
Research Article
Austin J Anal Pharm Chem. 2016; 3(3): 1071.
Development and Validation of High-Performance Thin- Layer Chromatography Method for the Simultaneous Densitometric Determination of Metformin and Rosiglitazone in Tablets
Susheel JV*, Paul D and Ravi TK
Department of Pharmaceutical Analysis, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore-641044, Tamil Nadu, India
*Corresponding author: Susheel John Varghese, Department of Pharmaceutical Analysis, College of Pharmacy, Sri Ramakrishna Institute of Paramedical Sciences, Coimbatore-641044, Tamil Nadu, India
Received: August 18, 2016; Accepted: September 02, 2016; Published: September 06, 2016
Abstract
This paper describes validated High-Performance thin-layer chromatography (HPTLC) method for the simultaneous determination of metformin (MET) and rosiglitazone (ROS) in combined tablet dosage forms. The HPTLC separation was carried out on aluminum backed silica gel 60F254 layers using methanoltetrahydrofuran- ammonia, 8:3:1.5 (v/v/v), as the mobile phase. Quantification was achieved with UV detection at 262nm. The developed analytical method was validated according to International Conference on Harmonization guidelines in terms of parameters like accuracy, precision, linearity, and specificity. Good linearity was observed in the concentrations ranging from 0.12 to 0.48 and 15 to 60 μg/band for ROS and MET, respectively. The recovery study results ranged from 98 to 103% for MET and ROS, showing the accuracy of the method. The method was successfully applied for the analysis of the drugs in laboratory prepared mixtures and commercial tablets. The developed method is simple, precise, and sensitive, and applicable for simultaneous determination of MET and ROS in pure powder and tablets.
Keywords: Metformin; Rosiglitazone; High-Performance Thin-Layer Chromatography (HPTLC); Densitometric determination
Introduction
Metformin hydrochloride (MET) (1-carbamimidamido-N, N-dimethylmethanimidamide) (Figure 1) is a biguanide antihyperglycemic agent used for treating non-insulin-dependent diabetes mellitus [1]. Rosiglitazone (ROS) (5-[(4-{2-[methyl (pyridin-2-yl) amino] ethoxy} phenyl) methyl]-1, 3-thiazolidine-2, 4-Dione) (Figure 1) is an anti-diabetic drug in the thiazolidinedione class of drugs [2]. Metformin hydrochloride and rosiglitazone is a combination of two oral diabetes medicines that help control blood sugar levels for people with type II diabetes who do not use daily insulin injections [3]. The complementary actions of the antidiabetic agent’s metformin hydrochloride and rosiglitazone maleate may maintain optimal glycemic control in patients with type 2 diabetes; therefore, their combined use may be indicated for patients whose diabetes is poorly controlled by metformin alone [4]. As first-line therapy in patients with uncontrolled type II diabetes, rosiglitazone/metformin fixed-dose combination therapy achieved significant reductions in A1C (glycated haemoglobin) and FPG (fasting plasma glucose) compared with either rosiglitazone or metformin monotherapy [5].
Figure 1: Chemical structures of metformin and rosiglitazone.
Different analytical methods like HPLC and LC-MS have been reported for the simultaneous determination of metformin and rosiglitazone in pharmaceutical preparations and plasma [6-13]. Spectrophotometric and HPTLC methods have been reported for the simultaneous determination of metformin and rosiglitazone in pharmaceutical preparations [14]. The developed method has advantage that it is more sensitive than the reported methods.
Experimental
Chemicals, reagents, and standards
Standard bulk drug samples of MET and ROS were provided by Microlabs Ltd., (Bengaluru, India). The pharmaceutical dosage form used in this study was REZULT- M4 tablets, labeled to contain rosiglitazone 4mg and metformin 500 mg (Sun Pharmaceuticals Ltd. Mumbai, India). Tetrahydrofuran (AR grade) was purchased from Qualigens Fine Chemicals (Mumbai, India). Ammonia (LR grade) and Methanol (AR grade) were procured from Merck (Mumbai, India).
Instrumentation and chromatography
The HPTLC instrumentation consisted of a Linomat V sample applicator with 100μL Hamilton syringe and TLC scanner III controlled by win CATS software (CAMAG, Switzerland). Merck TLC plates coated with silica gel 60F254 on aluminum sheets were used as the stationary phase. The plates were developed in a CAMAG twin trough chamber previously saturated for 30min with the mobile phase methanol-tetrahydrofuran-ammonia, 8:3:1.5 (v/v/v). Each chromatogram was developed over a distance of 8cm.
The spots were scanned in reflectance mode at 262nm with slit dimension of 5 mm×0.45mm. All weighing was done on a Shimadzu electronic balance, Model BL-220H.
Preparation of the standard stock solution
Standard stock solution containing 80 μg/mL of ROS and 10000 μg/mL MET was prepared in methanol.
Preparation of calibration curves
Standard solutions of ROS and MET (1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5 and 6.0 μL) prepared from the standard stock solution were applied on the TLC plate to obtain concentrations ranging from 0.12 to 0.48 and 15 to 60 μg/band for ROS and MET, respectively. The plate was developed in a chamber previously saturated with the mobile phase for 30min. After development, the plate was air-dried, and standard zones were quantified by scanning at 262nm with the deuterium lamp. After separation of the drugs on the TLC plate, UV spectra of the individual drugs in solid state were recorded using the deuterium lamp of the TLC scanner. The calibration curves were constructed by plotting peak areas versus concentrations for each drug.
Preparation of sample solution
Twenty tablets were weighed, and the average weight was calculated. An amount of powder equivalent to 8mg of ROS and 1000mg of MET was transferred to a 100mL volumetric flask; and 50mL methanol was added. The flask was shaken mechanically for 10min, followed by dilution to volume with methanol to provide a solution containing 80 μg/mL of ROS and 10000 μg/mL MET. This solution was filtered through a 0.45mm membrane filter before injection.
Method validation
The developed method was validated according to International Conference on Harmonization guidelines [15] for validation of analytical procedures.
The linearity was evaluated by linear regression analysis. The calibration graphs were plotted for various concentrations for ROS (0.12 to 0.48 μg/band; n=10) and MET (15 to 60 μg/band; n = 10).
The precision of the method was determined by repeatability (intraday precision) and intermediate precision (interday precision) and was expressed as R.S.D. of a series of measurements. Intraday precision was evaluated by six replicate readings at three concentration levels within the linearity range. Interday precision was studied by comparing the results on 3 different days.
To study the accuracy of the method, recovery studies were carried out by addition of standard drug to preanalyzed sample at 100% level. The resulting solutions were then reanalyzed by the proposed method.
LOD and LOQ were calculated using the S/N method. LOD was taken as the concentration of analyte at which S/N was 3. LOQ was taken as the concentration of analyte at which S/N was 10.
Robustness was evaluated by studying the influence of small deliberate variations of the analytical parameters on the Rf value, peak area, or peak shape. The method should be robust enough with respect to all critical parameters so as to allow routine laboratory use.
Specificity of the method was evaluated by studying the peak purity index values.
Results and Discussion
Experimental conditions, such as mobile phase and wavelength of detection, were optimized to provide accurate, precise, and reproducible results for simultaneous determination of ROS and MET. Good separation of both drugs (Rf values of ROS = 0.34 and MET = 0.54) was obtained by using methanol-tetrahydrofuranammonia, 8:3:1.5 (v/v/v), as the mobile phase. The presence of ammonia gave good compact spots for the drugs. The separated spots of the two drugs were scanned at 262 nm because both drugs showed considerable absorbance at this wavelength, figure 2. A typical densitogram obtained from the analysis of drugs using the proposed method is shown in Figure 3.
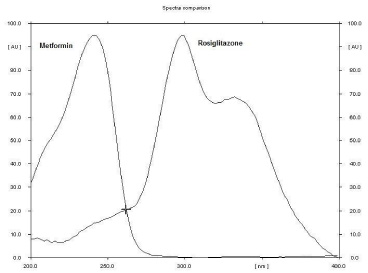
Figure 2: Overlain UV spectra of metformin and rosiglitazone on precoated
TLC plate.
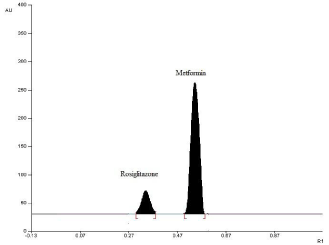
Figure 3: HPTLC densitogram of ROS (0.12 μg/band) and MET (15 μg/band).
The relationship between the concentration of ROS and MET and peak areas of the spots was investigated. Good linearity was observed in the concentration range of 0.12 to 0.48 and 15 to 60 μg/band for ROS and MET, respectively. The regression equations for the drugs were found by plotting peak areas in AU (y) versus the concentration (x) in μg/band. Table 1 summarizes the linearity ranges and linear regression equations for both drugs.
Parameters
ROS
MET
Linearity, µg/band
0.12 to 0.48
15 to 60
Linear regression equationa
Intercept
498.353
4670.868
Slope
6.970
163.97
Correlation coefficient
0.9992
0.9997
LOD, µg/band
0.04
0.1
LOQ, µg/band
0.12
0.5
Precision
Intraday(n=3), % RSDb
0.3863
0.6574
Interday (n=3), % RSDb
1.2673
1.5876
Repeatability of injection, % RSDb
0.7461
1.9456
ay = mx + c.
bAverage of six determinations.
Table 1: Summary of validation parameters for the developed method.
The LOD values for ROS and MET were found to be 0.04 and 0.1 μg/band, respectively, and their LOQ values were 0.12 and 0.5 μg/ band, respectively.
Robustness studies were done by making slight alterations in the detection wavelength and mobile phase composition. Influence of different conditions (different lots of precoated plates, different suppliers of reagents, etc.) were also studied. Not much change was found in the Rf values, area, or symmetry of the peaks.
The specificity of the developed method was evaluated by studying the peak purity index values. The peak purities of ROS and MET were assessed by comparing their respective in situ spectra at peak start (s), peak apex (m), and peak end (e) positions of the spots. Good correlation among the spectra indicated the peak purity for ROS [correlation r(s,m) = 0.9999 and r(m,e) = 0.9998] and MET [correlation r(s,m) = 0.9999 and r(m,e) = 0.9994], Figure 4. It could be concluded that no impurities or degradation products migrated with the spots obtained from solutions of the drugs.
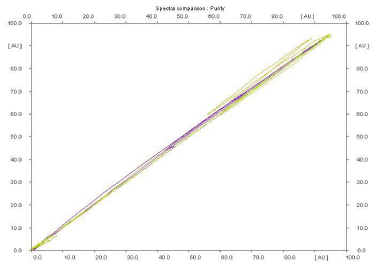
Figure 4: Purity graph showing correlation between peak maxima and slope
for drug peaks.
The precision of the method was determined by repeatability (intraday) and intermediate (interday) precision and was expressed as the R.S.D. of the results. The values obtained for the precision studies, presented in Table 1, indicate good repeatability and low interday variability. Stability of the analyte on the plate was studied at different time intervals and peak areas were compared with the peak area of freshly scanned plate. The developed plate showed stability upto 2.5 hours for ROS and 3 hours for MET.
The recovery study results ranged from 98 to 103% for ROS and MET, showing the accuracy of the method (Table 2). Low RSD values indicate that the method is also precise.
Drug
Recovery (%)a
R.S.D. (%)a
ROS
102.30
1.5983
MET
98.76
1.6875
a Average of six determinations.
Table 2: Accuracy Studies.
The proposed method was applied in the assay of the commercially available tablets containing ROS and MET. Six replicate determinations were performed on accurately weighed tablets. Experimental values obtained for determination of ROS and MET in samples is presented in Table 3.
Drug
Labeled amount,
mg/tabletAmount found, mg/tablet
Amount found, %a
R.S.D., %a
ROS
4
3.93
98.26
1.8338
MET
500
502.95
100.59
0.3591
a Average of six determinations.
Table 3: Analysis of the formulation.
Comparison between the Developed and Reported Methods
The proposed analytical method was compared with reported method using statistical analysis. Student’s t-test was applied to the results and did not show significant differences between the sample analyses by the two methods. The calculated t-value was found to be less than the critical t-value at the 0.05 significance level. This result shows that the two methods are equivalent for the quantitative simultaneous determination of drugs in formulations.
Conclusion
The proposed new HPTLC method is simple, precise, and accurate. The method uses simple reagents with minimal sample preparation procedures. Since the method has the advantages of short run time, large sample capacity, and use of minimal volumes of solvents, it is suitable for routine simultaneous analysis of ROS and MET in the formulations.
Acknowledgments
The authors wish to thank M/s SNR Sons Charitable Trust, Coimbatore, India, for providing the facilities to carry out the experiments.
References
- Metformin.
- Rosiglitazone.
- Common Side Effects of Celebrex (Celecoxib) Drug Center – RxList.
- Vivian F, Julio R, Rita P, Alan S. J. Am. Med. Assoc. 2000; 283: 1695-1702.
- Rosenstock J, Rood J, Cobitz A, Biswas N, Chou H, Garber A. Initial treatment with rosiglitazone/metformin fixed-dose combination therapy compared with monotherapy with either rosiglitazone or metformin in patients with uncontrolled type 2 diabetes. Diabetes. Obes. Metab. 2006; 8: 650-660.
- Sultana N, Arayne MS, Shafi N, Siddiqui FA, Hussain A. Development and Validation of New Assay Method for the Simultaneous Analysis of Diltiazem, Metformin, Pioglitazone and Rosiglitazone by RP-HPLC and its Applications in Pharmaceuticals and Human Serum. J. Chromatogr. Sci. 2011; 49: 774-779.
- Chen L, Zhou Z, Shen M, Ma A. Simultaneous determination and pharmacokinetic study of metformin and rosiglitazone in human plasma by HPLC-ESI-MS. J. Chromatogr Sci. 2011; 49: 94-100.
- Onal A. Spectrophotometric and HPLC determinations of anti-diabetic drugs, rosiglitazone maleate and metformin hydrochloride, in pure form and in pharmaceutical preparations. Eur. J. Med. Chem. 2009; 44: 4998-5005.
- Ali AR, Duraidi II, Saket MM, Abu-Nameh ES. Column High-Performance Liquid Chromatographic Method for the Simultaneous Determination of Rosiglitazone and Metformin in a Pharmaceutical Preparation. J. AOAC Int. 2009; 92: 119-124.
- Kolte BL, Raut BB, Deo AA, Bagool MA, Shinde DB. Simultaneous Determination of Metformin in Combination with Rosiglitazone by Reversed-Phase Liquid Chromatography. J. Chromatogr. Sci. 2004; 42: 70-73.
- Akteruzzaman M, Asma R, Zakir SM, Farhana I, Abdus SM, Rashid MA. Dhaka Univ. J. Pharm. Sci. 2012; 11:157-163.
- Zhang X, Peng Y, Wan P, Yin L, Wang G, Sun J. Simultaneous Determination and Pharmacokinetic Study of Metformin and Pioglitazone in Dog Plasma by LC–MS-MS. J. Chromatogr. Sci. 2014; 52: 52-58.
- Zhang L, Tian Y, Zhang Z, Chen Y. J. Chromatogr. B Analyt. Simultaneous determination of metformin and rosiglitazone in human plasma by liquid chromatography/tandem mass spectrometry with electrospray ionization: application to a pharmacokinetic study. Technol. Biomed. Life Sci. 2007; 854: 91-98.
- Mahgoub H, Youssef RM, Korany MA, Khamis EF, Kamal MF. Development and validation of spectrophotometric and HPTLC methods for simultaneous determination of rosiglitazone maleate and metformin hydrochloride in the presence of interfering matrix excipients. Drug. Dev. Ind. Pharm. 2014; 40: 1190-1198.
- ICH Harmonized Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2 (R1), (November 2005) International Conference on Harmonization, Geneva, Switzerland.