
Review Article
Austin J Anal Pharm Chem. 2016; 3(4): 1076.
Recent Progress in Chemistry and Biology of Indazole and its Derivatives: A Brief Review
Shrivastava A, Chakraborty AK, Upmanyu N and Singh A*
School of Pharmacy and Research, Peoples University, Bhopal, India
*Corresponding author: Singh Alka, School of Pharmacy & Research, People’s University, People’s campus, Bhopal, India
Received: November 01, 2016; Accepted: November 18, 2016; Published: November 21, 2016
Abstract
The biological and medicinal properties of indazoles have prompted enormous research aimed at developing synthetic routes to these heterocyles. This review focuses on the biological properties associated with this system, chemical reactions, functionalizations, and medicinal application of indazole nucleus. Moreover many useful drugs have emerged from the successful investigation carried out in this branch. Indazole ring system is not a common feature in nature but a large number of synthetically prepared compounds have shown desirable pharmacological properties, so efforts have been made in the last few decades to synthesize a different new and novel heterocyclic indazole compounds & its derivatives which were evaluated for various activity. Study of biological activity of substituted heterocyclic compounds represents a core area in the field of drug development and discovery.
This overview summarizes structures of pharmacologically interesting indazoles published during the last decade, as well as syntheses, reactions, and functionalizations.
Keywords: Heterocycles; indazoles; Indazole tautomer biological activities
Introduction
Indazoles refers to isomeric chemical compounds with molecular formula C7H6N2, having pyrazole ring condensed with the benzene ring. The indazole heterocycle is normally referred to as 1H-indazole, although it has two other potential tautomers 2H-indazole and 3H-indazole [1].
Three tautomeric forms of indazole can be discussed; the 1H-, 2H-, and 3H-form (Scheme 1). Tautomerisations of indazoles have been thoroughly investigated both from a theoretical and a synthetic view, and a review article summarizing the knowledge appeared in 2000 [2]. The tautomeric equilibrium between 1H- and 2H indazoles both in the ground state (S0) and in the excited state (S1) has been investigated by photophysical and thermochemical techniques, and have also been calculated. According to this study, the 1H tautomer is 2.3kcal mol–1 (9.63kcal/mol) more stable than the 2H tautomer, regardless of the ground state or excited state, and this trend is not reversed by solvent effects from water or formic acid. Correspondingly, 1-methylindazole is 3.2 kcalmol–1 (13.40 kcal/ mol) more stable than 2-methylindazole [3]. The MP2/6-31G* level of theory predicts an energy difference of 3.6 kcal/mol (15.1kcal/mol), which is characterized also by ΔG0 298.15 = 4.1 kcal/mol (17.2kcal/ mol), when thermal energy correction and entropy effects are taken into account [4]. Calculations on the tautomerism of substituted indazoles lead to similar results in the gas-phase [5] as well as in water [6]. AM1/B3LYP calculations, however, predict also some candidates of substituted and annulated indazoles which are more stable as 2H-tautomers [7]. The two tautomeric forms can be identified in solid-state substances by NMR-NQR spectroscopy [8]. In addition, theoretical 13C NMR studies have been carried out [9], as well as 1H, 13C and 15N NMR studies on indazoles in solution and in the solid state [10]. Only few examples of 3Hindazoles are known, which carry alkyl or aryl groups on the five-membered ring [1]. Indazol-3-ones, which can be regarded as 3H-indazole derivatives, however, are welldocumented. Some interest has also been focused on conformers of indazole derivatives. Thus, similar to 1-(formylamino)indazole, 1-(acetylamino)indazole (1) exists as an equimolar mixture of Eand Z-conformers in CDCl3 solution, as evidenced by 1H NMR spectroscopy (Scheme 2) [11].
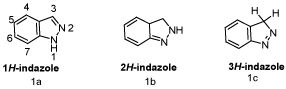
Scheme 1: Structures of various indazole nucleus.
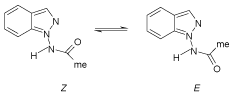
Scheme 2: Conformers.
NOESY NMR spectroscopy reveals that the imine 2 (Scheme 3) exists in form A in CDCl3 and in form B with disrupted hydrogen bond in [D6] DMSO. 13C and 15NCPMAS NMR studies show that in the solid state no hydrogen bonds exist, so that rotamer B is detected [12].
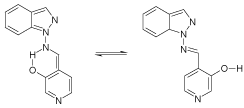
Scheme 3: Conformers and hydrogen bonds.
Two concomitant polymorphs of 3-phenyl-1H-indazole were examined by X-ray crystallography and their NMR properties were measured [13].
Indazoles as Natural Products
Indazoles are rare in Nature.[14] To date, only three natural products possessing the indazole ring have been isolated: Nigellicine (3), Nigeglanine (4), and Nigellidine (5) (Scheme 4) (Figure 1). The alkaloid Nigellicine (3), a 6,7,8,9-tetrahydropyridazino[ 1,2-a] indazolium-11-carboxylate, was isolated as yellow crystals from the widely distributed herbaceous plant Nigella sativa L. [Ranunculaceae; black cumin (engl.), Schwarzkümmel (name in German)] which is an annual flowering plant, native to southwest Asia [15]. The structure was determined by an X-ray crystal structure analysis. An intramolecular hydrogen bond was found between the carboxylate oxygen atom and the hydroxy group. Nigellicine (3) belongs to the class of heterocyclic mesomeric betaines (MB), as it can exclusively be represented by dipolar canonical formulae in which both the positive and negative charge are delocalized in a common p-electron system. More precise, Nigellicine is a pseudo-cross-conjugated heterocyclic mesomeric betaine (PCCMB). The biological, physical, and chemical consequences of the distinct types of conjugation have been surveyed recently [16]. The alkaloid Nigeglanine (4) was isolated from extracts of Nigella gland-ulifera [17]. Nigeglanine (4) and Nigellidine (5) (Nigella sativa) [18] can be represented by zwitter ionic as well as by neutral canonical formulae (Scheme 4), thus setting them apart from mesomeric betaines (Table 1).
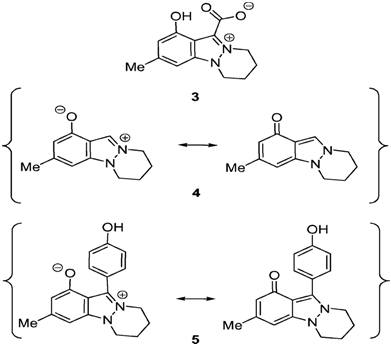
Scheme 4: The indazole alkaloids Nigellicine, Nigeglanine and Nigellidine.
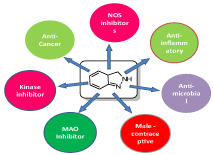
Figure 1: Graphical representation of Pharmacological activities of indazoles
moiety.
Chemical name
Structure
IUPAC name
Uses
Benzdac
(1-Benzyl-1H-indazole-3yloxy)-acetic acid
Anti-cataract, Anti-inflammatory
Benzylamine
Anti-inflammatory
Axitinib
Anti-cancer
Lonidamine

1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid
Anti-cancer
Quiniprole
(4aR,8aR)-5-propyl-4,4a,5,6,7,8,8a,9-octahydro-1H-pyrazolo[3,4-9] quinoline
D2 and D3 receptor agonist
Stanozolol
(1S,3aS,3bR,5aS,10aS,10bS,12aS)-1,10a,12a-trimethyl-1,2,3,3a,3b,4,5,5a,6,7,10,10a,10b,11,12,12a-hexadecahydrocyclopenta [5,6]naptho[1,2-F] indazole-1-ol
Anabolic steroids
Cortivazol

(11β,16α)-21-(Acetyloxy)-11,17-dihydroxy-6,16-dimethyl-2'-phenyl-2'H-pregna-2,4,6-trieno[3,2-c]pyrazol-20-one
Anabolic steroids
Table 1: Chemical name, structure, IUPAC name and uses of some important indazole derivatives.
Biologically Active Indazole Derivatives
The indazole ring system is of great current interest as partial structure of biologically active compounds. Some aspects of pharmacological properties of indazoles have been reviewed in 2005 [19]. As the molecular shape and electrostatic distribution play a crucial role in enzyme and receptor recognition and contribute extensively to binding affinity, a study on electroforms of molecules, including indazole, have been presented in order to help drug discovery [20]. Unsubstituted indazole was used as ligand in metal complexes. Thus, tumor-inhibiting [21,22], and redoxactive antineoplastic ruthenium complexes with indazole have been reported and a correlation between in vitro potency and reduction potential was examined [23]. An example is the water-soluble anionic complex 6 with trans-standing indazoles, in which bonding with the metal is achieved via N(2) (Scheme 5) [24].
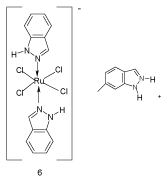
Scheme 5: Unsubstituted indazole as ligand.
Monosubstituted Indazoles
N To the best of our knowledge, relatively few examples of N(1)- monosubstituted indazoles were published as biologically interesting compounds during the last decade. As examples, the indazoles 7a,b were screened for antifungal activities as fluconazole analogues (Scheme 6) [25].
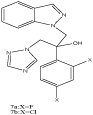
Scheme 6: An N(1)-monosubstituted indazole as biologically interesting
compound.
By contrast, several series of C (3)-monosubstituted indazoles were described as biologically interesting compounds (Scheme 7). Thus, 8 were tested as 5-HT4 receptor agonist [26]. The iodobenzamide derivative 9 shows antifungal activities [27] and derivatives such as 10 were examined in search of β3-adrenergic receptor agonists as potential drugs for the treatment of type II diabetes [28]. Indazole 11 is interesting as analogue of an dopamine D2 receptor antagonist [29], while 12 was tested as inhibitor of VEGFR-2 and cyclin dependent kinase 1 (CDK1) [30].
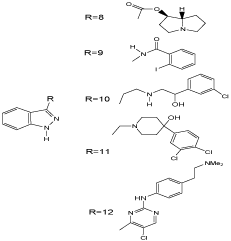
Scheme 7: C(3)-substituted indazoles.
Introduction of bromine at C(4) provides compound 13 which is almost as potent as the reference compound 7-nitroindazole as inhibitor of neuronal nitric oxide synthase, and 4-nitroindazole also proved to be a potent inhibitor of NOS activity (Scheme 8) [31]. ABT-102 14 has been identified as potent vanilloid receptor (VR1) antagonist and is currently undergoing advanced clinical development for the treatment of chronic pain [32]. 3-Aminopiperidinylsubstituted indazoles 15 with R«‘ substituents varying from n-propyl to 3-methylfuryl represent C(5)-mono substituted indazoles with potential biological activities as they were tested as Rho kinase inhibitors [33]. More examples are the selective Rho-kinase inhibitor 16 [34], the α7 nicotinic acetylcholine receptor agonist 17 [35], and the anti-HIV protease inhibitor18 [36]. No effect of 5- and 6-nitroindazole nucleosides of D-pinitol as antitumor agents, however, was found [37]. A series of substituted 6-anilinoindazoles as inhibitors of c-Jun N- terminal kinase-3 was recently described [38]. The latter mentioned compounds are examples for C(6)-monosubstituted indazoles of pharmacolocical interest, only few examples of this group are described [39]. Two additional examples are TAS-3–124 [46] and 19 [36]. Biologically interesting indazoles substituted at C(7) are represented by 20 and 21. 7-Methoxyindazole (20) [40] as well as 7-nitroindazole (21) [41–43], are nitric oxide synthase inhibitors. The latter mentioned (7- NI 21) and related indazoles were also shown to exhibit antinociceptive and cardiovascular effects [44]. Several substituted indazoles and potential analogues of 7-NI have been reported [39,45] from which 7-methoxyindazole proved to be the most active compound in in vitro enzymatic assays of nNOS activity [46]. N-(7-Indazolyl)benzenesulfonamide derivatives were prepared and investigated as cell cycle inhibitors [47].
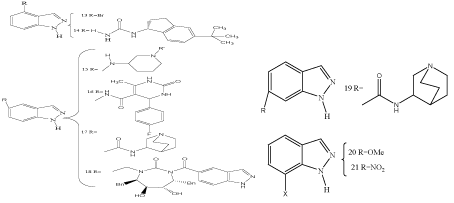
Scheme 8: C(4)-, C(5)-, C(6)-, and C(7)-substituted indazoles.
Disubstituted Indazoles
Numerous examples of N(1)–C(3)-disubstituted indazoles showing pharmacologically interesting properties have been published (Scheme 9). The selective 5-HT3 receptor antagonist Granisetron (22) has been used clinically to prevent nausea and emesis induced by cancer chemotherapeutic agents [48]. Gastrointestinal prokinetic and dopamine D2 receptor antagonists were observed with derivatives such as 23 [49]. A series of N(1)-benzyl-substituted indazoles is represented by 24–27. Bendazac (24), a topical antiinflammatory agent, impedes effects associated with lens opacification and photochemical modes of action have been suggested [50]. Benzydamine (25) is a widely applied locally-acting nonsteroidal antiinflammatory drug with local anaesthetic and analgesic properties. Bindarit (26) as well as Bendazac showed a selective inhibition of protein denaturation. The indazole derivative YC-1 (27) has been reported as activator of the physiological receptor for nitric oxide, sGC, which is a signalling molecule in the cardiovascular and central nervous system. YC-1 displays also some other biological activities such as inhibition of endothelial cell functions induced by angiogenic factors in vitro and angiogenesis in vivo [51]. It also effects cGMP metabolism by inhibiting the activity of phosphodiesterase isoforms 1–5 [52]. The corresponding 2-benzyl isomer showed no activity [53]. Syntheses of YC-1 analogues [54] as well as of structural variationsin search for new antithrombotics [55] have been reported.
Scheme 9: N(1)–C(3)-disubstituted indazoles.
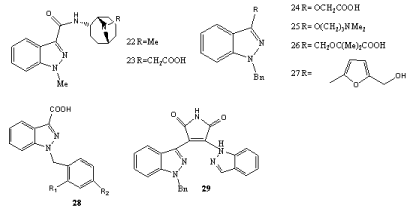
Indazol-3-carboxylic acid derivatives such as 28 compromises non-hormonal and non-steroidal, antispermatogenic agents and found interest in male contraception [56]. It was found that the presence of substituted benzyl groups at N(1) are essential for this specific activity, and so are halogen or methyl groups as R1. The activity increases when two substituents are present in o- and p-position (R1= R2 = Cl; or R1 = Me, R2 = Cl). AF 1311 (R1 = R2 = H) displays no anti-spermatogenic activity, but is able to reduce protein denaturation. By contrast, in Lonidamine (R1 = R2 = Cl) this property is decreased. The corresponding indazole- 3-carbohydrazide (R1 = R2 = Cl, AF2364) and 3- acrylic acid (R1 = R2 = Cl; AF 2785) showed anti-spermatogenic effects [57]. (Indolyl-indazolyl)maleinimides 29 with a broad variety of substituents Ar (vinyl, Ph, 2-thiazolyl, 2- naphthyl, 3-quinolyl etc.) and X (Me2N, morpholinyl etc.) were tested as inhibitors of protein kinase C-β [58].
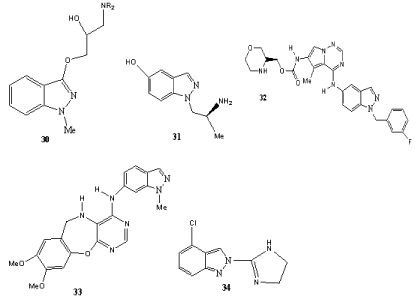
N(1)–C(X)-Substituted indazoles (X ? 3) are represented by the structures 30–33 (Scheme 10). Indazoles such as 30 exhibit antiarrhythmic, local anaesthetic and analgesic activities, [59] where as 31 was identified as a periphally acting potent 5-HT2 receptor agonist [60]. BMS-599626 (32) [61] is in clinical development as EGFR and HER2 protein tyrosine kinase inhibitor. Structural optimizations have been reported [62]. Compound 33 was reported as inhibitor of epidermal growth factor receptor tyrosine kinase [63]. Syntheses as well as I2/α2-adrenoceptor binding profiles of a series of 2-(4,5-dihydroimidazol-2-yl)indazoles (indazim) such as 34 have been described [64]. These receptors are involved in several diseases such as psychiatric disorders, Parkinson’s and Alzheimers’s diseases and Huntington’s chorea. Compound 34 is a typical N(2)–C(4)- disubstituted indazole.
Scheme 10: N(1)–C(X)-disubstituted [X ? 3] indazoles 40–43 and the N(2)–
C(4)-disubstituted indazole 44.
Trisubstituted Indazoles
Some trisubstituted indazoles have also been described as biologically active compounds (Scheme 11). Novel Combretastatin analogues endowed with antitumor activity [65] belong to this class of compounds. In addition, a series of indazoles such as 35 was rationally designed and synthesized as cannabinoid ligands (Scheme 16) [66]. The dopamine D1/D5 receptor antagonist 37, indazole 38 (a phenol bioisosteric analogue of benzazepine derivatives [67]), the orally efficacious melanin-concentrating hormone receptor 1 antagonist 39 (treatment of obesity [68]) and the indazole 40 (binds specifically to the colchicines binding site and inhibits tubulin polymerization in vitro [69]) are examples for active trisubstituted indazoles. In addition, 41 and related systems are potential DNA-intercalators and demonstrated significant antiproliferative activities [70]. The furoindazole YM348 (42) was identified as a new and potent 5-HT2c receptor agonist, which is one of the serotonine receptors [71].
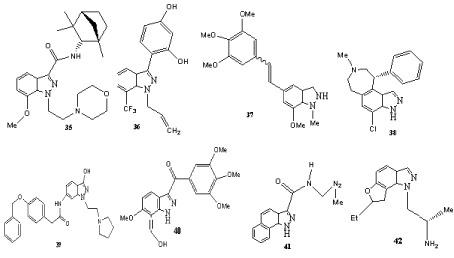
Scheme 11: Biologically active trisubstituted indazoles.
Tetrasubstituted Indazoles
The N(1)–C(3)–C(4)–C(6)-tetrasubstituted indazole 43 is interesting as sodium glucose co-transporter 2 inhibitor (Scheme 12) [72]. A number of benzothiopyrano-indazoles such as 44 demonstrate significant antitumor activities. A promising example is CL-958 which is in clinical evaluation for prostate cancer treatment. In this context, aza-bioisosters of CL-958 were prepared [73].
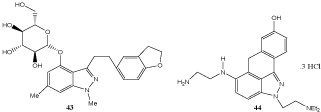
Scheme 12: Tetrasubstituted indazoles.
Routes for the Syntheses of 1H-Indazoles
Reactions from hydrazines, hydrazones, azo, and diazo compounds
By means of intermolecular coupling of diazo groups with o-methyl groups: A general route to indazoles is the intermolecular coupling of diazo groups with o-methyl groups (Scheme 13).
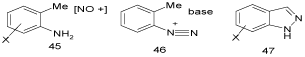
Scheme 13: General routes to indazoles I.
Some applications of this procedure were described recently [68,74,75]. It was also shown that o-alkynylanilines such as 48 can be diazotized to give indazoles 49 (Scheme 14) [69,76] and some studies were presented to elucidate the mechanisms leading to fivemembered (pyrazole) or six membered (pyridazine) partial structures [77]. A similar reaction leading to 1H-naphtho[2,3-g]indazole-6,11- diones was described before [78].
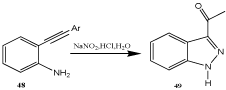
Scheme 14:
By means of nitrozation of N-acetyl derivatives (Jacobsen modification): Another general route to indazoles is the nitrozation of N-acetyl derivatives (Jacobsen modification) (Scheme 15).
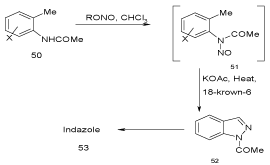
Scheme 15: General routes to indazoles II.
Thus, acetic anhydride preforms the diacetate of 54, which yields the indazole 55 on treatment with n-amyl nitrite under phase-transfer conditions (Scheme 16) [36].
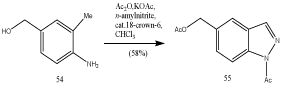
Scheme 16:
By means of cyclization of NH Boc-protected methoxyaniline: A related procedure was described recently. Thus, the NH Bocprotected methoxyaniline 56 cyclized to indazole 57 (Scheme 17) [66].
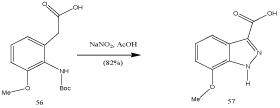
Scheme 17:
By means of condensation of o-substituted benzaldehyde with hydrazine: The condensation of o-substituted benzaldehydes with hydrazine is an alternative common synthetic route for the preparation of indazoles (Scheme 18).
Scheme 18: General routes to indazoles III.
By means of rearrangement reactions
Diphenylcarbamoyl azide 60 underwent a rearrangement to indazole 62 [79,80]. The mechanism is depicted in Scheme 19.
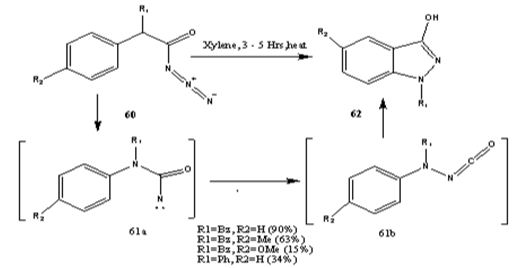
Scheme 19:
By means of cycloaddition reactions
The aryne mechanism was observed on [3+2] cycloaddition starting from fluorobenzenes 63 and trimethylsilyldiazomethane in a basic medium [81]. In some cases, regioisomers 64 and 65 were obtained (Scheme 20).
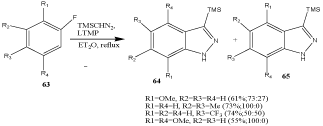
Scheme 20:
By means of metal-organic syntheses
A broad variety of substituted indazoles was prepared starting from 2-bromobenzaldehydes and arylhydrazines by palladium catalysis [82]. Phosphorous-chelating ligands such as 1,1_-bis(diphenylphosphanyl) ferrocene and 1,3-bis(diphenylphosphanyl) propane along with sodium tert-butox ide were used. A broad variety of substituted indazoles 67 were prepared via palladium-catalyzed intramolecular mination of aryl halides 66 (Scheme 21) [83,84].
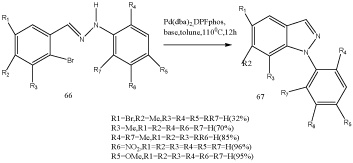
Scheme 21:
Syntheses starting from pyrazoles
A two-step [3+2] annulation of 1,3-diphenyl-5- cyanomethylpyrazole 68 with α-oxoketenes dithioacetals afforded functionalized indazoles 70 with high regioselectivity (Scheme 22) [85].
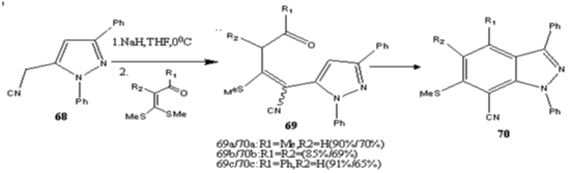
Scheme 22:
By means of oxidative N–N coupling reactions
Most syntheses of indazoles involve the formation of the pyrazole moiety. A suitable method is the oxidation of anthranilic acid amides 71 with iodine reagents such as the hypervalent iodine reagent phenyliodine(III) bis(trifluoroacetate) (PIFA) to 1,2-disubstituted indazolones 72 [86]. The key cyclization step embraces the PIFAmediated formation of an N-acylnitrenium intermediate (Scheme 23).
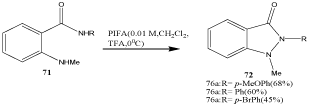
Scheme 23:
By means of carbocycle aromatization
Aromatization of 4-hydroxyimino-6,6-dimethyl-1-phenyl- 4,5,6,7-tetrahydroindazole (73) to 74 was accomplished by heating in PPA. The proposed mechanism proceeds via the formation of an iminium cation, followed by subsequent hydride and methyl migration, and final oxidation to indazole 74 (Scheme 24) [87].
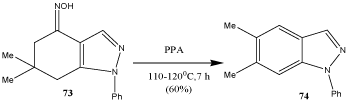
Scheme 24:
By means of nucleophilic ring transformation
A series of fluorinated indazoles 76 and 77 (Scheme 25) was synthesized by an ANRORC-type rearrangement of 5-tetrafluorophenyl-1,2,4-oxadiazoles 75 with hydrazine [88].
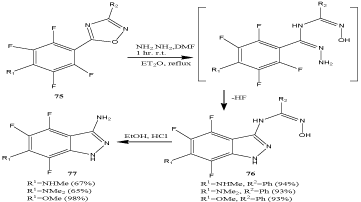
Scheme 25:
Routes for the Syntheses of 2H-Indazoles
By coarctate reactions
Coarctate[89] cyclizations are defined by the presence of a coarctate atom where two bonds are being broken and two are being made and that bond breaking and making do not occur in a cyclic array, thus setting them apart from pericyclic reactions. Analogous disconnections in coarctate reactions are termed pseudocoarctate. A coarctate mechanism formed 2-phenylisoindazoles 79 from (2-ethynylphenyl) phenyldiazenes 78 (Scheme 26-42); the intermediary carbene 78B was formed under neutral conditions via transition state 78A at moderate temperatures [90]. The free carbene could also be trapped as a [2+1] cycloadduct with 2,3-dimethyl-2- butene.
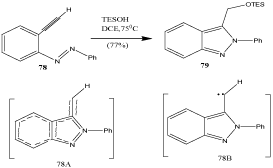
Scheme 26:
Scheme 27:
Scheme 28:

Scheme 29:
Scheme 30:
Scheme 31:
Scheme 32:
Scheme 33:
Scheme 34:

Scheme 35:
Scheme 36:
Scheme 37:
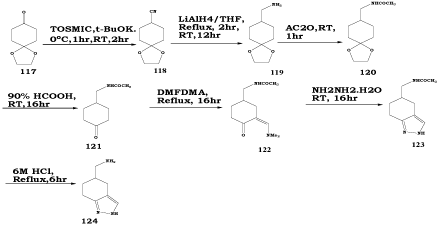
Scheme 38:
Scheme 39:
Scheme 40:
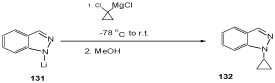
Scheme 41:
Scheme 42:
Scheme 43:
Scheme 44:
Scheme 45:
Scheme 46:
Different Scheme of Synthesis of Indazoles and Its Derivatives
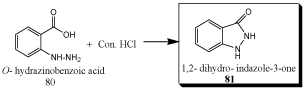
Synthesis of Schiff’s bases of Indazole 3-one derivatives using O-hydrozinobenzoic acid [91]
P. Muthumani et al synthesized and evaluated of some novel schiff’s bases of Indazole 3-one derivatives [81].
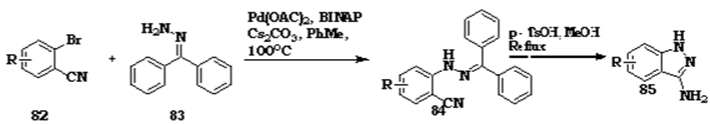
Bromobenzonitrile mediated synthesis of 3-aminoindazole
Frederic Fabis et al. reported synthesis of 3-amionoindazole from 2- bromobenzonitriles [92].
Microwave assisted synthesis of indazole
Scheme 7 Imine was mixed with triethylphosphite and irradiated with microwave irradiation to give the corresponding indazoles. Evelyn Cuevas Creencia et al [93].
Hydrazine and their derivative mediated synthesis of Indazoles [94]
Nesrin Gokhan-Kelekci et al synthesized 2-thiocarbamoyl- 2,3,4,5,6,7-hexahydro-1H-indazole derivatives by using arylidenecyclohexanones 88 as a starting material.
Synthesis of furan-fused indazole using 1,3-Cyclohexanedione [95]
Itsuro Shimada et al synthesized furan-fused indazole. 1,3-Cyclohexanedione [93] was reacted with chloroacetaldehyde to give 6,7-dihydrobenzofuran- 4(5H).
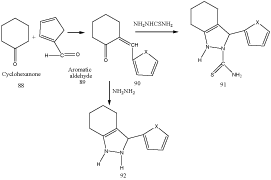
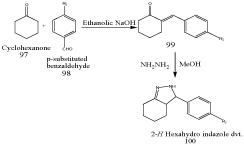
Synthesis of hexahydro-2-H-indazoles derivatives [96]
Maninder Minu et al synthesized and evaluated new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Synthesis of substituted benzylidenecyclohexanones (100) by the reaction of cyclohexanone (97) with p-substituted benzaldehydes (98) in the presence of ethanolic sodium hydroxide. Benzylidenecyclohexanones, on heating with hydrazine hydrate resulted in the formation of 100.
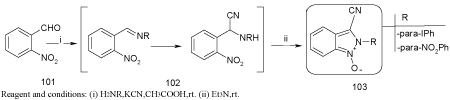
Synthesis of indazole N-oxide derivatives [97]
Alejandra Gerpe et al has been synthesized a series of indazole N-oxide derivatives and the synthesized are studied for their antichagasic and leishmanocidal properties.
Treatment with sodium cyanide converts the Schiff base to it’s a aminonitrile derivatives (102), which in turn undergo basic cyclization and forms indazole N-oxide derivatives (103). The cyclization occurs under extremely mild condition and indazole N1-oxide yield depends on the reaction solvent, being acetic acid the best condition.
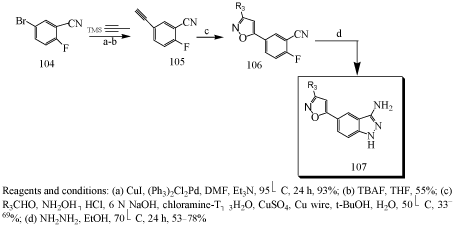
Synthesis of 5-substituted indazoles [98]
Irini Akritopoulou-Zanze et al synthesized and evaluated 5-substituted indazoles as kinase inhibitors. 5-substituted-3- amino indazoles (107) could be prepared by Sonogashira coupling of trimethylsilylacetylene with commercially available 2-fluoro- 5-iodobenzonitrile. Deprotected intermediate was subjected to cycloaddition reactions, followed by treatment with hydrazine to afford the final 5- substituted indazoles products (107).
Synthesis of tetrahydro-2H-indazoles from 2-benzoylcyclohexanone [99]
Ornelio Rosati et al. reported synthesis of 2,3-diaryl-4,5,6,7- tetrahydro-2H-indazoles from 2-benzoylcyclohexanone and substituted hydrazine in the presence of α-Zr(CH3PO3)1.2(O3PC6H 4SO3H)0.8.
Synthesis of indazole derivatives from 1- cyanocyclohexene [100]
Synthesis of indazole derivatives from 1- cyanocyclohexene was reported by Jan Bergman et al.
Synthesis of 2-(1H-furo[2,3-g]indazol-1-yl) ethylamine derivatives from 1,3-Cyclohexadione [101]
Itsuro Shimada et al. reported synthesis of series of substituted 2-(1H-furo[2,3-g]indazol-1-yl) ethylamine derivatives from 1,3-Cyclohexadione.
Synthesis of tetrahydroindazole from novel enamino ketone [102]
Synthesis of tetrahydroindazole was reported by Danijel Kikelj et al. from novel enamino ketone as the key intermediate.
Chemical Reactions of Indazoles and Its Derivatives
Alkylations and Arylations of N(1) and N(2)
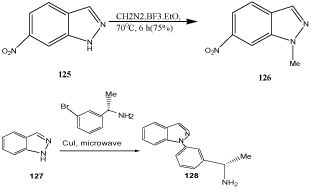
Indazoles are alkylated or acylated at N(1), N(2), or at both positions depending on the reaction conditions [49,103–107]. For example, Reaction of 1-lithioindazole 131 with cyclopropylmagnesium derivatives yields indazoles 132 which are cyclopropanylated at N(1) (Scheme 41) [108].
Functionalizations of C(3) position in indazoles
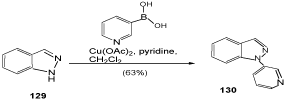
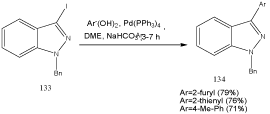
In view of the large number of biologically active indazoles substituted at C(3), functionalizations of this position are of great current interest. In this context, Heck [109] as well as Suzuki-type cross-couplings of 3-iodoindazoles has been described. As example for the latter mentioned procedure, 133 reacts with aryl- and hetarylboronic acids to give 3-arylated indazoles 134 (Scheme 42) [110].
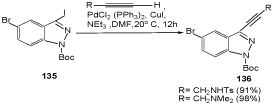
Sonogashira reactions for acetylenizations of C(3) of the starting material 135 were also described. The reaction products 136 undergo an additional Sonogashira coupling to afford bis-acetylenesubstituted indazoles (Scheme 43) [111,112].
N-Heterocyclic Carbenes of Indazole
Trapping reactions
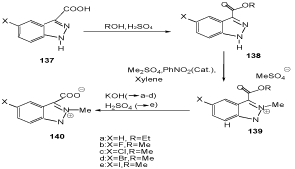
N-Heterocyclic carbenes of indazole are accessible by extrusion of heterocumulenes from pseudo-cross-conjugated heterocyclic mesomeric betaines (PCCMB). Indazolium-3- carboxylates are examples for those systems (Scheme 44). They were prepared starting from the indazole-3-carboxylic acids 137 which were first converted into the esters 138, then methylated to indazolium salts 139 with dimethyl sulfate in the presence of catalytic amounts of nitrobenzene, and finally saponified (140) [113,114].
Redox reactions of indazoles
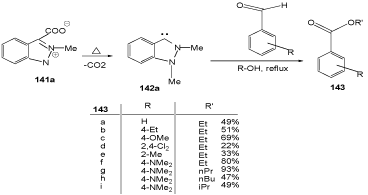
Redox esterifications of aldehydes to the benzoates 143 were found on generation of NHC 142a in the presence of aromatic aldehydes and alcohols (Scheme 43) [115].
Indazolium Salts
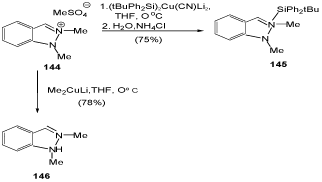
Lithium bis(silyl)cuprates react with indazolium salt 144 to give 3-silylindazolines such as 145 (Scheme 45). Variation of the cuprate results in reductive ring opening leading to benzo-β-enaminoimines such as 146, or mixtures of both [116].
Pharmacological Activities of Indazole and Its Derivatives
See Table 2.
Sl. No.
Pharmacological Activity
Indazole derivatives
Structures
Reference
1.
Anti-inflammatory activity.
Novel Schiff’s bases of Indazolone derivatives.
P. Muthumani et al
[117],[91]
7-Benzylidene-2, 3-diphenyl-4, 5, 6, 7-tetrahydro-2H-indazole derivatives.
Richa Kaur Bhatia et al [118]
2,3-disubstituted tetrahydro-2H-indazols
Ornelio Rosati et al [90]
4,5-dihydro-1H-benzo[g]indazole-3-carboxamides
Gabriele Murineddu et. Al [119]
I-(pyrimidin-2-yl)-3-pyrazolin-5-ones and 2-(pyrimidin-2-yl)-1,2,4,5,6,7-hexahydro-3H indazol-3-ones.
El-Sayed A.M. Badawey & Ibrahim M. El-Ashmawey [120]
N-methyl and N-ethyl substitutions
Demetrio Raffa et al [121]
2.
Anti-cancer activity
N-[(1-aryl-1H-indazol-5 yl) methyl]amide
Gabriella Dessole et al [122]
N-(6(4)-indazolyl) benzenesulfonamides and 7-ethoxy-N-(6(4)-indazolyl) benzenesulfonamides.
Najat Abbassi et al [123]
N-phenyl-1H-indazole-1-carboxamides

Benedetta Maggio et al [124]
2-alkylamino- and 2-alkylthiothiazolo[5,4-e]- and -[4,5-g]indazoles
Manas Chakrabarty et al [125]
Diarylureas
Cui-rong Zhao et al [126]
4-(4-hydroxyphenyl)-6-phenylpyrimidin-2(1H)-ones
Cynthia M. Shafer et al [127]
N-(7-indazolyl) benzenesulfonamide
L. Bouissane et al [128]
2-phenyl-2H-indazole-7-carboxamides
Rita Scarpelli et al [129]
Table 2: Pharmacological activities of Indazole and its derivatives.
3-amino-N-phenyl-1H-indazole-1-carboxamides
Demetrio Raffa et al [121]
N-phenyl-imidazo[4,5-b]pyridin-2-amines, 4-indazolyl-N-phenylpyrimidin-2-amines and N-phenyl-4-pyrazolo[3,4-b]pyridin-pyrimidin-2-amines
Pawel M. Lukasik et al [130]
5-HT2C receptor agonists
Novel indazole derivatives
Itsuro Shimada et al [131]
5-HT6 antagonists
3-sulfonylindazole
Kevin G. Liu et al [132]
Orally efficacious melanin concentrating hormone receptor-1 antagonists
3-amino indazole melanin concentrating hormone
Anil Vasudevan et al [133]
Male Contraceptive
Series of benzoimidazole, benzothiazole, pyrazole, and indazole derivatives.
Qianqian Chen et al [134]
1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide
C. Yan Cheng et al [135]
Anti-oxidant activity
6-carbethoxy-2-cyclohexen-1-one and 2H-indazol-3-ol derivatives
N.A. Shakil et al [136]
Nitric Oxide Synthase (NOS) inhibitors
Halo-1-H-indazoles
Valérie Collot et al [137]
Fluoro indazoles

Rosa M. Claramunt et al [138]
Substituted imidazoles or other mono or bicyclic nitrogen-containing heterocycles
Loredana Salerno et al [139]
NOS inhibitors
Rosa M. Claramunt et al [140]
Methoxyindazoles
Pascale Schumann et al [141]
Analgesic activity
Schiff’s bases of Indazolone
P. Muthumani et al [91]
1 (pyrimidin-2-yl)-3-pyrazolin-5-ones and 2-(pyrimidin-2-yl)-1,2,4,5,6,7-hexahydro-3H- indazol-3-ones.
El-Sayed A.M. Badawey, Ibrahim M. El-Ashmawey [142]
Monoamine Oxidase Inhibitors
2-thiocarbamoyl-2,3,4,5,6,7-hexahydro-1H-indazole and 2-substituted thiocarbamoyl- 3,3a,4,5,6,7-hexahydro-2H- indazoles
Nesrin Gokhan-Kelekci et al [94]
Antimicrobial activity
N-[(4-oxo-2-substituted aryl-1, 3-thiazolidine) –acetamidyl]-5-substituted indazoles
Chirag Parekh et al [143]
N-[(4-oxo-2-substituted aryl-1, 3-thiazolidine)-acetamidyl]-5-nitroindazoles
Apoorva Upadhya et al [144]
N'-(1H-indazole-3-carbonyl)-hydrazide derivatives
T. Chandrasekhar [145]
2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazole derivatives
Maninder Minu et al [146]
a-aminophosphonates containing Indazole
Nasir ali Shafakat Ali et al [147]
Schiff’s bases of Indazolone
P. Muthumani et al [91]
Series of fluconazole analogues
N. Lebouvier et al [148]
(2R,3R)-2-(2,4-difluorophenyl)-3-(substituted indazol-1-yl)-1-(1H-1,2,4- triazol-1-yl)butan-2-ol
Joon Seok Park et al [149]
Chalcone based 6-carbethoxy-2-cyclohexen-1-one and 2H-indazol-3-ol derivatives
N.A. Shakil et al [136]
N-(1-Phenyl-4-carbetoxypyrazol-5-yl)-, N-(indazol-3-yl)- and N-(indazol-5-yl)-2-iodobenzamides
Demetrio Raffa et al [150]
Anti-Protozoal activity
Indazole N-oxide derivatives
Alejandra Gerpe et al [151]
Anti-Fungal activity
Nicolas Lebouvier et al [150]
(2R, 3R)-2-(2,4-difluorophenyl)-3-(substituted indazol-1-yl)-1-(1H-1,2,4- triazol-1-yl)butan-2-ol
Joon Seok Park et al [149]
Anticonvulsant Activity
Indazole substituted-1,3,4-thiadiazole
Kikkeri P. et al [152]
Immunomodulatory
[2-(arylamino)-4,4-dimethyl-6-oxo-cyclohex-1-ene] carbodithioates and 6,6-dimethyl-4-(2-(propan-2-ylidene)hydrazinyl)-6,7-dihydro-2H-indazole-3(5H)-thione
El Sayed H. El Ashry et al [120]
Table 2 & off:
Conclusion
The above literature survey indicate that indazole and its derivatives have pivot role in the medicinal chemistry, as most of the essential drugs are having these heterocycles in their compositions. Indazole is an important heterocyclic system which has great significance in pharmaceutical industry as well as being a useful synthon for the synthesis of many bridgehead heterocycles. This review describes new strategies and the development of novel concepts along with conventional methods to synthesize a wide variety of substituted indazoles. The large number of biologically active indazoles substituted at C(3), functionalizations of this position are of great current interest. In this context, Heck as well as Suzukitype cross-couplings of 3-iodoindazoles has been described in review, multi-component synthesis and ring transformations provide useful synthetic routes to unsubstituted, monosubstituted, disubstitued, trisustituted & tetrasustituted indazole derivatives. By means of cycloaddition reactions regioisomers have formed.
References
- Gabriel E Büchel , Iryna N Stepanenko, Michaela Hejl, Michael A Jakupec, Bernhard K Keppler, Petra Heffeter, et al. Osmium (IV) complexes with 1H- and 2H-indazoles: Tautomer identity versus spectroscopic properties and antiproliferative activity. J Inorganic Biochem. 2012; 113: 47–54.
- VI Minkin, DG Garnovskii, J Elguero, AR Katritzky, OV Denisko. The Tautomerism of Heterocycles: Five-membered Rings with Two or More Heteroatoms. Adv. Heterocycl. Chem. 2000; 76: 157-323.
- J Catalán, JC del Valle, RM Claramunt, G Boyer, J Laynez, J Gómez, et al. Acidity and Basicity of Indazole and its N-Methyl Derivatives in the Ground and in the Excited State. J Phys Chem.1994; 98: 10606-10612.
- J Catalán, JLG de Paz, J Elguero. The Structure Of NH-Benzazoles (1H-Benzimidazoles, 1H- and 2H-Indazoles, 1H- And 2H-Benzotriazoles). J Chem Soc Perkin Trans. 1996; 2: 57.
- C Ogretir, N Funda Kaypak. Quantum chemical calculations on the annular tautomerism of some indazole derivatives. 1. A gas phase study. 2002; 583: 137-144.
- C Ogretir, N Funda (Kaypak) Tay. Investigation of the structure and properties of some indazole derivatives using the AM1, PM3 and MNDO semiempirical methods. 2. An aqueous phase study. J Molecular Structure THEOCHEM. 2002; 588: 145-153.
- I Alkorta, J Elguero. Theoretical estimation of the annular tautomerism of indazoles. J Phys Org Chem. 2005; 18: 719.
- JN Latosinska. Thermodynamic stability of indazole studied by NMR–NQR spectroscopy and ab initio calculations. Magn Reson Chem. 2000; 38: 192-196.
- K Anandan, P Kolandaivel, R Kumaresan. Ab initio and DFT studies on tautomerism of indazole in gaseous and aqueous phases. J Molecular Structure THEOCHEM. 2004; 686: 83-89.
- RM Claramunt, MD Santa María, D Sanz, I Alkorta. A 1H, 13C and 15N NMR study in solution and in the solid state of six N-substituted pyrazoles and indazoles. J Elguero Magn Reson Chem. 2006; 44: 566-570.
- OV Dyablo, AV Mikhailus, AF Pozharskii, DS Trishkin. Synthesis and Investigation of the Conformational Mobility of Certain (N-Benzyl-N-Nitrosoamino) azoles. Chem Heterocycl Compd. 2005; 41: 329-339.
- A Perona, D Sanz, RM Claramunt. Syntheses and Structural Studies of Schiff Bases Involving Hydrogen Bonds. J Elguero Molecules. 2006; 11: 453-463.
- M A García, C López, RM Claramunt, A Kenz, M Pierrot, J Elguero. Polymorphism vs. Desmotropy: The Cases of 3-Phenyl- and 5-Phenyl- 1H-pyrazoles and 3-Phenyl-1H-indazole. Helv Chim Acta. 2002; 85: 2763-2776.
- A Schmidt. Heterocyclic Mesomeric Betaines and Analogs in Natural Product Chemistry. Betainic Alkaloids and Nucleobases. Adv Heterocycl Chem. 2003; 85: 67-171.
- Atta-ur-Rahman, S Malik, H Cun-heng, J Clardy. Isolation and structure determination of nigellicine, a novel alkaloid from the seeds of nigella sativa Tetrahedron Lett. 1985; 26: 2759-2762.
- A Schmidt. Biologically Active Mesomeric Betaines and Alkaloids, Derived from 3- Hydroxypyridine, Pyridin-N-oxide, Nicotinic Acid and Picolinic Acid: Three Types of Conjugation and Their Consequences. Curr Org Chem. 2004; 8: 653.
- YM Liu, JS Yang, QH Liu. A new alkaloid and its artificial derivative with an indazole ring from Nigella glandulifera. Chem Pharm Bull. 2004; 52: 454-455.
- Atta-ur-Rahman, S Malik, SS Hasan, MI Choudhary, CZ Ni, J Clardy. Nigellidine — A new indazole alkaloid from the seeds of Nigella sativa. Tetrahedron Lett. 1995; 36: 1993-1996.
- H Cerecetto, A Gerpe, M González, VJ Arán, C Ochoa de Ocáriz. Pharmacological properties of indazole derivatives: recent developments. Mini-Rev Med Chem. 2005; 5: 869-878.
- A Jennings, M Tennant. Selection of molecules based on shape and electrostatic similarity: proof of concept of “electroforms”. J Chem Inf Model. 2007; 47: 1829-1838.
- M Hartmann, ME Sommer, BK Keppler, F Kratz, TJ Einhäuser. New tumor-inhibiting ruthenium compounds: Investigations into their mode of action in human blood plasma and binding towards DNA. J Inorg Biochem.1995; 59: 214.
- MJ Clarke. Ruthenium metallopharmaceuticals. Coord Chem Rev. 2003; 236: 69-93.
- MA Jakupec, E Reisner, A Eichinger, M Pongratz, VB Arion, M Galanski, et al. Redox-Active Antineoplastic Ruthenium Complexes with Indazole: Correlation of in Vitro Potency and Reduction Potential. J Med Chem. 2005; 48: 2831-2837.
- KG Lipponer, E Vogel. Synthesis, Characterization and Solution Chemistry of trans-Indazoliumtetrachlorobis(Indazole)Ruthenate(III), a New Anticancer Ruthenium Complex. IR, UV, NMR, HPLC Investigations and Antitumor Activity. Crystal Structures of trans-1-Methyl-Indazoliumtetrachlorobis-(1-Methylindazole)Ruthenate(III) and its Hydrolysis Product trans-Monoaquatrichlorobis-(1-Methylindazole)-Ruthenate(III). Met Based Drugs. 1996; 3: 243–260.
- N Lebouvier, F Pagniez, M Duflos, P Le Pape, YM Na, GLe Baut et al. Synthesis and antifungal activities of new fluconazole analogues with azaheterocycle moiety. Bioorganic & Medicinal Chemistry Letters. 2007; 17: 3686-6389.
- DP Becker, DL Flynn, AE Moormann, R Nosal, CI Villamil, R Loeffler, et al. Pyrrolizidine Esters and Amides as 5-HT4 Receptor Agonists and Antagonists. J Med Chem. 2006; 49: 1125.
- D Raffa, G Daidone, F Plescia, D Schillaci, B Maggio, L Torta. Synthesis and antifungal activity of new N-(1-phenyl-4-carbetoxypyrazol-5-yl)-, N-(indazol-3-yl)- and N-(indazol-5-yl)-2-iodobenzamides. Farmaco. 2002; 57: 183-187.
- H Harada, Y Hirokawa, K Suzuki, Y Hiyama, M Oue, H Kawashima, et al. Discovery of a novel and potent human and rat beta3-adrenergic receptor agonist, [3-[(2R)-[[(2R)-(3-chlorophenyl)-2-hydroxyethyl]amino]propyl]-1H-indol-7-yloxy]acetic acid. Chem Pharm Bull. 2005; 53: 184-198.
- P Grundt, S Little Jane Husband, RR Luedtke, M Taylor, A Hauck Newman. Analogues of the dopamine D2 receptor antagonist L741,626: Binding, function, and SAR , Bioorg. Med Chem Lett. 2007; 17: 745-749.
- S Huang, R Li, PJ Connolly, S Emanuel, A Fuentes-Pesquera, M Adams, et al. Synthesis and biological study of 2-amino-4-aryl-5-chloropyrimidine analogues as inhibitors of VEGFR-2 and cyclin dependent kinase 1 (CDK1). Bioorg Med Chem Lett. 2007; 17: 2179-2183.
- M Boulouard, P Schumann-Bard, S Butt-Gueulle, E Lohou, S Stiebing, V Collot, et al. 4-Substituted indazoles as new inhibitors of neuronal nitric oxide synthase. Bioorg. Med. Chem Lett. 2007; 17: 3177-3180.
- A Gomtsyan, EK Bayburt, JR Koenig, CH Lee. US. Patent Application. 2004; 254188.
- M Iwakubo, A Takami, Y Okada, T Kawata, Y Tagami, H Ohashi, et al. Design and synthesis of Rho kinase inhibitors (II). Bioorg Med Chem. 2007; 15: 350-364.
- KB Goodman, H Cui, SE Dowdell, DE Gaitanopoulos, RL Ivy, CA Sehon. et al. Development of Dihydropyridone. Indazole Amides as Selective Rho-Kinase Inhibitors. J Med Chem. 2007; 50: 6–9.
- DP Walker, DG Wishka, DW Piotrowski, S Jia, SC Reitz, KM Yates, et al. Design, synthesis, structure–activity relationship, and in vivo activity of azabicyclic aryl amides as α-7 nicotinic acetylcholine receptor agonists, Bioorg. Med. Chem. 2006; 14: 8219-8248.
- JH Sun, CA Teleha, JS Yan, JD Rodgers, DA Nugiel. Efficient Synthesis of 5-(Bromomethyl)- and 5-(Aminomethyl)-1-THP-Indazole. J Org Chem. 1997: 62: 5627-5629.
- T Zhan, H Lou. Synthesis of azole nucleoside analogues of d-pinitol as potential antitumor agents. Carbohydr Res. 2007; 342: 865-869.
- BM Swahn, F Huerta, E Kallin, J Malmstrom, T Weigelt, J Viklund, et al. Design and synthesis of 6-anilinoindazoles as selective inhibitors of c-Jun N-terminal kinase-3. Bioorg Med Chem Lett. 2005; 15: 5095-5099.
- M Liu, S Wang, JE Clampit, RJ Gum, DL Haasch, CM Rondinone et al. Discovery of a new class of 4-anilinopyrimidines as potent c-Jun N-terminal kinase inhibitors: Synthesis and SAR studies. Bioorg Med Chem Lett. 2007; 17: 668-672.
- P Schumann, V Collot, Y Hommet, W Gsell, F Dauphin, J Sopkova, et al. Inhibition of neuronal nitric oxide synthase by 7-methoxyindazole and related substituted indazoles. Bioorg Med Chem Lett. 2001; 11: 1153-1156.
- BE Kalisch, BP Connop, K Jhamandas, RJ Beninger, RJ Boegman, Neurosci. Lett. 1996, 219, 75; Crystal structure: J Sopková-de Oliveira Santos, V Collot, S Rault, Acta Crystallogr. Sect. C. 2000; 56: 1503.
- R Van Leeuwen, R De Vries, MR Dzoljic. 7-Nitro indazole, an inhibitor of neuronal nitric oxide synthase, attenuates pilocarpine-induced seizures. Eur J Pharmacol. 1995; 287: 211-213.
- IT Uzbay, H Lal. Effects of NG-nitro-l-arginine methyl ester, 7-nitro indazole, and agmatine on pentylenetetrazol-induced discriminative stimulus in Long–Evans rats. Progress in Neuro-Psycopharmacology & Biol Psychiatry. 2002; 26: 567-573.
- PK Moore, P Wallace, Z Gaffen, SL Hart, RC Babbedge. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects. Br J Pharmacol. 1993; 110: 219-214.
- H Akabane, N Miyagawa, H Nii, Y Inami, M Togawa, H Tanaka, et al. The Effect of a Newly Synthesized Indazole Compound, TAS-3-124, on Experimental Autoimmune Disease. Biol Pharm Bull. 2004; 27: 1218-1223.
- J. Sopková-de Oliveira Santos, V. Collot, S. Rault. A view of the trimer in the crystal packing of 7-methoxy-1H-indazole. Dashed lines indicate hydrogen bonds. Acta Cryst. 2002; C58: 688-690.
- L. Bouissane, S. El Kazzouli, S. Léonce, B. Pfeiffer, E. M. Rakib, M. Khouili, et al. Synthesis and biological evaluation of N-(7-indazolyl)benzenesulfonamide derivatives as potent cell cycle inhibitors. Bioorg. Med. Chem. 2006. 14: 1078.
- P. J. Hesketh, D. R. Gandara. Serotonin Antagonists: A New Class of Antiemetic Agents. J. Natl. Cancer Inst. 1991; 83: 613.
- J. Sakaguchi, N. Iwasaki, Y. Iwanaga, T. Saito, E. Takahara, H. Kato, et al. Synthesis and Gastrointestinal Prokinetic Activity of Novel Benzamide Derivatives with Amphoteric Side Chains Chem. Pharm. Bull. 2001; 49: 424.
- J. M. Jez, J. M. Vanderkooi, A. M. Laties. Spectroscopic characterization of bendazac and benzydamine: possible photochemical modes of action. Biochem. Biophys. Res. Commun. 1996; 221: 266.
- S.L. Pan, J.H. Guh, C.Y. Peng, S.W. Wang, Y.L. Chang, F.C. Cheng, et al. YC-1 [3-(5'-Hydroxymethyl-2'-furyl)-1-benzyl Indazole] Inhibits Endothelial Cell Functions Induced by Angiogenic Factors in Vitro and Angiogenesis in Vivo Models. J. Pharmacol. Exp. Ther. 2005; 314: 35-42.
- J. Galle, Z. Zabel, U. Hübner, A. Hatzelmann, B. Wagner, C. Wanner, et al. Schmidt, Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br. J. Pharmacol. 1999; 127: 195-203.
- P. A. Fernandez, T. Bellamy, M. Kling, D. J. Madge, D. L. Selwood. A Convenient Route to the Soluble Guanylate Cyclase Activator YC-1 and Its N2 Regioisomer. Heterocycles 2001; 55: 1813-1816.
- K. W. Hering, J. D. Artz, W. H. Pearson, M. A. Marletta, The design and synthesis of YC-1 analogues as probes for soluble guanylate cyclase. Bioorg. Med. Chem. Lett. 2006; 16: 618-621.
- K. Yildiz, K. Rehse, J.-P. Stasch, E. Bischoff. New Antithrombotics with an Indazole Arch. Pharm. Pharm. Med chem. 2004; 337: 311.
- M. Bonanomi, G. Lucente, B. Silvestrini. Male fertility: core chemical structure in pharmacological research. Contraception 2002; 65: 317.
- C.Y.Cheng, M.Y. Mo, J. Grima, L. Saso, B. Tita, D. Mruk, et al. Indazole carboxylic acids in male contraception. Contraception.2002; 65: 265.
- H.C. Zhang, C. K. Derian, D. F. McComsey, K. B. White, H. Ye, L. R. Hecker et al. Novel Indolylindazolylmaleimides as Inhibitors of Protein Kinase C-β: Synthesis, Biological Activity, and Cardiovascular Safety. J. Med. Chem. 2005; 48:1725.
- L. Mosti, G. Menozzi, P. Fossa, W. Filippelli, S. Gessi, B. Rinaldi et al. Synthesis and preliminary biological evaluation of novel N-substituted 1-amino-3-[1-methyl(phenyl)-1H-indazol-4-yloxy]-propan-2-ols interesting as potential antiarrhythmic, local anaesthetic and analgesic agents. Arzneim.-Forsch. Drug Res. 2000; 50: 963-972.
- J. A. May, A. P. Dantanarayana, P. W. Zinke, M. A. McLaughlin, N. A. Sharif. 1-((S)-2-Aminopropyl)-1H-indazol-6-ol: A Potent Peripherally Acting 5-HT2 Receptor Agonist with Ocular Hypotensive Activity. J. Med. Chem. 2006; 49: 318.
- T. W. Wong, F. Y. Lee, C. Yu, F. R. Luo, S. Oppenheimer, H. Zhang et al. Preclinical Antitumor Activity of BMS-599626, a pan-HER Kinase Inhibitor That Inhibits HER1/HER2 Homodimer and Heterodimer Signaling Clin. Cancer Res. 2006; 12: 6186-6193.
- H. Mastalerz, M. Chang, P. Chen, P. Dextraze, B. E. Fink, A. Gavai, et al. New C-5 substituted pyrrolotriazine dual inhibitors of EGFR and HER2 protein tyrosine kinases. Bioorg. Med. Chem. Lett. 2007; 17: 2036.
- L. Smith II, W. C. Wong, A. S. Kiselyov, S. Burdzovic-Wizemann, Y. Mao, Y. Xu, et al. Novel tricyclic azepine derivatives: Biological evaluation of pyrimido[4,5-b]-1,4-benzoxazepines, thiazepines, and diazepines as inhibitors of the epidermal growth factor receptor tyrosine kinase. Bioorg. Med. Chem. Lett. 2006; 16: 5102.
- F. Saczewski, A. L. Hudson, R. J. Tyacke, D. J. Nutt, J. Man, P. Tabin, et al. 2-(4,5-Dihydro-1H-imidazol-2-yl)indazole (indazim) derivatives as selective I2 imidazoline receptor ligands. Eur. J. Pharm. Sci. 2003; 20: 201.
- D. Simoni, R. Romagnoli, R. Baruchello, R. Rondanin, M. Rizzi, M. G. Pavani, et al. Novel Combretastatin Analogues Endowed with Antitumor Activity. J. Med. Chem. 2006; 49: 3143.
- S. T. Wrobleski, P. Chen, J. Hynes Jr, S. Lin, D. J. Norris, C. R. Pandit, et al. Rational Design and Synthesis of an Orally Active Indolopyridone as a Novel Conformationally Constrained Cannabinoid Ligand Possessing Antiinflammatory Properties. J. Med. Chem. 2003; 46: 2110.
- W.L. Wu, D. A. Burnett, R. Spring, W. J. Greenlee, M. Smith, L. Favreau, et al. Dopamine D1/D5 Receptor Antagonists with Improved Pharmacokinetics: Design, Synthesis, and Biological Evaluation of Phenol Bioisosteric Analogues of Benzazepine D1/D5 Antagonists. J. Med. Chem. 2005; 48: 680.
- J. Souers, J. Gao, M. Brune, E. Bush, D. Wodka, A. Vasudevan, et al. Identification of 2-(4-Benzyloxyphenyl)-N- [1-(2-pyrrolidin-1-yl-ethyl)-1H-indazol-6-yl]acetamide, an Orally Efficacious Melanin-Concentrating Hormone Receptor 1 Antagonist for the Treatment of Obesity. J. Med. Chem. 2005; 48: 1318.
- J.X. Duan, X. Cai, F. Meng, L. Lan, C. Hart, M. Matteucci. Potent Antitubulin Tumor Cell Cytotoxins Based on 3-Aroyl Indazoles. J. Med. Chem. 2007; 50: 1001-1006.
- G. A. Pinna, M. A. Pirisi, J.M. Mussinu, G. Murineddu, G. Loriga. A. Pau, et al. Chromophore-modified bis-benzo[g]indole carboxamides: synthesis and antiproliferative activity of bis-benzo[g]indazole-3-carboxamides and related dimmers. Farmaco. 2003; 58: 749-763.
- Shimada I, Maeno K, Kimizuka T, Goto S, Takahashi T, Nakamura A, et al. An Efficient Preparative Route to 7-Ethyl-1H-furo[2,3-g]indazole. Heterocycles. 2004; 62: 807-813.
- X. Zhang, M. Urbanski, M. Patel, R. E. Zeck, G. G. Cox, H. Bian, et al. Heteroaryl-O-glucosides as novel sodium glucose co-transporter 2 inhibitors. Part 1. Bioorg. Med. Chem. Lett. 2005; 15: 5202.
- Krapcho A.P, Haydar S.N. Synthesis of regioisomeric 2,5-bis-substituted-aza-benzothiopyranoindazoles. J. Heterocycl. Chem. 2001; 38: 1153-1166.
- Crestey F, Collot V, Stiebing, Rault S. A new and efficient synthesis of 2-azatryptophans. Tetrahedron. 2006; 62: 7772-7775.
- J. Souers, J. Gao, D. Wodka, A. S. Judd, M. M. Mulhern, J. J. Napier, et al. Synthesis and evaluation of urea-based indazoles as melanin-concentrating hormone receptor 1 antagonists for the treatment of obesity. Bioorg. Med. Chem. Lett. 2005; 15: 2752.
- L. G. Fedenok, N. A. Zolnikova. On the cyclization of ortho-alkynylbenzene diazonium salts. Tetrahedron Lett. 2003; 44: 5453.
- L. G. Fedenok, I. I. Barabanov, V. S. Bashurova, G. A. Bogdanchikov. Mechanism of the heterocyclization of vic-alkynylanthra- and vic-alkynylnaphthoquinone diazonium salts. Tetrahedron. 2004; 60: 2137-2145.
- M. S. Shvartsberg, I. D. Ivanchikova, L. G. Fedenok. An unusual direction of the Richter synthesis. 1H-naphtho[2,3-g]indazole-6,11-diones. Tetrahedron Lett. 1994; 35: 6749.
- D. L. Selwood, D. G. Brummell, J. Budworth, G. E. Burtin, R. O. Campbell, S. S. Chana, et al. Synthesis and biological evaluation of novel pyrazoles and indazoles as activators of the nitric oxide receptor, soluble guanylate cyclase. J. Med. Chem. 2001; 44: 78-93.
- N. Koga, G. Koga, J.-P. Anselme. The photolysis of diphenylcarbamoyl azide. Tetrahedron. 1972; 28: 4515-4521.
- Y. Shoji, Y. Hari, T. Aoyama. Facile synthesis of 3-trimethylsilylindazoles by [3+2]cycloaddition reaction of lithium trimethylsilyldiazomethane with benzynes. Tetrahedron Lett. 2004; 45: 1769.
- Cho CS, Lim DK, Heo NH, Kim TJ, Shim SC. Facile palladium-catalysed synthesis of 1-aryl-1H-indazoles from 2-bromobenzaldehydes and arylhydrazines. Chem. Commun. 2004; 1: 104.
- Y. Lebedev, A. S. Khartulyari, A. Z. Voskoboynikov. Synthesis of 1-Aryl-1H-indazoles via Palladium-Catalyzed Intramolecular Amination of Aryl Halides. J. Org. Chem. 2005; 70: 596.
- K. Inamoto, M. Katsuno, T. Yoshino, I. Suzuki, K. Hiroya, T. Sakamoto. Efficient Synthesis of 3-Substituted Indazoles Using Pd-Catalyzed Intramolecular Amination Reaction of N-Tosylhydrazones . Chem. Lett. 2004; 33: 10261027.
- S. Peruncheralathan, T. A. Khan, H. Ila, H. Junjappa. α-Oxoketene dithioacetals mediated heteroaromatic annulation protocol for benzoheterocycles: an efficient regiocontrolled synthesis of highly substituted and annulated indazoles. Tetrahedron. 2004: 60; 3457.
- Correa, I. Tellitu, E. Domínguez, R. SanMartin. Novel Alternative for the N-N Bond Formation through a PIFA-Mediated Oxidative Cyclization and Its Application to the Synthesis of Indazol-3-ones. J. Org. Chem. 2006; 71: 3501.
- L. G. Delyatitskaya, M. V. Petrova, S. Grinberga, N. N. Tonkikh, A. Y. Strakov. 3,6,6-trimethyl-4-oxo-1-(2-pyridyl)-4,5,6,7-tetrahydroindazole in Schmidt reaction and Beckmann rearrangement conditions for the 4-hydroxyimino derivative .Chem. Heterocycl. Compd. 2000; 36: 728.
- Palumbo Piccionello, A. Pace, I. Pibiri, S. Buscemi, N. Vivona. Mild method for the synthesis of 1H-indazoles through oxime-phosphonium ion intermediate. Tetrahedron. 2014; 55: 2480.
- R. Herges, Angew. Organizing Principle of Complex Reactions and Theory of Coarctate Transition States. Angew. Chem. Int. Ed. Engl. 1994; 33: 255-276.
- L. D. Shirtcliff, T. J. R. Weakly, M. M. Haley, F. Köhler, R. Herges. Experimental and Theoretical Investigation of the Coarctate Cyclization of (2-Ethynylphenyl) phenyldiazenes. J. Org. Chem. 2004; 69: 6979.
- P. Muthumani, R. Meera, S.Venkatraman, Murugan, P. Devi. Synthesis and biological study of some novel schiff’s bases of indazolone derivatives. J. Chem. Pharm. Res. 2010; 2: 433-443 .
- Aran V. J, Ochoa, C, Boiani L. Buccino P. Synthesis and biological properties of new 5-nitroindazole derivatives. Bioorg. Med. Chem. 2005; 13: 3197-3207.
- Creencia E. C, Kosaka M, Muramatsu T, Horaguchi T. Microwave-assisted synthesis of 2-pyridinylethyl indazoles, Tetrahedron Letters. 2015; 56: 4811–4814.
- Nesrin Gökhan-Kelekçi, O. Ozgun Simsek, Ayse Ercan, Kemal Yelekçi, Z. Sibel Sahin, Samil Isik, Gülberk Uçar, et al. Synthesis and molecular modeling of some novel hexahydroindazole derivatives as potent monoamine oxidase inhibitors. Bioorganic & Medicinal Chemistry. 2009; 17: 6761–6772.
- Itsuro Shimada, et al. synthesized furan-fused indazole. 1,3-Cyclohexanedione I.; Maeno, K.; Kazuta, K.; Kubota H. Synthesis and structure–activity relationships of a series of substituted 2-(1H-furo[2,3-g]indazol-1-yl)ethylamine derivatives as 5-HT2C receptor agonists. Bioorg. Med. Chem. 2008; 16: 1966-1982.
- Maninder Minu, Ananda Thangadurai, Sharad Ramesh Wakode, Shyam Sundar Agrawal, Balasubramanian Narasimhan, et al. Synthesis, antimicrobial activity and QSAR studies of new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Bioorganic & Medicinal Chemistry Letters. 2009; 19: 2960–2964.
- Gerpe, A, Aguirre, G, Boiani, L, Cerecetto H. Indazole N-oxide derivatives as antiprotozoal agents: Synthesis, biological evaluation and mechanism of action studies. Bioorg. Med. Chem. 2006; 14: 3467-3480.
- Irini Akritopoulou-Zanze, Brian D. Wakefield, Alan Gasiecki, Douglas Kalvin, Eric F. Johnson, Peter Kovar, et al. Scaffold oriented synthesis. Part 3: Design, synthesis and biological evaluation of novel 5-substituted indazoles as potent and selective kinase inhibitors employing [2+3] cycloadditions. Bioorganic & Medicinal Chemistry Letters. 2011; 21: 1476–1479.
- Ornelio Rosati, Massimo Curini, Maria Carla Marcotullio, Antonio Macchiarulo, Marina Perfumi, Laura Mattioli, Francesco Rismondo and Giancarlo Cravotto. Synthesis, docking studies and anti-inflammatory activity of 4,5,6,7-tetrahydro-2H-indazole derivatives. Bioorg. Med. Chem. 2007; 15: 3463-3473.
- Jan Bergman et al. Nakhai, A.; Bergman. Synthesis of hydrogenated indazole derivatives starting with α,β-unsaturated ketones and hydrazine derivatives. J. Tetrahedron. 2009; 65: 2298-2306.
- Itsuro Shimada, et al. Synthesis and structure–activity relationships of a series of substituted 2-(1H-furo[2,3-g]indazol-1-yl)ethylamine derivatives as 5-HT2C receptor agonists. Bioorg. Med. Chem. 2008; 16: 1966-1982.
- Danijel Kikelj, et al. Synthesis of α,β-unsaturated aryl esters via Heck reaction of unsymmetrical aryl tellurides. Tetrahedron Lett. 2000; 41: 5589-5592.
- Q Han, C Dominguez, PFW Stouten, JM Park, DE Duffy, RA Galemmo Jr, et al. Design, Synthesis, and Biological Evaluation of Potent and Selective Amidino Bicyclic Factor Xa Inhibitors. J. Med. Chem. 2000; 43: 4398.
- DG Batt, JJ Petraitis, GC Houghton, DP Modi, GA Gain, MH Corjay, et al. Disubstituted Indazoles as Potent Antagonists of the Integrin αvβ3. J. Med. Chem. 2000; 43: 41.
- HR Tsou, EG Overbeek-Klumpers, WA Hallett, MF Reich, M Brawner Floyd, BD Johnson, et al. Optimization of 6,7-Disubstituted-4-(arylamino)quinoline-3-carbonitriles as Orally Active, Irreversible Inhibitors of Human Epidermal Growth Factor Receptor-2 Kinase Activity. J. Med. Chem. 2005; 48: 1107.
- ER Perez, A Loupy, M Liagre, AM de Guzzi Plepis, PJ Cordeiro. Clean and efficient microwave-solvent-free synthesis of 1-(2',4'-dichlorophenacyl) azoles. Tetrahedron. 2003; 56: 865-870.
- FC Teixeira, H Ramos, IF Antunes, MJM Curto, MT Duarte, I Bento. Synthesis and Structural Characterization of 1- and 2-Substituted Indazoles: Ester and Carboxylic Acid. Molecules. 2006; 11: 867-889.
- T Sato, M Miura, K Sakai, Y Yokoyama. Reaction of magnesium cyclopropylidene with N-lithio arylamines: a method for generation of α-amino-substituted cyclopropylmagnesiums and a study for their reactivity with electrophiles. Tetrahedron. 2006; 62: 3771-3938.
- Crestey F, Collot V, Stiebing S, Rault S, Francois C, Valerie C et al. Synthesis of New Dehydro 2-Azatryptophans and Derivatives via Heck Cross-Coupling Reactions of 3-Iodoindazoles with Methyl 2-(Acetylamino)acrylate. Synthesis. 2006; 20: 3506-3514.
- V Collot, P Dalemagne, PR Bovy, S Rault. Suzuki-type cross-coupling reaction of 3-iodoindazoles with aryl boronic acids: A general and flexible route to 3-arylindazoles. Tetrahedron. 1999; 55: 6917.
- A Arnautu, V Collot, J Calvo Ros, C Alayrac, B Witulski, S Rault. Sonogashira cross-coupling reaction of 3-iodoindazoles with various terminal alkynes: a mild and flexible strategy to design 2-aza tryptamines. Tetrahedron Lett. 2002; 43: 2695.
- B Witulski, J Ramon Azcon, C Alayrac, A Arnautu, V Collot, S Rault, et al. Sequential Sonogashira and Suzuki Cross-Coupling Reactions in the Indole and Indazole Series. Synthesis. 2005; 5: 771.
- Schmidt A, Snovydovych B, Habeck T, Drottboom P, Gjikaj M, Adam A. N-Heterocyclic Carbenes of 5-Haloindazoles Generated by Decarboxylation of 5-Haloindazolium-3-carboxylates. Eur. J. Org. Chem. 2007; 29: 4909-4916.
- A Schmidt, L Merkel, W Eisfeld. Nucleophilic Carbenes and Pseudo-Cross-Conjugated Mesomeric Betaines of Indazole Starting from Analogues of the Alkaloid-Betaine Nigellicine. Eur. J. Org. Chem. 2005; 10: 2124-2130.
- A Schmidt, T Habeck, B Snovydovych, W Eisfeld. Addition Reactions and Redox Esterifications of Carbonyl Compounds by N-Heterocyclic Carbenes of Indazole. Org. Lett. 2007; 9: 3515-3518.
- Catalán J, Palomar J, de Paz JLG. On the acidity and basicity of azoles: the Taft scheme for electrostatic proximity effects. Int. J. Mass Spectrom. Ion Processes. 1998; 175: 51-59.
- AM González-Nogal, M Calle, P Cuadrado. Reactions of Lithium Silylcuprates with Pyrazolium and Indazolium Salts Ana M. González-Nogal, Mariola Calle and Purificación Cuadrado. Eur. J. Org. Chem. 2007; 37: 6089-6096.
- Richa Kaur Bhatia, P Bhoja Raju, Upendra K Jain, P Praveen Kumar and K Satyavathi. Synthesis and anti-inflammatory activities of 7-Benzylidene-2, 3-diphenyl-4, 5, 6, 7-tetrahydro-2H-indazole derivatives. RJPBCS. 2011; 2: 118-129.
- Gabriel E Büchel, Iryna N Stepanenko, Michaela Hejl, Michael A Jakupec, et al. Osmium(IV) complexes with 1H- and 2H-indazoles: Tautomer identity versus spectroscopic properties and antiproliferative activity. Journal of Inorganic Biochemistry. 2012; 113: 47–54.
- El Sayed H, El Ashry, Mohammad R, Amer, Omer M. Abdalla, et al. Synthesis, biological evaluation, and molecular docking studies of benzyl, alkyl and glycosyl [2-(arylamino)-4,4-dimethyl-6-oxo-cyclohex-1-ene]carbodithioates,as potential immunomodulatory and immunosuppressive agents. Bioorganic & Medicinal Chemistry. 2012: 20: 3000–3008.
- Raffa D, Maggio B, Cascioferro S, Raimondi M V, Schillaci D, Gallo G, et al. Synthesis and antiproliferative activity of 3-amino-N-phenyl- 1H-indazole-1-carboxamides. Eur. J.Med. Chem. 2009; 44: 165-178.
- Dessole G, Branca D, Ferrigno F, Kinzel O, Muraglia E, Palumbi M C, et al. Discovery of N-[(1-aryl-1H-indazol-5-yl)methyl]amides derivatives as smoothened antagonists for inhibition of the hedgehog pathway. Bioorganic & Medicinal Chemistry Letters. 2009; 19: 4191–4195.
- Najat Abbassi, Hakima Chicha, El Mostapha Rakib, Abdellah Hannioui, Mdaghri Alaoui , Abdelouahed Hajjaji, et al. Synthesis, antiproliferative and apoptotic activities of N-(6(4)-indazolyl)- benzenesulfonamide derivatives as potential anticancer agents. European Journal of Medicinal Chemistry. 2012; 57: 240-249.
- Benedetta Maggio, Maria Valeria Raimondi, Demetrio Raffa, Fabiana Plescia, Stella Cascioferro, Salvatore Plescia, et al. Synthesis of substituted 3-amino-N-phenyl-1H-indazole-1-carboxamides endowed with antiproliferative activity. European Journal of Medicinal Chemistry. 2011; 46: 168-174.
- Chakrabarty M, Kundu T, Arima S, Harigaya Y. An expedient, regioselective synthesis of novel 2-alkylamino- and 2-alkylthiothiazolo[5,4-e]- and -[4,5-g]indazoles and their anticancer potential. Tetrahedron. 2008; 64: 6711–6723.
- Cui-rong Zhao, Rui-qi Wanga, Gang Li, Xiao-xia Xue, Chang-jun Sun, Xian-jun Qua, et al. Synthesis of indazole based diarylurea derivatives and their antiproliferative activity against tumor cell lines. Bioorganic & Medicinal Chemistry Letters. 2013; 123: 1989-1992.
- Cynthia M. Shafer, Mika Lindvall, Cornelia Bellamacina, Thomas G. Gesner, Asha Yabannavar, Weiping Jia, et al. 4-(1H-Indazol-5-yl)-6-phenylpyrimidin-2(1H)-one analogs as potent CDC7 inhibitors. Bioorganic & Medicinal Chemistry Letters. 2008; 18: 4482–4485.
- L. Bouissane, S. El Kazzouli, S. Le´once, B. Pfeiffer, E. M. Rakib, et al. Synthesis and biological evaluation of N-(7-indazolyl)benzenesulfonamide derivatives as potent cell cycle inhibitors. Bioorganic & Medicinal Chemistry. 2006; 14: 1078–1088.
- Rita Scarpelli, Julia K. Boueres, Mauro Cerretani, Federica Ferrigno, Jesus M. Ontoria, Michael Rowley. Synthesis and biological evaluation of substituted 2-phenyl-2H-indazole-7-carboxamides as potent poly(ADP-ribose) polymerase (PARP) inhibitors. Bioorganic & Medicinal Chemistry Letters. 2010; 20: 488–492.
- Pawel M. Lukasik, Sherifa Elabar, Frankie Lam, Hao Shao, Xiangrui Liu, Abdullah Y, et al. Synthesis and biological evaluation of imidazo[4,5-b]pyridine and 4-heteroarylpyrimidine derivatives as anti-cancer agents. European Journal of Medicinal Chemistry. 2012; 57: 311-322.
- Itsuro Shimada, Kyoichi Maeno, Ken-ichi Kazuta, Hideki Kubota, Tetsuya Kimizuka, Yasuharu Kimura, et al. Synthesis and structure–activity relationships of a series of substituted 2-(1H-furo[2,3-g]indazol-1-yl)ethylamine derivatives as 5-HT2C receptor agonists. Bioorganic & Medicinal Chemistry. 2008; 16: 1966–1982.
- Kevin G. Liu, Albert J. Robichaud, Alexander A. Greenfield, Jennifer R. Lo, Cristina Grosanu, et al. Identification of 3-sulfonylindazole derivatives as potent and selective 5-HT6 antagonists. Bioorganic & Medicinal Chemistry. 2011; 19: 650–662.
- Anil Vasudevan, Andrew J. Souers, Jennifer C. Freeman, Mary K. Verzal, Ju Gao, Mathew M. Mulhern, et al. Aminopiperidine indazoles as orally efficacious melanin concentrating hormone receptor-1 antagonists. Bioorganic & Medicinal Chemistry Letters. 2005; 15: 5293–5297.
- Qianqian Chen, Wei Tian, Guangqian Han, Jingjing Qi, Canhui Zheng, Youjun Zhou, et al. Design and synthesis of novel benzoheterocyclic derivatives as human acrosin inhibitors by scaffold hopping. European Journal of Medicinal Chemistry. 2013; 59: 176-182.
- C. Yan Chenga, T Dolores Mruka, Bruno Silvestrinib, Michele Bonanomib, Ching-Hang Wonga, Michelle K.Y. Siua, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005; 72: 251–261.
- N.A. Shakil, Manish K. Singh, M. Sathiyendiran, J. Kumar, Jasdeep C. Padaria. Microwave synthesis, characterization and bio-efficacy evaluation of novel chalcone based 6-carbethoxy-2-cyclohexen-1-one and 2H-indazol-3-ol derivatives. European Journal of Medicinal Chemistry. 2013; 59: 120-131.
- Elodie Lohou, Jana Sopkova-de Oliveira Santos, Pascale Schumann-Bard, Michel Boulouard, Silvia Stiebing, Sylvain Rault , et al. New hypotheses for the binding mode of 4- and 7-substituted indazoles in the active site of neuronal nitric oxide synthase. Bioorganic & Medicinal Chemistry. 2012; 20: 5296–5304.
- Rosa M. Claramunt, Concepción López, Carlos Pérez-Medina, Marta Pérez-Torralba, José Elguero, Germaine Escames, et al. Fluorinated indazoles as novel selective inhibitors of nitric oxide synthase (NOS): Synthesis and biological evaluation. Bioorganic & Medicinal Chemistry. 2009; 17: 6180–6187.
- Loredana Salerno, Maria N. Modica, Giuseppe Romeo, Valeria Pittala, Maria A. Siracusa , Maria E. Amato, et al. Novel inhibitors of nitric oxide synthase with antioxidant properties. European Journal of Medicinal Chemistry. 2012; 49: 118-126.
- Rosa M. Claramunt, Concepción López , Ana López, Carlos Pérez-Medina, Marta Pérez-Torralba, Ibon Alkorta, et al. Synthesis and biological evaluation of indazole derivatives. European Journal of Medicinal Chemistry. 2011; 46: 1439-1447.
- Pascale Schumann, Valerie Collot, Yannick Hommet, Willy Gsell, Francois Dauphin, Jana Sopkova, et al. Inhibition of Neuronal Nitric Oxide Synthase by 7-Methoxyindazole and Related Substituted Indazoles. Bioorganic & Medicinal Chemistry Letters. 2001; 11: 1153–1156.
- El-Sayed A.M. Badaweya, Ibrahim M. El-Ashmawey. Nonsteroidal antiinflammatory agents - Part 1: Antiinflammatory, analgesic and antipyretic activity of some new 1-(pyrimidin-2-yl)-3-pyrazolin-5ones and 2-(pyrimidin-2-yl)-l,2,4,5,6,7-hexahydro-3H-indazol-3-ones. Eul: J. Med. Chem.1998; 33: 349-361.
- Parekh C, Modi A, Pillai J, Patel B. A novel synthesis of series of indazole derivative as potent antimicrobial agents. Int. J. Drug Res. Tech. 2012; 02: 279-288.
- Upadhyay A. Srivastava S.K. Srivastava S.D. Conventional and microwave assisted synthesis of Some new N-[(4-oxo-2-substituted aryl 1, 3-thiazolidine)-acetamidyl]-5-nitroindazoles and its antimicrobial activity. European Journal of Medicinal Chemistry. 2010; 45: 3541-3548.
- Chandrasekhar T. Vinay Kumar L. Reddy A.B. Naik P.J. Swamy G.N. Synthesis and biological evaluation of some new Aryl acid N'-(1Hindazole- 3-carbonyl)-hydrazide derivatives. Journal of Chemical and Pharmaceutical Research. 2012; 04: 2795-2802.
- Minu M. Thangadurai A. Ramesh S. Wakode. Agrawal S.S. Narasimha B. Synthesis, antimicrobial activity and QSAR studies of new 2,3-disubstituted-3,3a,4,5,6,7-hexahydro-2H-indazoles. Bioorganic & Medicinal Chemistry Letters. 2009; 19: 2960–2964.
- Ali N, Ali S, Zakir S, Patel M, Farooqui M. Synthesis of new a aminophosphonate system bearing Indazole moiety and their biological activity. Eu Journal of Medi Chem. 2012; 50: 39-43.
- Lebouvier N. Pagniez F. Duflos M. Pape P.L. Min Na Y. Bauta G.L, et al. Synthesis and antifungal activities of new fluconazole analogues with azaheterocycle moiety. Bioorganic & Medicinal Chemistry Letters. 2007; 17: 3686–3689.
- Seok P. J. Kyung A. Yu. Kang T. H. Kima S. Suha Y.G. Discovery of novel indazole-linked triazoles as antifungal agents. Bioorganic & Medi Chem Lett. 2007; 17: 3486–3490.
- Raffa D. Daidone G. Plescia F. Schillaci D. Maggio B. Torta L. Synthesis and antifungal activity of new N-(1-phenyl-4-carbetoxypyrazol-5-yl)-, N-(indazol-3-yl)- and N-(indazol-5-yl)-2-iodobenzamides. IL Farmaco. 2002; 57: 183–187.
- Gerpe A. Aguirre G. Boiani L. Cerecetto H. Gonzalez M. Olea-Azar C. Indazole N-oxide derivatives as antiprotozoal agents: Synthesis, biological evaluation and mechanism of action studies. Bioorganic & Medi Chem. 2006; 14: 3467–3480.
- Kikkeri P. H. Kikkeri N.M. Lingappa M. Synthesis of indazole substituted-1,3,4-thiadiazoles and their anticonvulsant activity. Drug Invention Today. 2013; 5: 92-99.
















































