
Special Article - Infrared Spectroscopy
Austin J Anal Pharm Chem. 2016; 3(2): 1065.
In Situ Infrared Spectroscopy as a PAT Tool of Great Promise for Real-Time Monitoring of Animal Cell Culture Processes
Li M1, Ebel B1*, Courtès F2, Guedon E1 and Marc A1
1Laboratoire Réactions et Génie des Procédés, UMR 7274, CNRS-Université de Lorraine, France
2National Biopharmaceutical Facility, King Mongkut’s University of Technology Thonburi, Thailand
*Corresponding author: Bruno Ebel, Laboratoire Réactions et Génie des Procédés, UMR 7274, CNRSUniversité de Lorraine, 2 av. Forêt de Haye, TSA 40602, 54518 Vandoeuvre-lès-Nancy Cedex, France
Received: April 27, 2016; Accepted: May 17, 2016; Published: May 20, 2016
Abstract
Animal cell culture bioprocesses have become essential in pharmaceutical field for the production of recombinant therapeutic proteins, such as monoclonal antibodies. Consequently, the Process Analytical Technology approach recommends the use of in situ characterization tools, such as infrared (IR) spectroscopy, to control production processes and ensure the quality of endproducts. This review presents the current status of applying IR spectroscopy for animal cell culture processes. IR spectroscopy appears to be a very promising tool due to its flexibility, simplicity, rapidity and its inherent ability to provide simultaneous multi-analyte information with a single spectrum. Taken into account the main characteristics of animal cell bioprocesses, the advantages and challenges of IR spectroscopy applications are discussed. Then, published works underlining the interest of IR spectroscopy for animal cell bioprocesses are presented, which clearly demonstrated that IR spectroscopy is not only applicable for global supervision of process, but is also suitable for real-time monitoring of key parameters in animal cell cultures. Finally, this review also highlights some future improvement needed to strengthen IR spectroscopy as a reliable industrial PAT tool for feed-back control and process optimization, towards guaranteed end-product quality.
Keywords: Animal cells; Culture process; In situ monitoring; Infrared spectroscopy; Bioreactor
Abbreviations
ATR: Attenuated Total Reflectance; CHO: Chinese Hamster Ovary; CPP: Critical Process Parameters; CQA: Critical Quality Attributes; FDA: Food and Drug Administration; FT-IR: Fournier Transformed Infrared; HEK: Human Embryonic Kidney; IR: infrared; mAb: Monoclonal Antibody; MSCs: Mesenchymal Stem Cells; MIR: Mid Infrared; MVDA: Multivariate Data Analysis; NIR: Near Infrared; NS0: Non-secreting 0 Cells; PAT: Process Analytical Technology; PCA: Principal Component Analysis; PCR: Principal Component Regression; PLS: Partial Least-squares; PTMs: Posttranslational Modifications; QbD: Quality by Design.
Introduction
The past decade witnessed a great expansion of the production of recombinant therapeutic proteins such as mAbs. Animal cells, mainly CHO cells, are the most used host cell lines because of their innate capacity to perform human-like PTMs, which are crucial for functionality and efficiency of the therapeutic proteins [1]. Therefore, to assure the quality of final products which are subject to high variabilities, the QbD approach has been outlined by the FDA in 2002, urging manufacturers to monitor their process CQAs and optimize CPPs within a specified design space during the process [2]. In this context, the PAT concept is considered as the most important tool for the implementation of QbD, since it encourages the biopharmaceutical industries to adopt modern monitoring tools based on real-time analysis of key variables during all stages of the manufacturing processes [3]. To meet this need, spectroscopic methods, particularly IR spectroscopies, have gained great attentions over the last decade. The aim of this review is to present the current status of IR spectroscopies in animal cell bioprocesses as monitoring tools.
Specificities for Implementation of IR Spectroscopy in Animal Cell Bioprocesses
Industrial production of therapeutic proteins is mainly performed inside stirred and aerated reactors [4] (Figure 1). Several specificities of animal cell cultures in reactor must be taken into consideration for a successful IR spectroscopy implementation: (i) the nutritional requirements of animal cells is generally fulfilled with complex media [5]; (ii) strict sterility is needed to avoid any microbial contamination; (iii) animal cells can be cultured either in single cell suspension or after adhesion on spherical microcarriers [4].
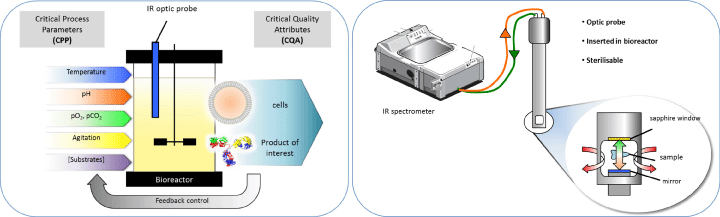
Figure 1: QbD approach using in situ IR probe as a PAT tool
For bioprocesses performed in reactors, IR spectroscopy exhibits major advantages compared to other classical analytical techniques. It exploits the fact that molecules absorb specific frequencies that are characteristic of their structure. NIR (750-2,500 nm) and MIR (2,500- 25,000 nm) are the main IR spectroscopies used in bioprocesses due to their simplicity and rapidity of measurement, great flexibility in application and the possibility to provide simultaneous multianalyte information from a single spectrum. However, to perform analysis directly inside bioreactors, in situ optical probes providing information of key variables in real-time are needed. Strategies coupling IR spectroscopies with in situ probes have been recently developed, and represent a significant advance because none of the previously existing sensors were able to monitor medium compounds in real-time, therefore requiring sampling with increased risks of contamination (Figure 1) [6]. Nevertheless, it has to be noticed that in situ probes can be affected by interfering with solid particles, gas bubbles, stirring rate, high cell densities or bulk viscosity, which often occur in bioprocesses [7], but these effects are generally reduced in animal cell culture since cell density as well as agitation and aeration rates, are rather low. Despite these benefits, several limitations have to be considered before implementing IR spectroscopy in animal cell processes.
One challenge is related to complexity of animal cell culture media and to the fact that there is no single and obvious IR spectral region for one particular component. Consequently, IR spectra will display overlapping peaks, leading to complex signals and hindering the assignment of specific features to individual compounds [8]. Moreover, the concentration of some compounds are often inversely correlated (e.g. glucose and lactate) which requires additional decorrelation experiments to perform real-time monitoring [9]. Additionally, culture medium compositions change greatly during the culture due to nutrient consumptions and product accumulations. Throughout the cell death phase, the spectroscopic characteristics of the culture bulk become even more complex due to cell debris, thereby increasing the challenge to monitor target bioprocess variables [8].
Another challenge lies in monitoring components that are present at only moderate concentrations over the time-course of mammalian cell bioprocess, since the molar absorptivity of molecules in NIR range is typically small. In MIR range, the vibration of the chemical bonds gives stronger signals and better degree of resolution [10]. However, water bonds absorption is also much stronger in MIR region, which often masks important information of key-molecule variations.
Most of the applications of IR spectroscopies for animal cell cultures reported in literature are applied to single cells cultivated in suspension. However, in the case of adherent cell culture, it is essential to take into consideration the presence of microcarriers in the sensing space, increasing greatly the complexity of spectral information analysis [9].
Finally, all these characteristics and complexities related to animal cell processes require sophisticated MVDA techniques, such as chemometrics, to reduce data dimensions and to relate IR spectral information with the target compounds. The chemometric methods most widely used for spectral data analysis in bioprocess monitoring are PCA, PCR and PLS [11].
Reported Works Highlighting the Interest of IR Spectroscopy for Animal Cell Bioprocesses
In recent years, the use of IR spectroscopy in the field of bioprocesses has grown and evolved rapidly from raw material testing and final product quality control to process monitoring [12]. Advances in instrumentation and chemometric techniques have largely contributed to this expansion in a wide range of bioprocesses [11]. In the case of mammalian cell cultures, the use of IR spectroscopy mainly focuses on two different purposes: process supervision and process monitoring. Table 1 summarizes the reported works displaying off-line and in situ applications of IR spectroscopies in animal cell cultures.
Cell line
IR tool
Analyse mode
Monitored component
Reference
Insectcells
NIR
Off-line
Ala, Glc, Leu, Gln
[32,33]
N.A.
NIR
Off-line
Glc, Lact, Ammo
[20]
CHO
FT-NIR
Off-line
Glc, Gln, Glu, Lact, Ammo, Pyr, 14AA
[21]
CHO, NS0
FTIR
Off-line
mAb, Glc, Lact
[34]
CHO
NIR
Off-line
Gln, Glu, Glc, Lact, Ammo, VCD, mAb, LDH, Osmolality
[35]
NS0
FTIR-ATR
Off-line
mAb, HCP
[36]
CHO
MIR
Off-line
mAb, Lact, Glu, LDH, VCD, DCD, viability
[37]
MSCs
MIR
Off-line
Glc, Lact, Gln, Ammo
[25]
CHO
NIR
In situ
Gln, Glc, Lact, Ammo
[6]
CHO
NIR
In situ
Glu, Lact
[24]
HEK
NIR
In situ
Gln, Glu, Glc, Lact, Ammo, VCD, pH
[38]
Vero
NIR
In situ
Glc, Lact
[9]
N.A.
NIR
In situ
Glc, Lact, Ammo, TCD
[13]
CHO
NIR
In situ
Glc, mAb, PCV, ivPCV, VCD, iVCC, Osmolality
[24]
CHO
NIR
In situ
Glc
[23]
CHO
NIR
In situ
LDH
[27]
PER.C6®
NIR
In situ
Glc, Lact, TCD
[26]
AA: Amino Acid; AA: Ammo Ammonium; DCD: Dead Cell Density; Glc: Glucose; Glu: Glutamate; Gln: Glutamine; HCP: Host Cell Protein; iVCC: Integrated Viable Cell Count; ivPCV: Integrated Viable Packed Cell Volume; LL: Lact Lactate; LDH: Lactate Dehydrogenase; N.A: Non Available; PCV: Packed Cell Volume; Pyr: Pyruvate; TCD: Total Cell Density; VCD: Viable Cell Density.
Table 1: Reported works with off-line and in situ IR monitoring of animal cell culture processes.
IR Spectroscopy as a global supervision tool for animal cell processes
Taking advantage of the multivariate nature of IR data, which contain both physical and chemical information, process trajectories can be analyzed using IR spectra coupled with MVDA techniques. This approach is based on a global comparison of spectra patterns with standard ones, without quantification of any particular medium component. It is mostly relevant to analyze differences in historical culture batches, to detect abnormal runs and to guarantee that process remains inside the design space. For example, NIR spectroscopy was used to characterize a mAb production process in which several cultures were compared according to their overall behavior and some batch deviations could be successfully identified [13]. Another study reported the possibility of using NIR combined with PCA to identify batch homogeneity between lots and to detect early stage contamination [14]. Additionally, NIR was also found to be able to detect variabilities in raw materials of cell culture media [15]. Indeed, such variabilities can lead to large unpredictability of cell culture process performances, which impact the process CQA consequently. A combined NIR/MVDA approach, analyzing the fingerprint of raw materials, allows a robust selection of medium lot while providing a biological link between chemical composition of raw materials and cell culture performances [16].
IR Spectroscopy as a real-time monitoring tool for animal cell process
In order to quantify concentrations of components in cell culture supernatants, it is crucial to establish beforehand calibration models based on off-line measurements with reference methods. Subsequently, these models must be validated in order to perform accurate predictions of target compound concentrations [17]. The first works reporting the use of IR spectroscopy for animal cell culture monitoring were published in late 1990s. Mostly based on off-line analysis, these preliminary studies demonstrated that IR spectroscopy can be a reliable tool to perform simultaneous measurement of different key-parameters in cell culture supernatant (e.g. nutrients and products concentration), with a relative high accuracy compared to reference methods [18,19]. Some later works aimed to improve model predictions. For example, NIR spectral information of various samples, including cell culture samples and aqueous mixtures, were combined to reach calibration models which display an higher accuracy [20]. In another work, NIRS was used to quantify 19 cellular nutrients and waste products simultaneously in culture medium [21]. Formulated synthetic samples were prepared to provide a wider range of component concentrations and to prevent data from being influenced by correlated phenomena. All these results were proved to be an important step toward further in situ IR applications within animal cell processes.
In 2002, the first paper describing in situ IR monitoring of mammalian cell bioprocess was published: a MIR spectroscopy was used in association with an ATR diamond probe plunged into bioreactor to monitor real-time concentrations of glucose and lactate during a CHO cell culture [22]. A following work proposed NIR spectroscopy coupled with a fiber optic probe to monitor in situ concentrations of four key analyte, glucose, lactate, glutamine, and ammonia, over a CHO cell culture [6]. Some later works focused on calibration model constructions, and demonstrated that model performances can be improved using semi-synthetic sample data to extend the calibration to wider range of process conditions [23]. In another study, a calibration strategy employing a multiplexing NIR for the monitoring of multiple reactors simultaneously has been proposed [24]. This method allowed improving and speeding up the model construction.
Latest studies have mainly focused on the expansion of IR spectroscopy applications in culture processes with wide range of operating conditions at different process scales, from laboratory to pilot and industrial ones. In one recent study, in situ NIR was applied on large production bioreactors of 12,500L, and seven keyparameters were successfully monitored in real-time, including mAb concentration, which is one of the most important CQAs of protein production bioprocesses [14]. As far as we know, this was the first in situ mAb titer measurement. Furthermore, IR applications have been expanded to different cell types, from continuous to primary cell lines. Most recently, MIR was implemented in MSCs cultures, and glucose, lactate, and ammonia concentrations were monitored with low prediction errors [25]. IR monitoring of cell cultures have also been tested in the case of different feeding strategies and culture modes. One successful example of in situ NIRS monitoring of a perfused cell culture was demonstrated, indicating that IR techniques have great potentials for future process control [26].
Conclusion and Future Challenges
This review highlights the potential of IR spectroscopy as a promising and reliable PAT tool to supervise and monitor animal cell culture bioprocesses. Besides global process supervision, it allows multi parametric, non-invasive, and real-time concentration measurements of key supernatant compounds throughout cell culture processes. In the near future, further developments should make it possible to monitor and control CPPs to ensure CQAs. This will lead to higher process stabilities, and thus better process performances and more stable product qualities.
Currently, the objective of the most published works remains in real-time estimation of medium compound concentrations. However, one future challenge lies, not only in additional compounds supervision but also in in situ monitoring of cell physiological state parameters as well as product qualities such as glycosylation of recombinant proteins. While IR based measurements are clearly useful, additional values for implementation of PAT approach can be obtained by combining different types of in situ/off-line monitoring tools, such as Raman spectroscopy, dielectric spectroscopy, flow cytometry, mass spectrometry,… For example, a new strategy based on NIR and dielectric spectroscopies combination has been recently proposed to monitor and characterize the different viable, dead and lysed cell populations of CHO cell cultures [27].
It should be noticed that, despite all the attractive benefits of IR spectroscopy, there are still little applications in biopharmaceutical industries. Indeed, while several successful examples have been reported in the case of microbial fermentations, to our knowledge, there is still no application of IR spectroscopy for control of animal cell bioprocesses [28,29,30]. However, many authors have suggested the great interest of IR implementation in control systems of animal cell cultures, in order to obtain a fully automatic and self-regulating production system [26,31]. Future works should focus on IR spectroscopy adaptation to industrial bioprocesses, especially for close-loop feedback control of CPP, which is of great importance to control process CQA.
Acknowledgment
This work was supported by the French Ministry of Research.
References
- Abès R, Teillaud JL. Impact of glycosylation on effector functions of therapeutic IgG. Pharmaceuticals. 2010; 3: 146–157.
- Mandenius CF, Graumann K, Schultz TW, Premstaller A, Olsson IM, Petiot E, et al. Quality-by-design for biotechnology-related pharmaceuticals. Biotechnol J. 2009; 4: 600-609.
- US FDA. Current good manufacturing practices for drugs: Reports, guidances and additional information - Pharmaceutical cGMPs for the 21st Century - A Risk-based approach. 2004.
- Chu L, Robinson DK. Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol. 2001; 12: 180-187.
- Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serumfree cell culture: the serum-free media interactive online database. ALTEX. 2010; 27: 53-62.
- Arnold SA, Crowley J, Woods N, Harvey LM, McNeil B. In-situ near infrared spectroscopy to monitor key analytes in mammalian cell cultivation. Biotechnol Bioeng. 2003; 84: 13-19.
- Arnolda S A, Crowley J, Vaidyanathan S, Matheson L, Mohan P, Hall JW, et al. At-line monitoring of a submerged filamentous bacterial cultivation using near-infrared spectroscopy. Enzyme Microb Technol. 2000; 27: 691–697.
- Teixeira AP, Oliveira R, Alves PM, Carrondo MJ. Advances in on-line monitoring and control of mammalian cell cultures: Supporting the PAT initiative. Biotechnol Adv. 2009; 27: 726-732.
- Petiot E, Bernard-Moulin P, Magadoux T, Gény C, Pinton H, Marc A. In situ quantification of microcarrier animal cell cultures using near-infrared spectroscopy. Process Biochem. 2010; 45: 1832–1836.
- Zhao L, Fu HY, Zhou W, Hu WS. Advances in process monitoring tools for cell culture bioprocesses. Eng Life Sci. 2015; 15: 459–468.
- Lourenço ND, Lopes JA, Almeida CF, Sarraguça MC, Pinheiro HM. Bioreactor monitoring with spectroscopy and chemometrics: a review. Anal Bioanal Chem. 2012; 404: 1211-1237.
- Roychoudhury P, Harvey LM, McNeil B. The potential of mid infrared spectroscopy (MIRS) for real time bioprocess monitoring. Anal Chim Acta. 2006; 571: 159-166.
- Henriques JG, Buziol S, Stocker E, Voogd A, Menezes JC. Monitoring mammalian cell cultivations for monoclonal antibody production using nearinfrared spectroscopy. Adv Biochem Eng Biotechnol. 2009; 116: 73-97.
- Clavaud M, Roggo Y, Von Daeniken R, Liebler A, Schwabe JO. Chemometrics and in-line near infrared spectroscopic monitoring of a biopharmaceutical Chinese hamster ovary cell culture: Prediction of multiple cultivation variables. Talanta. 2013; 111: 28–38.
- Kirdar AO, Chen G, Weidner J, Rathore AS. Application of near-infrared (NIR) spectroscopy for screening of raw materials used in the cell culture medium for the production of a recombinant therapeutic protein. Biotechnol Prog. 2010; 26: 527-531.
- Lee HW, Christie A, Liu JJ, Yoon S. Estimation of raw material performance in mammalian cell culture using near infrared spectra combined with chemometrics approaches. Biotechnol Prog. 2012; 28: 824-832.
- Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. 6th edn. Harlow: Pearson Education. 2010.
- Harthun S, Matischak K, Friedl P. Process control of antithrombin III production by near-infrared spectroscopy. MJT Carrondo et al., editors. In Animal cell technology. Springer Netherlands, 1997: 417-421.
- Riley MR, Rhiel M, Zhou X, Arnold MA, Murhammer DW. Simultaneous measurement of glucose and glutamine in insect cell culture media by near infrared spectroscopy. Biotechnol Bioeng. 1997; 55: 11-15.
- Mcshane MJ, Cote GL. Near-infrared spectroscopy for determination of glucose, lactate, and ammonia in cell culture media. Appl Spectrosc. 1998; 52: 1073–1078.
- Riley MR, Crider HM, Nite ME, Garcia RA, Woo J, Wegge RM. Simultaneous measurement of 19 components in serum-containing animal cell culture media by Fourier transform near-infrared spectroscopy. BiotechnolProg. 2001; 17: 376–378.
- Rhiel M, Ducommun P, Bolzonella I, Marison I, von Stockar U. Real-time in situ monitoring of freely suspended and immobilized cell cultures based on mid-infrared spectroscopic measurements. Biotechnol Bioeng. 2002; 77: 174-185.
- Milligan M, Lewin-Koh N, Coleman D, Arroyo A, Saucedo V. Semisynthetic model calibration for monitoring glucose in mammalian cell culture with in situ near infrared spectroscopy. Biotechnol Bioeng. 2014; 111: 896-903.
- Roychoudhury P, O’Kennedy R, McNeil B, Harvey LM. Multiplexing fibre optic near infrared (NIR) spectroscopy as an emerging technology to monitor industrial bioprocesses. Anal Chim Acta. 2007; 590: 110-117.
- Rosa F, Sales KC, Carmelo JG, Fernandes-Platzgummer A, da Silva CL, Lopes MB. Monitoring the ex-vivo expansion of human mesenchymal stem/stromal cells in xeno-free microcarrier-based reactor systems by MIR spectroscopy. Biotechnol Prog. 2016; 32: 447-455.
- Mercier SM, Rouel PM, Lebrun P, Diepenbroek B, Wijffels RH, Streefland M. Process analytical technology tools for perfusion cell culture. Eng Life Sci. 2016; 16: 25–35.
- Courtès F, Ebel B, Guedon E, Marc A. A dual near-infrared and dielectric spectroscopies strategy to monitor populations of Chinese hamster ovary cells in bioreactor. Biotechnol Lett. 2016; 38: 745–750.
- González-Vara y R A, Vaccari G, Dosi E, Trilli A, Rossi M, Matteuzzi D. Enhanced production of L-(+)-lactic acid in chemostat by Lactobacillus casei DSM 20011 using ion-exchange resins and cross-flow filtration in a fully automated pilot plant controlled via NIR. Biotechnol Bioeng. 2000; 67: 147–156.
- Tosi S1, Rossi M, Tamburini E, Vaccari G, Amaretti A, Matteuzzi D . Assessment of in-line near-infrared spectroscopy for continuous monitoring of fermentation processes. Biotechnol Prog. 2003; 19: 1816-1821.
- Navrátil M, Norberg A, Lembrén L, Mandenius C-F. On-line multi-analyzer monitoring of biomass, glucose and acetate for growth rate control of a Vibrio cholerae fed-batch cultivation. J Biotechnol. 2005; 115: 67–79.
- Cervera AE, Petersen N, Lantz AE, Larsen A, Gernaey KV. Application of near-infrared spectroscopy for monitoring and control of cell culture and fermentation. Biotechnol Prog. 2009; 25: 1561-1581.
- Sandor M, Rüdinger F, Bienert R, Grimm C, Solle D, Scheper T. Comparative study of non-invasive monitoring via infrared spectroscopy for mammalian cell cultivations. J Biotechnol. 2013; 168: 636-645.
- Riley MR, Arnold MA, Murhammer DW, Walls EL, DelaCruz N. Adaptive calibration scheme for quantification of nutrients and byproducts in insect cell bioreactors by near-infrared spectroscopy. Biotechnol Prog. 1998; 14: 527-533.
- Sellick CA, Hansen R, Jarvis RM, Maqsood AR, Stephens GM, Dickson AJ, et al. Rapid monitoring of recombinant antibody production by mammalian cell cultures using fourier transform infrared spectroscopy and chemometrics. Biotechnol Bioeng. 2010; 106: 432-442.
- Hakemeyer C, Strauss U, Werz S, Jose GE, Folque F, Menezes JC. At-line NIR spectroscopy as effective PAT monitoring technique in Mab cultivations during process development and manufacturing. Talanta. 2012; 90: 12-21.
- Capito F, Skudas R, Stanislawski B, Kolmar H. Matrix effects during monitoring of antibody and host cell proteins using attenuated total reflection spectroscopy. Biotechnol Prog. 2013; 29: 265-274.
- Capito F, Zimmer A, Skudas R. Mid-infrared spectroscopy-based analysis of mammalian cell culture parameters. Biotechnol Prog. 2015; 31: 578-584.
- Card C, Hunsaker B, Smith T, Hirsch J. Near-infrared spectroscopy for rapid, simultaneous monitoring. Bio Process Int. 2008; 6: 59–67.