
Research Article
Austin J Anal Pharm Chem. 2016; 3(4): 1073.
New Method for Determination of Mercury in Contaminated Water by using Nano Composite Carbon Paste Electrode
Gohari H*
Department of Applied Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran
*Corresponding author: Hadi Gohari, Department of Applied Chemistry, Mashhad Branch, Islamic Azad University, Mashhad, Iran
Received: September 16, 2016; Accepted: October 06, 2016; Published: October 10, 2016
Abstract
In this research, we constructed chemical sensor for determining mercury in contaminated water because we needed fast, simple, low-cost, and accurate determination of mercury in different environmental systems. Although several methods have been developed for determination of mercury ion in contaminated water, there is no a cheap, simple, accurate and rapid method to measure this ion. Aim of this study is to develop a new method to measure the mercury based on using nanosilica, multi-walled carbon nanotube (MWCNT) and 1,13-Bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane (Kryptofix 5) as an ionophore for modification of a CPE. The optimum composition of modified CPE was determined as 63.6% of graphite powder, 25% of paraffin oil, 4% of multiwalled carbon nanotube (MWCNT), 0.4% nanosilica and 7% of ionophore. This optimum composition was shown high selectivity, with appropriate Nernestian slope (29.5 ± 0.4mV/decade), linear range (from 1.0×10-2to 1.0×10-8M), with a detection limit of (2.0×10-9M), The response of the sensor is independent of pH in the range of 3.1 – 8.4, and The interference of different ionic species with the response of the electrode shows a good selectivity of the proposed sensor The proposed sensor is successfully applied in determination of mercury ions in some contaminated water samples. The results of this study to introduce a cheap accurate and simple method for determination of mercury ion in contaminated water.
Keywords: Multi-walled carbon nanotubes; Kryptofix 5; Sensor; Carbon paste ion selective; Potentiometry
Introduction
Mercury is generally found at very low concentration in the environment. Mercuric ion can be absorbed readily by humans and other organisms. It may cause kidney toxicity, neurological damage, paralysis, chromosome breakage, and birth defects [1]. Due to its serious hazardous effect on human health and toxicity in the environment, it is important to control its levels in natural and potable water. Thus, it is very necessary to monitor the mercury levels in our environment in the past decades, numerous methods have been developed to determine heavy metals, including spectrophotometry, inductively coupled plasma mass spectrometry (ICP-MS), inductively coupled plasma optical emission spectrometry (ICP-OES), atomic absorption spectrometry (AAS), electro thermal atomic absorption spectrometry (ETAAS) [2-8]. However, the majority of the above techniques require complex devices, training special skills and time consuming operations, which result in high costs. Electrochemical methods such as potentiometric is one of the most favorable approaches for the determination of heavy metal ions, due to its high sensitivity, ability for portability, low cost and time saving. Also they are able to preconcentrate the analytes on the electrode surface and have ability to analyze element speciation with no or minimum sample change [9-14]. Carbon paste electrodes (CPEs) have gained much attraction as ion selective electrodes of potentiometric mostly due to their benefits over membrane electrodes such as fixed response, renewability, low ohmic resistance and no necessity for internal solution [15-18]. Graphite powder usually becomes dispersed in a non-conductive mineral oil, as a binder, to make carbon paste [19]. Newly, CPEs have been chemically modifying to boost the sensitiveness, selectivity, detection limit and other aspects of these electrodes. For example, some matters such as functionalized nanoparticles and appropriate ligands, have been applied within the electrode structure [20-23]. In this area multiwalled carbon nanotube (MWCNT), have attractive physicochemical properties that make them favorable for using as electrode modifiers, like ultra-light weight, high mechanical strength, high electrical conductivity, high thermal conductivity, ordered structure with high aspect ratio, high surface area and metallic or semi-metallic behavior [24-25]. Also, the ability of electron transfer between the electrodes and the electro active species offers great potential for creating electrochemical sensors. These aspects make CNTs unique materials for diverse electrochemical applications [26-28]. In this work, the application of Kryptofix5 as an ionophore shown in Figure 1 have been discussed for the detection of Hg(II) ions in drinking water samples. The sensor responds to Hg(II) ions. The recognition of small molecules in binding with heavy metals has gained importance in the field of research. The Kryptofix5 has two donating nitrogen atoms and five oxygen atoms, low molecular weight and flexible structure were expected to act as a suitable ionophore in the preparation of carbon paste sensors for mercury ions of proper size and charge. Structure of 1,13-Bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane (Kryptofix5) (Figure 1). The main aim of this study is to develop a new method for determination of mercury ion in contaminated water based on using multi-walled carbon nanotube (MWCNT) and Kryptofix 5 as an ionophore for modification of a CPE.
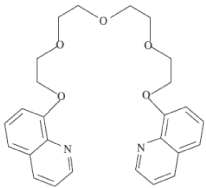
Figure 1: Structure of 1,13-Bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane
(Kryptofix 5).
Experimental Parts
Apparatus
The cell used for the measurements included the Hg2+ carbon paste electrode as the working electrode and an Ag/AgCl electrode (Azar electrode, Iran) as a reference electrode both of which were connected to a mili-voltmeter. The cell assembly constructed for the conduction of the electromotive force (Emf) measurements is as follows; Carbon paste electrode | sample solution (Contaminated Water) | Ag/AgCl–KCl (satd.).
Reagents and materials
The ionophore Kryptofix 5 was purchased from Sigma-Aldrich. The graphite powder with a 1–2 μm particle size purchased from Merck. High purity paraffin oil obtained Aldrich. The multi-walled carbon nanotubes (MWCNTs) with 10–40 nm diameters, 1–25 μm length and with 95% purity were purchased from Research Institute of the Petroleum Industry (Iran). Nitrate and chloride salts of all the used cations (all from Merck Co.) were of the highest available purity and were used without any further purification. Doubly distilled water was used during the experiments.
Carbon paste electrode preparation
For the purpose of preparing the carbon paste electrode the following proceeding was carried out respectively: First, different amounts of the paste ingredients such as ionophore (Kryptofix 5), binder (paraffin oil), graphite powder as an inert matrix, NS and MWCNTs as modifier entirely were pooled. Next, the resulting mixture was stirred to get homogenization paste about 30-40 min. Then, the final paste was transferred to the tip of a tube while a copper wire was put into the opposite side of the tube to electrical contact. It should be noted, in order to avoid possible air gaps which mostly lead to boost the electrode resistance, it is necessary to pack up the paste carefully and thoroughly into the tube tip. Last, to replace the new smoothed surface CPE by the old one, the external layer of the carbon paste was polished with soft paper. In the final stage, the electrode was conditioned for 48h by soaking it in a 1.0×10-3 mol L-1 Hg(NO3)2 solution. Unassayed Contaminated Water samples were analyzed for Mercury Ions.
Results and Discussion
Optimization of the CPEs
The ionophore used as the main ingredient of any ion-selective sensors is known to strongly influence the selectivity of such devices [29-33]. (Kryptofix 5) (Figure 1) was used in constructing a set of CPEs with a variety of compositions some of which were modified using the MWCNT, and nanosilica having the results of solutions studies in hand. The CP electrodes were then tested as sensing devices for Hg2+ ion selective sensors and the results are illustrated in Table 1. The results show that the absence of the ionophore (Kryptofix 5) (compositions 1-3) in the composition of the carbon paste electrode leads to rather constant responses that are probably caused by the inherent (and probably unselective extraction of the cation into the sensing CPE surface). It was also observed that changing the amounts of the graphite powder as the filler and the nanosilica from 73.6-73.3 and 0.4-0.7 respectively, does not change the potential response of the sensor. It is noteworthy that graphite powder being a hydrophobic, high surface area compound improves the extraction of the ions into the surface of the CPE and also the mechanical resistant of the electrode.
Dynamic linear range (M)
Slope (mV/decade)
Composition of Carbon Paste (wt.%)
Electrode No.
Nano-Silica
MWCNTs
Graphite Powder
Kryptofi5
Binder
(Paraffin oil)
3.1±0.2
0.4
0
74.6
0
25
1
4.0×10-4-1.0×10-2
2.5±0.3
0.5
0
74.5
0
25
2
1.0×10-4-1.0×10-2
2.0±0.4
0.7
0
74.3
0
25
3
1.0×10-7-1.0×10-2
18.7±0.5
0.4
0
70.6
4
25
4
24.9±0.1
0.4
0
68.6
6
25
5
1.0×10-8 - 1.0×10-2
28.9±0.3
0.4
0
67.6
7
25
6
1.0×10-8-1.0×10-2
33.4±0.5
0.4
0
66.6
8
25
7
1.0×10-8-1.0×10-2
27.6±0.5
0.4
2
65.6
7
25
8
32.5±0.2
0.4
3
64.6
7
25
9
1.0×10-8-1.0×10-2
29.5 ± 0.4
0.4
4
63.6
7
25
10
1.0×10-6-1.0×10-2
34.5 ± 0.4
0.4
4
58.6
7
30
11
Table 1: The optimization of the carbon paste ingredients.
The introduction of (Kryptofix 5) however leads to a meaningful increase in the potential response of the CP electrode which is, based on the previous observations, due to the enhanced and as was later observed, selective extraction of Hg2+ ion into the sensing surface of the electrode. This was a further support to the initial assumptions made about the selective tendency of (Kryptofix 5) towards Hg2+ ions. This trend further continued by increasing the amount of (Kryptofix 5) in the composition of CPE to 6 (no. 5) and 7 (no. 6) percents increasing the potential response to 24.9±0.1 mV decade-1 and 28.9±0.3 respectively. Further increase in the amount of the amount of the ionophore to 8% wt. (no. 7), however, did not lead to any considerable changes in the response of the electrode. It was hence decided that 7% wt. is the optimum amount of the ionophore in the CPE. The electrode compositions were further modified by adding %2, %3 and %4 wt. of MWCNT to the composition (Nos. 8-10) which led to improvements in the potential response of the sensor from the sub Nernstian value of 27.6±0.5 mV decade-1 to the Nernstian values of 32.5±0.2 mV decade-1 and 29.5 ± 0.4 mV decade-1 due to improving the conductivity of the composition.
Calibration curve
The potential response of the Hg2+-CP electrode based on Kryptofix 5, Figure 2, which is known in terms of calibration curve displays the widish working linear range from 1.0×10-9 to 1.0×10-2 mol L-1 for optimized Kryptofix 5-based mercury (II) carbon paste electrode, while according to Nernstian equation the slope of linear part of calibration curve is 29.5 ± 0.4 mV per decade with detection limit of 2.0×10−9 mol L-1 of mercury ions concentration which pursuant to the IUPAC recommendations is calculated by crossing of two extrapolated segments of the calibration curve [34-46]. The standard deviation for ten replicate measurements was ±0.4mV.
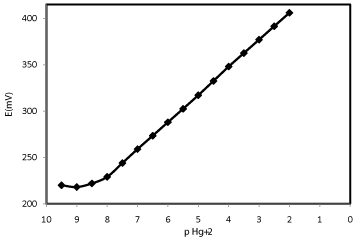
Figure 2: Calibration curves of the Kryptofix 5-based Hg2+ sensor.
The effect of pH
The influence of pH on the potential of the electrodes was investigated by measuring the Electro Motive Force (EMF) of the cell 1.0×10-3 M of mercury (II) solutions. The pH values of the cell were adjusted by the addition of very small volumes of (0.01-0.1 mM) Hydrochloric acid or sodium hydroxide. The results are shown in Figure 3 it is evident that the electrode does not respond to pH changes in the range (3.1-8.4). Under more acidic conditions, the ligand may be protonated thereby losing its capacity to complex with the metal ions. The drift in potential at pH 8.5 is attributed to formation of mercury (II) hydroxide [47].
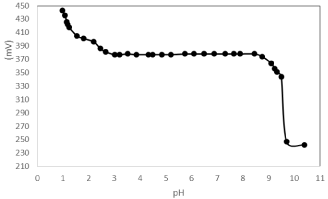
Figure 3: pH effect of the test solution (1.0×10-3 mol L-1 of Hg2+) of the Hg2+
sensor based on Kryptofi 5.
Response time
Response time of the electrochemical sensors is evaluated through measuring the average time required to achieve potential values within ±0.1mV of the steady state potential of the electrode after its immersion in a series of solutions of the target ions, each having a tenfold difference in concentration. Experimental parameters including stirring or the flow rate, the concentration and composition of each test solution, any previous application or preconditioning of the electrode, and the testing temperature can all affect the experimental response time of a sensor [48-54]. The response time of the proposed modified CPE was found to be less than 7s Figure 4.
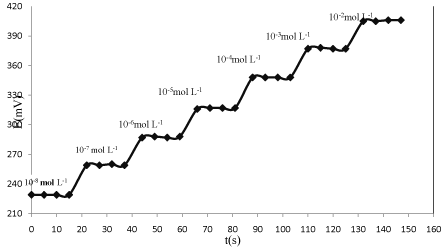
Figure 4: Dynamic response time of Hg2+ sensor based on Kryptofi 5.
Selectivity of the Hg2+-CPE
One of the most important characteristics of the CPEs is its relative response towards the primary ion over other ions present in the solution, which is usually expressed in terms of potentiometric selectivity coefficients. In this work, the matched potential method was used for the evaluation of the selectivity of the sensor. The matched potential method selectivity coefficient, KMPM, is then given by the resulting primary ion to interfering ion activity (concentration) ratio, KMPM = aA/aB. The resulting values are listed in Table 2. For all tested ions, the selectivity coefficients are in the order of 1.0×10-3 or smaller, indicating they would not radically disturb the function of the developed Hg2+-CPE.

Interfering Ion

4.0 × 10-4
Yb+3
8.0 × 10-4
Pr+3
8.0 × 10-4
Mg+2
8.0 × 10-4
La+3
8.0 × 10-4
Pb+2
4.0 × 10-4
Tm+3
4.0 × 10-4
Na+
9.0 × 10-4
Nd+3
6.0 × 10-4
K+
6.0 × 10-4
Eu+3
9.0 × 10-4
Co+2
×9.0 10-4
Ho+3
1.0 × 10-3
Cd+2
4.0 × 10-4
Gd+3
8.0 × 10-4
Ca+2
8.0 × 10-4
Sm+3
2.0 × 10-3
Fe+3
6.0 × 10-4
Er+3
1.7 × 10-4
Cr+3
8.0 × 10-4
Tb+3
6.0 × 10-4
Lu+3
4.0 × 10-4
Dy+3
Table 2: Selectivity coefficients () of proposed Hg2+ -CPE.
Titration with EDTA
The proposed Hg2+-CPE was successfully applied as an indicator electrode in the titration of 25mL of contaminated water samples with a standard EDTA solution (1.0×10-2mol L-1) and the resulting titration curve is shown in Figure 5. As can be seen from Figure 5, the endpoint of the titration is sharp and the sensor can monitor the amount of Mercury ions with good accuracy from the resulting titration curve.
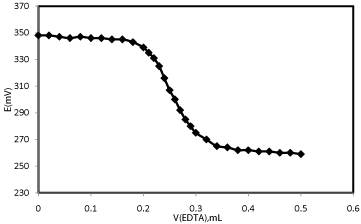
Figure 5: Potential titration curves 25mL of contaminated water samples with
1.0 × 10-2 mol L-1 of EDTA.
Conclusions
The proposed Hg2+ selective electrode based on1, 1,13-Bis(8- quinolyl) 1,4,7,10,13-pentaoxatridecane (Kryptofix 5) as the electro active compound might be a useful analytical tool for the determinations of Hg(II) ions in the range from 1.0×10-8 to 1.0×10-2 M, and therefore an alternative to spectrophotometric methods. The proposed electrode was applied as indicator electrode and successfully used to determine mercury (II) in tap water samples with satisfactory results.
Acknowledgement
The authors gratefully acknowledge the financial support of this research proposal, provided by the Research Council of the Mashhad Islamic Azad University.
References
- Wang J, Feng X, Anderson CW, Xing Y, Shang L. Remediation of mercury contaminated sites-A review. J Hazard Mater. 2012; 221-222: 1-18.
- Longerich HP, Fryer BJ, Strong DF. Determination of lead isotope ratios by inductively coupled plasma-mass spectrometry (ICP-MS). Spectrochimica Acta Part B. 1987; 42: 39-48.
- Bispo MS, Korn MG, Morte ES, Teixeira LS. Determination of lead in seawater by inductively coupled plasma optical emission spectrometry after separation and pre-concentration with cocrystallized naphthalene alizarin. Spectrochimica Acta Part B. 2002; 57: 2175-2180.
- Cho HJ, Myung SW. Determination of Cadmium, Chromium and Lead in Polymers by ICP-OES Using a High Pressure Asher (HPA), Bull. Korean Chem. Soc. 2011; 32: 489.
- Bagheri H, Afkhami A, Saber-Tehrani M, Khoshsafar H. Preparation and characterization of magnetic nanocomposite of Schiffbase /silica/ magnetite as a preconcentration phase for the trace determination of heavy metal ions in water, food and biological samples using atomic absorption spectrometry. Talanta 2012; 97: 87-95.
- Sohrabi MR, Matbouie Z, Asgharinezhad AA, Dehghani A. Solid phase extraction of Cd(II) and Pb(II) using a magnetic metal-organic frame work, and their determination by FAAS. Microchim. Acta. 2013; 180: 589-597.
- Shiri S, Delpisheh A, Haeri A, PoornajafA,Golzadeh B, Shiri S. Determination of Trace Amounts of Lead Using the Flotation-spectrophotometric method. Anal Chem Insights. 2011; 6: 15–20.
- Grinberg P, de Campos R. Iridium as permanent modifier in the determination of lead in whole blood and urine by electrothermal atomic absorption spectrometry. Spectrochim Acta Part B. 2001; 56: 1831-1843.
- Munoz E, Palmero S. Determination of heavy metals in milk by potentiometric stripping analysis using a home-made flow cell. Food Control. 2004; 15: 635-641.
- Abbasi S, Bahiraei A, Abbasai F. A highly sensitive method for simultaneous determination of ultra trace levels of copper and cadmium in food and water samples with luminol as a chelating agent by adsorptive stripping Voltammetry. Food Chem. 2011; 129: 1274-1280.
- Cao L, Jia J, Wang Z. Sensitive determination of Cd and Pb by differential pulse stripping voltammetry with in situ bismuth modified zeolite doped carbon paste electrodes. Electrochim Acta. 2008; 53: 2177-2182.
- Javanbakht M, Fard SE, Abdouss M, Mohammadi A, Ganjali MR, Norouzi P, et al. A biomimetic potentiometric sensor using molecularly imprinted polymer for the cetirizine assay in tablets and biological fluids. Electroanalysis. 2008; 20: 2023-2030.
- Kaličanin B, Velimirović D. Potentiometric Stripping Analysis of Zinc, Cadmium and Lead in Tobacco Leaves (Nicotian a Tabacum) and Soil Samples. Int. J. Electrochem. Sci. 2012; 7: 313-323.
- Gupta VK, Jain AK, Kumar P. PVC-based membranes of N, N_-dibenzyl-1, 4, 10, 13-tetraoxa- 7, 16-diazacyclooctadecane as Pb (II)-selective sensor. Sens. Actuators B. 2006; 120: 259-265.
- Guo J, Chai Y, Yuan R, Song Z, Zou Z. Lead (II) carbon paste electrode based on derivatized multi-walled carbonnanotubes: Application to lead content determination in environmental samples. Sens. Actuators B. 2011; 155: 639–645.
- Afkhami A, Ghaedi H, Madrakian T, Rezaeivala M. Highly sensitive simultaneous electrochemical determination of trace amounts of Pb(II) and Cd(II) using a carbon paste electrode modified with multiwalled carbon nanotubes and a newly synthesized Schiff base. Electrochimica Acta, 2013; 89: 377– 386.
- Shams E, Torabi R. Determination of nanomolar concentrations of cadmium by anodic-stripping voltammetry at carbon paste electrode modified with zirconiumphosphatedmorphous silica. Sens. Actuators B. 2006; 117: 86-92.
- Kokkinos C, Economou A, Raptis I, Efstathiou CE. Lithographically fabricated disposable bismuthfilm electrodes for the trace determination of Pb(II) and Cd(II) by anodic stripping Voltammetry. Electrochim Acta. 2008; 53: 5294-5299.
- Hu C, Wu K, Dai X, Hu S. Simultaneous determination of lead(II) and cadmium(II) at a diacetyldioxime modified carbon paste electrode by differential pulse stripping voltammetry. Talanta. 2003; 60: 17-24.
- Ganjali MR, Motakef-Kazami N, FaridbodF, Khoee S, Norouzi P. Determination of Pb2+ ions by a modified carbon paste electrode based on multiwalled carbon nanotubes (MWCNTs) and nanosilica. J Hazard Mater. 2010; 173: 415-419.
- Wang Y, Wei WZ, Liu XY, Zeng XD. Fabrication of a copper nanoparticle/chitosan/carbon nanotube-modified glassy carbon electrode for electrochemical sensing of hydrogen peroxide and glucose. Michrochimica Acta. 2008; 160: 253-260.
- Javanbakht M, Ganjali MR, Norouzi P, Hashemi-Nasa A, Badei AR. Carbon paste electrode modified with functionalized nanoporous silica gel as a new sensor for determination of silver ion. Electroanalysis. 2007; 19: 1307-1314.
- Afkhami A, Ghaedi H, Madrakian T, Rezaeivala M. Highly sensitive simultaneous electrochemical determination of trace amounts of Pb(II) and Cd(II) using a carbon paste electrode modified with multiwalled carbon nanotubes and a newly synthesized Schiff base. Electrochimica Acta. 2013; 89: 377– 386.
- Ganjali MR, Motakef-Kazami N, Faridbod F, Khoee S, Norouzi P. Determination of Pb2+ ions by a modified carbon paste electrode based on multiwalled carbon nanotubes (MWCNTs) and nanosilica. J. Hazard. Mater. 2010; 173: 415–419.
- Ajayan PM. Nanotubes from carbon. Chem. Rev. 1999; 99: 1787–1799.
- Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005; 9: 674-679.
- Chou A, Bocking T, Singh NK, Gooding JJ. Demonstration of the importance of oxygenated species at the ends of carbon nanotubes for their favorable electrochemical properties. Chem. Commun. 2007; 7: 842–844.
- Hu C, Wu K, Dai X, Hu S. Simultaneous determination of lead(II) and cadmium(II) at a diacetyldioxime modified carbon paste electrode by differential pulse stripping Voltammetry. Talanta. 2003; 60: 17-24.
- MR Ganjali, F Faridbod, P Norouzi and M Adib. A novel Er(III) sensor based on a new hydrazone for the monitoring of Er(III) ions. Sens. Actuators B. 2006; 120: 119.
- MR Ganjali, Z Memari, R Dinarvand, F Faridbod and P Norouzi. Uranyl Plasticized Membrane Sensor Based on a New Calix[4]arene. Sensor Lett. 2009; 7: 1156.
- HA Zamani, MR Ganjali, P Norouzi, A Tadjarodi, and E Shahsavani. Determination of terbium(III) ions in phosphate rock samples by a Tb3+–PVC membrane sensor based on N, N-Dimethyl-N′, N″-bis(4-methoxyphenyl)phosphoramidate. Mater. Sci. Eng. C. 2006; 28: 1489.
- VK Gupta, AK Singh, and B Gupta. A cerium(III) selective polyvinyl chloride membrane sensor based on a Schiff base complex of N,N'-bis[2-(salicylideneamino)ethyl]ethane-1,2-diamine. Anal Chim. Acta. 2006; 575: 198.
- VK Gupta, S Jain, S Chandra. Chemical sensor for lanthanum(III) determination using aza-crown as ionophore in poly(vinyl chloride) matrix. Anal. Chim. Acta. 2003; 486: 199.
- R De Marcol, G Clarke, and B Pejcic. Ion-Selective Electrode Potentiometry in Environmental Analysis. Electroanalysis. 2007; 19: 1987.
- JS Kim, A Ohki, R Ueki, T Ishizuka, T Shimotashiro and S Maeda. Cesium-ion selective electrodes based on calix[4]arene dibenzocrown ethers. Talanta. 1999; 48: 705.
- MR Ganjali, P Norouzi, F Faridbod, S Riahi, J Ravanshad, J Tashkhourian, et al. Determination of Vanadyl Ions by a New PVC Membrane Sensor Based on N, N'-bis-(Salicylidene)-2,2-Dimethylpropane-1,3-Diamine. Ieee Sensors J. 2007; 7: 544.
- HA Zamani, MS Zabihi, M Rohani, A Zangeneh-Asadabadi, MR Ganjali, F Faridbod, et al. Quantitative monitoring of terbium ion by a Tb3+ selective electrode based on a new Schiff's base. Mater. Sci. Eng. C. 2011; 31: 409.
- JE Harwood. The use of an ion-selective electrode for routine fluoride analyses on water samples. Water Research. 1969; 3: 273.
- HA Zamani, MR Ganjali, H Behmadi, and MA Behnajady. Fabrication of an iron(III) PVC-membrane sensor based on bis-benzilthiocarbohydrazide as a selective sensing material. Mater. Sci. Eng. C. 2009; 29: 1535.
- RK Mahajan, I Kaur, TS Lobana. A mercury(II) ion-selective electrode based on neutral salicylaldehyde thiosemicarbazone. Talanta. 2003; 59: 101.
- MR Ganjali, P Norouzi, FS Mirnaghi, S Riahi and F Faridbod. Lanthanide Recognition: Monitoring of Praseodymium(III) by a Novel Praseodymium(III) Microsensor Based on N′-(Pyridin-2-Ylmethylene)Benzohydrazide. Ieee Sensors J. 2007; 7: 1138.
- HA Zamani, G Rajabzadeh, M Masrornia, A Dejbord, and MR Ganjali. Determination of Cr3+ ions in biological and environmental samples by a chromium(III) membrane sensor based on 5-amino-1-phenyl-1H-pyrazole-4-carboxamide. Desalination. 2009; 249: 560.
- A Craggs, GJ Moody, and JDR Thomas. PVC matrix membrane ion-selective electrodes. Construction and laboratory experiments. J. Chem. Educ. 1974; 51: 541.
- JF Coetzee, WK Istone. SIEMENS. Anal. Chem. 1980; 52: 53.
- HA Zamani, MR Ganjali, P Norouzi and S Meghdadi. Application of Novel Praseodymium (III) PVC‐Membrane Electrode for Determination of Pr(III) Ions in Soil and Sediment Samples. Anal. Lett. 2008; 41: 902.
- BT Altura, BM Altura. Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased human subjects. Magnes Trace Elem. 1991; 10: 90.
- Mahajan RK, Sood P, Pal Mahajan M, Marwaha A. Mercury(II) ionselective electrodes based on heterocyclic systems. Ann Chim. 2007; 97: 959-971.
- MR Ganjali, H Shams, F Faridbod, L Hajiaghababaei and P Norouzi. Lanthanide recognition: A Ho3+ potentiometric membrane sensor as a probe for determination of terazosin. Mater. Sci. Eng. C. 2009; 29: 1380.
- F Faridbod, MR Ganjali, R Dinarvand, S Riahi, P Norouzi, and MB Am. Olia. Determination of avonoids in pharmaceutical preparations using Terbium sensitized uorescence method. J. Food Drug Anal. 2009; 17: 264.
- AK Singh, VK Gupta, B Gupta. Chromium(III) selective membrane sensors based on Schiff bases as chelating ionophores. Anal. Chim. Acta. 2007; 585: 171.
- SK Srivastava, VK. Gupta, S Jain. A PVC-based benzo-15-crown-5 membrane sensor for cadmium. Electroanalysis B. 1996; 8: 938.
- HA Zamani, MR Ganjali, P Norouzi, M Adib, M Aceedy. Synthesis of N′-(1-Pyridin-2-ylmethylene)-2-furohydrazide and Its Application in Construction of a Highly Selective PVC-Based Membrane Sensor for La(III) Ions. Anal. Sci. 2006; 22: 943.
- VK Gupta, R Mangla, U Khurana, and P Kumar. Determination of Uranyl Ions Using Poly(vinyl chloride) Based 4-tert-Butylcalix[6]arene Membrane Sensor. Electroanalysis. 1999; 11: 573.
- VK Gupta, AK Singh, B Gupta. Electroanalytical performance of a terbium(III)-selective sensor based on a neutral ionophore in environmental and medicinal samples. Anal. Bioanal. Chem. 2008; 390: 2171.