartment while the solution is being stirred continuously using magnetic stirrer, replacing it with fresh 2ml medium each time. The experiment was carried out at 37±1°C (Figure 3).

Figure 3: Assembly for ex-vivo permeability study.
Ex-vivo residence time [17,18]: The tablet was applied on the porcine buccal mucosa which was fixed on the glass slide with cyanoacrylate glue. The slide was tied to the disintegration apparatus and suspended in the beaker filled with 800 ml simulated saliva, pH 6.8. The slide was allowed to reciprocate in the medium until the tablet got detached or eroded from the mucosa. The test was performed in triplicate. Time for the detachment of the tablet was recorded as exvivo residence time.
Short-term stability study: Stability studies were carried out for Sumatriptan Succinate formulation as per ICH guidelines. The best unidirection Bucco-adhesive tablet (D6) was sealed in high density polyethylene bottles and stored at 4±1 °C/Ambient, 25±2 °C/60±5 % RH %, 40±2 °C/75±5 % RH for 90d. The samples were periodically evaluated for entrapment efficiency and percentage mucoadhesion [19-23].
Results and Discussion
Melting point
The observed melting point was found to be 169-171 °C. This melting point resembles to melting point given in article.
FTIR Spectra of Ivabradine hydrochloride
IR spectra of drug are shown in following Figure 4. The peaks obtained in the spectra of drug correlates with functional groups of Sumatriptan Succinate which confirms the purity of drug.
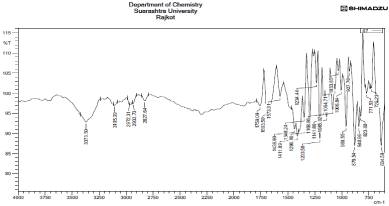
Figure 4: FTIR spectra of Sumatriptan succinate.
DSC study
From the observation of thermogram of pure drug, the melting point of the sample was found at 168.30 °C that is nearly same with documented melting point (167-168 °C) proving the identity and purity of drug (Figure 5).
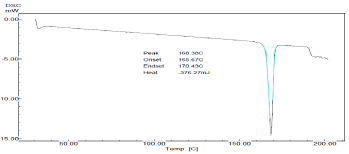
Figure 5: DSC thermogram of Sumatriptan succinate.
Compatibility studies
Physicochemical property of “Core-in-Cup” tablet.: The prepared tablets were smooth and white in color. Weight variation in case of all tablets was acceptable. The weight variation in case of all the tablets was within ±7.5% of theoretical tablet weight. This falls well within the acceptance criteria. Friability in case of all the designed tablets was less than 1% w/w indicating suitability of the method used for manufacturing the tablets. The prepared tablets showed maximum thickness of 1.65 mm. Hardness value of all the formulation was in the range of 5-5.5 Kg/cm2 (Figure 6).
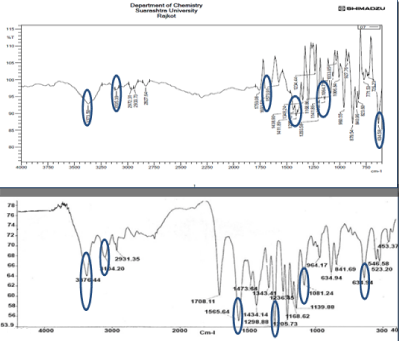
Figure 6: Comparision of FTIR study of drug and drug + polymer mixture.
To evaluate a tablet‘s potential for efficacy the amount of drug in the tablet need to be monitored from tablet to tablet and batch to batch. The mean drug content was found to be in between range of 96.89% to 98.99% (Table 1, 2 & 3).
Ingredients (mg)
D1
D2
D3
D4
D5
D6
D7
D8
D9
Sumatriptan succinate
10
10
10
10
10
10
10
10
10
PEO WSR 301
10
20
30
10
20
30
10
20
30
CP 934p
2.5
2.5
2.5
5
5
5
7.5
7.5
7.5
Lactose
32.5
22.5
12.5
30
20
10
27.5
17.5
7.5
Ethyl cellulose
10
10
10
10
10
10
10
10
10
PEG 4000
10
10
10
10
10
10
10
10
10
Mg stearate
2.5
2.5
2.5
2.5
2.5
2.5
2.5
2.5
2.5
Talc
2.5
2.5
2.5
2.5
2.5
2.5
2.5
2.5
2.5
Total
80
80
80
80
80
80
80
80
80
Ethyl cellulose (Backing layer)
70
70
70
70
70
70
70
70
70
Total
150
150
150
150
150
150
150
150
150
Table 1: Composition table of formulations.
Batch No.
Bulk density (g/cm3)
Tapped density
(g/cm3)
Carr’s index (%)
Hausner’s ratio
Angle of repose
Core bland
D1
0.28±0.010
0.32±0.002
12.5±1.15
1.14±0.020
26.33±0.12
D2
0.28±0.021
0.31±0.04
9.67±1.25
1.11±0.03
24.44±0.19
D3
0.28±0.010
0.320.055
9.81±1.21
1.14±0.010
22.68±0.21
D4
0.29±0.010
0.34±0.001
14.7±1.05
1.17±0.010
23.25±0.34
D5
0.28±0.015
0.33±0.005
15.1±1.24
1.18±0.014
26.78±0.41
D6
0.3±0.015
0.34±0.001
11.7±1.35
1.13±0.005
27.63±0.38
D7
0.3±0.023
0.33±0.002
9.09±1.12
1.10±0.01
25.55±0.26
D8
0.28±0.010
0.33±0.003
15.1±1.18
1.18±0.030
26.46±0.14
D9
0.27±0.006
0.31±0.051
12.90±1.20
1.15±0.035
23.88±0.35
Table 2: Composition of tablet for Factorial design batches (“Core-in-Cup”).
Batch no.
Hardness
( kg/cm2)
Thickness
(mm)
Weight variation (mg)
Friability
(%)
Drug Content
(%)
D1
5.3±0.286
1.64±0.110
152.5±1.71
0.20±0.03
97.11±0.14
D2
5.2±0.350
1.65±0.110
150.7±1.32
0.24±0.07
98.29±0.29
D3
5.35±0.521
1.64±0.109
151.3±1.23
0.23±0.05
97.23±.51
D4
5.3±0.230
1.62±0.123
153.0±1.21
0.25±0.06
98.21±0.25
D5
5.3±0.249
1.67±0.135
150.1±1.19
0.22±0.02
98.99±0.27
D6
5.3±0.520
1.63±0.125
152.1±1.16
0.26±0.05
98.42±0.31
D7
5.3±0.430
1.64±0.130
150.7±1.78
0.24±0.02
98.20±0.77
D8
5.3±0.470
1.64±0.118
152.3±1.31
0.23±0.08
96.89±0.45
D9
5.2±0.286
1.67±0.150
151.0±1.52
0.23±0.06
98.18±0.18
Table 3: Physicochemical property of “Core-in-Cup” tablet (D1-D9).
Ex Vivo mucoadhesive strength
Force of adhesion (N) = Mucoadhesive strength / 1000 × 9.81
Mucoadhesive strength is an important parameter of the mucoadhesive buccal tablet. It shows the capability of the dosage form for adhering to the buccal mucosa. When the concentration of polymer is low, the number of penetrating polymeric chains per unit volume of the mucous is low resulting in weaker interaction. Increase in adhesion with increase the concentration of polymer used can be attributed to higher strength of gel formed by PEO WSR 301 resulting in stronger entanglement of polymeric chains with glycoprotein chains of mucous. Mucoadhesive strength was found to be increased as PEO WSR 301 concentration increases (Figure 7, Table 4).
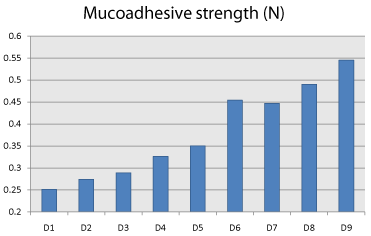
Figure 7: Comparision of Ex Vivo mucoadhesive strength of factorial batches.
Formulation code
Mucoadhesive strength (N)
Mean ±SD
D1
0.251±0.0025
D2
0.274±0.0026
D3
0.289±0.0015
D4
0.326±0.0015
D5
0.350±0.0050
D6
0.454±0.0030
D7
0.447±0.0021
D8
0.490±0.0020
D9
0.545±0.0042
Table 4: Comparision of Ex Vivo mucoadhesive strength of factorial batches.
Percentage swelling index:
% Swelling Index = [(W2-W1)/ W1] ×100
Where, W1 = initial weight
W2= final weight
Buccal tablets were weighed individually (designated as W1) and placed separately in Petri dishes containing 15mL of phosphate buffer (pH 6.8) solution. At regular intervals (1, 2, 3, 4, 5 and 6 hr) the buccal tablets were removed from the Petri dishes and excess surface water was removed carefully using the filter paper. The swollen tablets were then reweighed (W2) (Figure 8, Table 5).
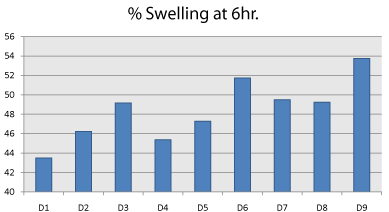
Figure 8: Comparision of Percentage Swelling index of factorial batches.
Formulation code
Percentage Swelling index at 6 hrs
Mean ±SD
D1
43.50±1.10
D2
46.23±0.64
D3
49.17±0.58
D4
45.37±0.96
D5
47.27±0.97
D6
51.73±1.19
D7
49.50±1.35
D8
49.23±0.96
D9
53.73±0.35
Table 5: Comparision of Percentage Swelling index of factorial batches.
Surface pH study
The maximum and minimum pH values of the formulations were found to be 6.8 and 6.2 respectively. The acceptable pH of saliva is in the range of 5-7 and the surface pH of all tablets is within limits. Hence, the formulations may not produce any irritation to the buccal mucosa (Figure 9, Table 6).
Formulation code
Surface Ph
Mean ±SD
D1
6.2
D2
6.3
D3
6.8
D4
6.4
D5
6.8
D6
6.8
D7
6.7
D8
6.8
D9
6.7
Table 6: Comparision of Surface pH study of factorial batches.
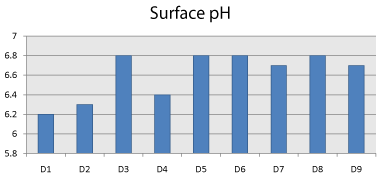
Figure 9: Comparision of Surface pH study of factorial batches.
Ex-vivo residence time
The Ex vivo residence time is one of the important physical parameter of buccal mucoadhesive tablets. The Ex vivo residence time was determined by using specially designed disintegration apparatus.
As the concentration of bioadhesive polymer increased, the residence time also increased. This examination reveals the mucoadhesive capacity of polymers used in formulations. PEO WSR301 had much more effect on the retention time (Table 7).
Formulation code
Residence time (hour)
Mean ±SD
D1
6.03±1.10
D2
6.12±0.66
D3
6.18±0.54
D4
6.10±0.87
D5
6.21±0.85
D6
6.24±1.20
D7
6.17±1.25
D8
6.28±0.83
D9
6.25±0.41
Table 7: Comparision of Ex-vivo Residence time of factorial batches.
In-vitro drug release profile of experimental batches
In-vitro drug release study was carried out in 6.8 pH buffer using IP apparatus type I at 37 ± 5º C temperature. Being a delayed release dosage form factorial batches D1, D2 and D3 showed drug release approximately 74.13, 72.15 & 70.35 % in 360 min. respectively. D4, D5, D8 and D9 showed drug release approx.86.16, 91.92, 92.33 & 88.15 % in 360 min. respectively. While D6 and D7 showed drug release at approx94.44 & 93.20 % in 360 min respectively (Figure 10, Table 8).
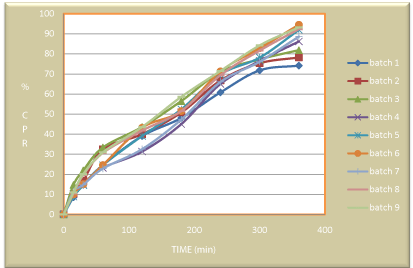
Figure 10: Dissolution data of experimental batches.
time (min)
% CPR of Experimental Batches
D1
D2
D3
D4
D5
D6
D7
D8
D9
0
0
0
0
0
0
0
0
0
0
15
11.23
10.15
9.42
9.9
8.85
9.96
11.35
10.49
10.92
30
14.77
16.71
19.92
14.73
14.53
15.02
14.74
19.61
20.05
60
24.61
32.40
33.32
23.12
24.56
24.53
22.85
30.65
30.90
120
39.02
40.01
43.58
31.31
39.07
43.14
32.48
41.76
43.50
180
48.01
50.79
56.47
44.97
52.35
51.44
47.26
51.54
58.56
240
60.78
66.71
60.01
65.01
69.74
71.24
66.02
69.40
71.43
300
71.74
70.32
65.24
76.50
78.14
82.78
76.07
81.65
84.18
360
74.13
72.15
70.35
86.16
91.92
94.44
93.20
92.33
88.15
Table 8: In-vitro drug release profile of experimental batches.
3² model for Mucoadhesive strength
For Mucoadhesive strength, as seen from figure 11 of the counter plot and response surface plot revealed that the Mucoadhesive strength was change in case of concentration of PEO WSR 301 and Carbopol 934p was varies.
The Polynomial equation generated from Microsoft excels for Mucoadhesive strength Was Mucoadhesive strength=
0.36+0.044X1+0.11X2+0.014X11-0.006X22+0.015X1X2
From polynomial equation and coefficient of X1 and X2 it was observed that the Concentration of and PEO WSR 301 showed positive effect on Mucoadhesive strength (Figure 11, Figure 12).
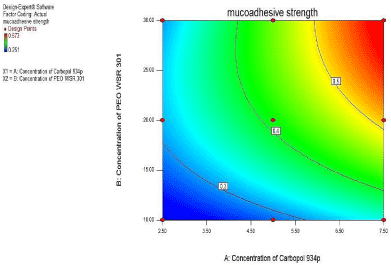
Figure 11: Contour plot showing the effect of Carbopol 934p (X1) and PEO
WSR301 (X2) on Mucoadhesive strength (Y1).
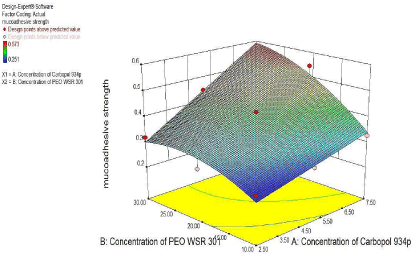
Figure 12: Response Surface plot showing the effect of Carbopol 934p (X1)
and PEO WSR 301 (X2) on Mucoadhesive strength(N) (Y1).
3² model for % swelling index
For % Swelling index, as seen from Figure 13 of the contour plot and response surface plot revealed that the %Swelling index was change in case of concentration of and PEO WSR 301 was varies.
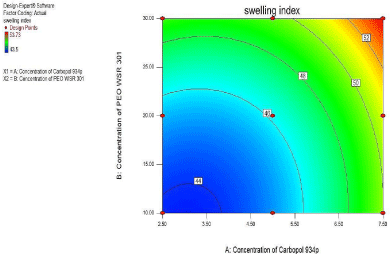
Figure 13: Contour plot showing the effect of Carbopol 934P(X1) and PEO
WSR 301 (X2) on %Swelling index (Y3).
The Polynomial equation generated from Microsoft excel for %Swelling index was %Swelling index =
47.28+2.711X1+2.261X2+1.25X11+0.43X22-0.36X1X2
From polynomial equation and coefficient of X1 and X2 it was observed that the concentration of carbopol 934p showed positive effect on %Swelling index. Increase in their concentration would increase the %Swelling index and concentration of it, was more significant than concentration of PEO WSR 301 (Figure 13, Figure 14).
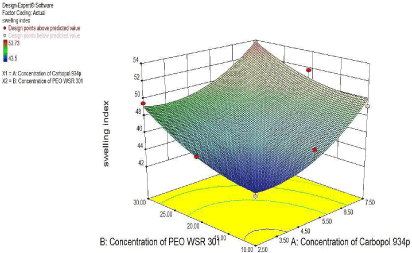
Figure 14: Response Surface plot showing the effect of Carbopol 934p (X1)
and PEO WSR 301 (X2) on %Swelling index (Y3).
3² model for %Drug release at 6hr
For %Drug release at 6 hr. as seen from Figure 15 of the contour plot and response surface plot revealed that the %Drug release at 6hr. was change in case of concentration of PEO WSR 301 and Carbopol 934p was varies.
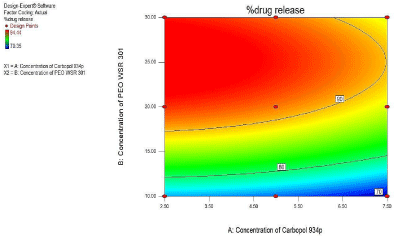
Figure 15: Contour plot showing the effect of Carbopol 934P (X1) and PEO
WSR 301 (X2) on %Drug release at 6hr (Y2).
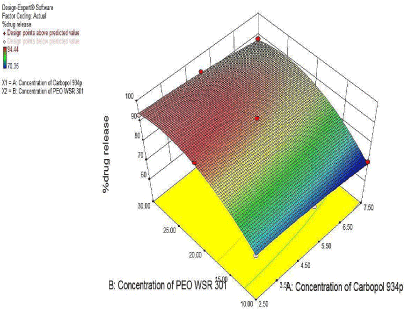
Figure 16: Response Surface plot showing the effect of Carbopol 934p (X1)
and PEO WSR 301 (X2) on %Drug release at 6hr. (Y2).
The Polynomial equation generated from Microsoft excel for %Drug release at 6hr. was %Drug release at 6hr. = 91.54-5.95X1- 0.85X2-1.06X11-0.61X22-4.53X1X2
From polynomial equation and coefficient of X1 and X2 it was observed that the concentration of PEO N80 showed negative effect on %Drug release while the concentration of crospovidone showed positive effect on %Drug release. So, optimum concentration of both polymers required for the desired %Drug release (Figure 15, Figure 16).
Desirability approach or optimization of experimental design
Concentration of Carbopol 934p (X1) and PEO WSR 301(X2) have an important bearing on the formulation of mucoadhesive buccal tablet. Dependent variables as indicators of the properties of buccal tablet were Mucoadhesive strength (Y1), %Swelling index (6hr). (Y2) and % drug release (6hr) (Y3).
The optimum formulation was selected based on the criteria of attaining the minimum, target and maximum range of the dependent variables. An overall desirability function dependent on all the investigated formulation variables was used to predict the ranges of variables where optimum formulation might occur. The desirable ranges are from zero to one (least two more desirable, respectively). The restriction value chosen (minimum, target, and maximum) put in Minitab software to obtain optimized batch. Optimized batch was prepared by using concentration of X1(Carbopol 934p) 30mg and Concentration of X2(PEO WSR 301) 5mg. Optimized batch was validated by F-test between the experimental results and calculated Value (Figure 17).
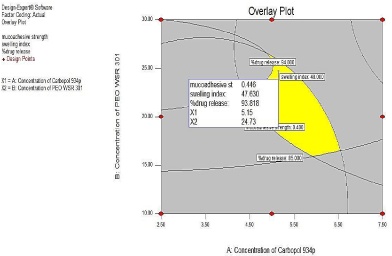
Figure 17: Profile of desirability graph.
Conclusion
The unidirection bucco-adhesive tablet devices of Sumatriptan succinate provides good concept to bypass the extensive hepatic firstpass metabolism. Formulated tablet using PEO-WSR 301 and Carbopol 934p showed good mucoadhesion, drug release profile (unidirection), swelling index and permeation behavior. For the sake of getting a better mucoadhesion and drug release both the polymers i.e Carbopol 934P and PEO WSR301 (1:4) were used in the combination. The PEO WSR301 provided mucoadhesion whereas the Carbopol 934P provided controlled drug release. Moreover Sumatriptan succinate is a BCS class III drug so its permeability is low, so PEG 4000 (10mg) was used as an effective permeation enhancer and the obtained results also revealed the same characteristic of the permeation behavior. So it can be concluded that Sumatriptan Succinate unidirectional bucco- adhesive tablet is a promising approach for the effective treatment of disease as it provides control drug release at 6hr.
References
- Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011; 153: 106-116.
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery--a promising option for orally less efficient drugs. J Control Release. 2006; 114: 15-40.
- Tripathi KD. Advances in oral transmucosal drug delivery. Journal of controlled release? 2011; 15: 106-116.
- Woertz C, Preis M, Breitkreutz J, Kleinebudde P. Assessment of test methods evaluating mucoadhesive polymers and dosage forms: an overview. Eur J Pharm Biopharm. 2013; 85: 843-853.
- Garg K and Singhvi I. Mucoadhesive buccal drug delivery system. Int. J. Pharm. Investig. 2015; 1: 11–19.
- Rao R, Shravani B and Reddy S. Overview on Buccal Drug Delivery Systems. Journal of pharmaceutical science and research. 2013; 5: 80–88.
- Andrews GP, Laverty TP, Jones DS. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm. 2009; 71: 505-518.
- Swamy P, Kinagi B, Biradar S, Gada S, and Shilpa H. Design and evaluation of buccoadhesive bilayer tablets of granisetron hydrochloride. IJPSR. 2010; 1: 104-110.
- Sumatriptan Succinate. Pub Chem.
- Sumatriptan. "drug bank".
- Shidhaye SS, Saindane NS, Sutar S, Kadam V. Mucoadhesive bilayered patches for administration of sumatriptan succinate. AAPS PharmSciTech. 2008; 9: 909-916.
- Singh K, Sharma V, and Pathak K. Formulation and evaluation of taste masked rapid release tablets of sumatriptan succinate. Int. J. Pharm. Investig. 2012; 4: 2–8.
- Bayrak Z, Tas C, Tasdemir U, Erol H, Ozkan CK, Savaser A, et al. Formulation of zolmitriptan sublingual tablets prepared by direct compression with different polymers: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2011; 78: 499-505.
- Shiledar RR, Tagalpallewar AA, Kokare CR. Formulation and in vitro evaluation of xanthan gum-based bilayered mucoadhesive buccal patches of zolmitriptan. Carbohydr Polym. 2014; 101: 1234-1242.
- Sheshala R, Khan N, Darwis Y. Formulation and optimization of orally disintegrating tablets of sumatriptan succinate. Chem Pharm Bull (Tokyo). 2011; 59: 920-928.
- Patel SR, Zhong H, Sharma A, Kalia YN. In vitro and in vivo evaluation of the transdermal iontophoretic delivery of sumatriptan succinate. Eur J Pharm Biopharm. 2007; 66: 296-301.
- Perioli L, Ambrogi V, Rubini D, Giovagnoli S, Ricci M, Blasi P, et al. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J Control Release. 2004; 95: 521-533.
- Onishi H, Yumoto K, Sakata O. Preparation and evaluation of ritodrine buccal tablets for rational therapeutic use. Int J Pharm. 2014; 468: 207-213.
- Varshosaz J, Dehghan Z. Development and characterization of buccoadhesive nifedipine tablets. Eur J Pharm Biopharm. 2002; 54: 135-141.
- Emami J, Shetabboushehri MA, Varshosaz J, Eisaei A. Preparation and characterization of a sustained release buccoadhesive system for delivery of terbutaline sulfate. Res Pharm Sci. 2013; 8: 219-231.
- Das S, Rajabalaya R, David S, Gani N, Khanam J, and Nanda A. Research Journal of Pharmaceutical. Biological and Chemical Sciences Cyclodextrins-The Molecular Container. Res J Pharm Biol Chem Sci Cyclodextrin. 2013; 4: 1694–1720.
- Emami J, Shetabboushehri MA, Varshosaz J, Eisaei A. Preparation and characterization of a sustained release buccoadhesive system for delivery of terbutaline sulfate. Res Pharm Sci. 2013; 8: 219-231.
- Ranade A, Ranpise N, Sanap G, and Kulkarni R. Development and In Vitro Evaluation of Buccal Tablet of Quinapril Hydrochloride. Res J Pharm Technol. 2011; 45: 364–369.