
Research Article
Austin J Anal Pharm Chem. 2017; 4(2): 1083.
Development and Validation of RP-HPLC Method for Simultaneous Estimation of Levocloperastine Fendizoate and Chlorpheniramine Maleate in their Combined Dosage Form
Sharma B¹, Satone D², Pradhan P¹, Jain H¹ and Meshram D¹*
¹Department of Quality Assurance, Pioneer Pharmacy Degree College, India
²University Department of Pharmaceutical Sciences, RTM Nagpur University, India
*Corresponding author: Dhananjay Meshram, Department of Quality Assurance, Pioneer Pharmacy Degree College, India
Received: March 20, 2017; Accepted: April 12, 2017; Published: April 19, 2017
Abstract
A simple and rapid RP-HPLC method has been developed and validated for the simultaneous determination of Levocloperastine fendizoate and Chlorpheniramine maleate syrup formulation. Resolution of the analytes was achieved within 10min, employing a mixture of 10mM mobile phase Buffer (pH 6.5): Acetonitrile (50:50, % v/v) as isocratic mobile phase, pumped at 1.0mL/ min through a C18 column (5μm particle size). The detection wavelength for the analytes was 227nm. The system suitability parameters were found to be acceptable. The linearity of response (r2>0.999) in the appropriate ranges (from 50% up to 150% of the expected concentrations of the analytes in the formulations), method accuracy (RSD<2.0%), repeatability and intermediate precision (RSD<2.0%), were confirmed. Robustness result indicates that the methods performance can withstand small variations in method parameters. Satisfactory results obtained in terms of analyte recovery and RSD, while analyzing marketed pharmaceutical preparations. Hence the method can be useful for regular analysis of this combination in marketed syrup formulation.
Keywords: Levocloperastine fendizoate; Chlorpheniramine maleate; RPHPLC
Introduction
Chlorpheniramine maleate (CPM), 3-(p-chlorophenyl)- 3-(2-pyridyl)-N,N-dimethyl propylamine (Figure 1) [1] is a firstgeneration alkyl amine antihistamine, act by antagonizing H1- receptors. It is commonly used for symptomatic relief of the common cold and allergic rhinitis with mild sedative property [2].
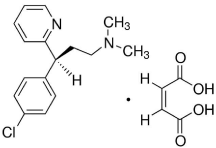
Figure 1: Chemical structure of Chlorpheniramine maleate.
Levocloperastine (LCF) i.e. 1-{2-[(4-chloro-phenyl)- phenylmethoxy]- ethyl}-piperidine (Figure 2) [3], is a drug with a central antitussive effect and it is also endowed with an antihistaminic (sharing an ethylamine moiety with H1 receptor antagonists) and papaverine like activity similar to codeine, but without its narcotic effects. Pharmacological studies have revealed LCF acts on the cough center, without any depression of respiratory center in brain [4].
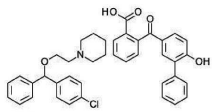
Figure 2: Structure of Levocloperastine fendizoate.
Literature survey revealed few quantitative techniques like RPHPLC [5-16], UV spectroscopy [17-19], TLC [20] methods for CPM and RP-HPLC method [21], UV spectroscopy [22] for LCF are available. So far to our present knowledge, no validated analytical high performance liquid chromatography (HPLC) method was available in literature for this combination in syrup formulation. Therefore, the purpose of this study was to develop and validate a HPLC methodology for the simultaneous determination of LCF and CPM in their combined syrup formulations, which has no literature precedents.
Materials and Methods
Apparatus
The chromatographic system utilised for development of method consists of a pump (Shimadzu LC 10AT VP) with a universal loop injector (Rheodyne 7725i) with a capacity of 20μL, SPD 20A UV Detector and Hypersil BDS C18 (25cm × 4.6mm i.d. × 5μm) column. The equipment was controlled by a PC work station equipped with CLASS M 10-VP software (Shimadzu, Kyoto, Japan). A UV/ Visible double beam spectrophotometer (Shimadzu Model 1800) was employed with a spectral bandwidth of 1nm and a wavelength accuracy of 0.2nm (with automatic wavelength correction using a pair of 1cm matched quartz cells).
Chemicals and solvents
Chlorpheniramine maleate was procured from Oasis laboratory and Levocloperastine fendizoate was obtained as gift sample from LGM Pharma. HPLC grade methanol, acetonitrile and water bought from Merck India. Marketed formulation (Levotus syrup, Shasun Pharmaceuticals Pvt. Ltd, India) containing LCF 20mg and CPM 4mg, bought from local pharmacy.
Preparation of standard solutions
Standard solutions of CPM (40μg/mL), LCF (200μg/mL) were prepared in MeOH and stored in volumetric flask and utilized as stock solution. Solution was found to be stable for 15 days. Working standard solutions of CPM (4μg/mL), LCF (20μg/mL) were freshly prepared by dilution of the stock standard solutions with mobile phase. Mixture solutions containing analytes were freshly prepared, by mixing appropriate volumes of the corresponding working standard solutions in volumetric flask and made up to the mark with mobile phase.
Commercial syrups
Syrup equivalent to 4mg of CPM and 20mg of LCF was transferred to a 100ml volumetric flask and volume was made up mark with methanol. An aliquot (1ml) of the solution was transferred to 10m volumetric flask and diluted to the mark with mobile phase. The solutions were filtered through 0.45-mm Millipore filter before injection. All preparations were performed in triplicate for each brand.
Chromatographic conditions
Chromatographic separation was performed on C18 (25cm × 0.46cm) Hypersil BDS column. The mobile phase comprised of buffer (pH 6.5)-acetonitrile (50:50, %v/v). The mobile phase was delivered at a flow rate of 1mlmin-1. Analysis was performed at ambient temperature. Injection volume was 20μl and detection was carried out at 227nm.
Results and Discussion
Selection of wavelength
Figure 3, depicts the UV spectra of CPM and LCF, dissolved in the mobile phase. CPM and LCF are in the ratio found in pharmaceutical formulations; both drugs show iso-absorptive absorption at 227nm, so considered to be the most desirable detection wavelength.
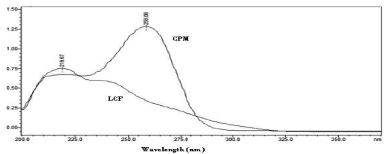
Figure 3: Absorption spectra of the analytes in the 200-400nm region. CPM
(4.0μg/mL), LCF (20.0μg/mL) in the optimized mobile phase.
Optimization of mobile phase
Factors such as solubility and pKa (9.47 for CPM and 8.82 for LCF) of both the drugs were utilised to develop mobile phase. Only CPM was found to be eluting in initial trials with water: methanol and water: acetonitrile. This tempted to have buffer in mobile phase. With the convention that pH of mobile phase should (ideally) be at least 2pH units below or above the sample pKa 10mM phosphate buffer of pH 6 was used [23]. With water (pH 6.0): methanol (40:60 %v/v) LCF eluted, but both peak for CPM and LCF were poorly resolved with retention time (Rt) 2.73 and 2.87min respectively. When strength of organic modifier methanol was decreased to 30% gradually, capacity factor (k) for both increases i.e. Rt increases to 3.1 and 4.5min. Selectivity (a) was increased by replacing methanol with acetonitrile, results in increased k value for LCF, Rt changes to 6.257min with tailing. On changing pH of buffer to pH 6.5 tailing decreases. Hence mobile phase Buffer (pH 6.5, 10mM): Acetonitrile (50:50, %v/v) with a flow rate of 1ml/min was finalized as it resulted in good peak shapes and a significant amount of resolution (Figure 4).
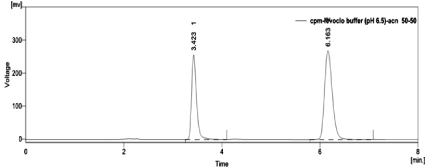
Figure 4: Chromatogram of Standard CPM and LCF in Buffer (pH 6.5):
Acetonitrile (50:50, %v/v) with flow rate 1ml/min.
Method validation
The method was validated in agreement with the ICH guideline [24]. So, method linearity in the relevant working ranges, precision, accuracy, specificity, and robustness were evaluated. System suitability was also determined.
Range and linearity
Method range and linearity were evaluated with seven mixtures of standards at the concentrations: 2.0–6.0 μg/mL for CPM and 10.0- 30.0 μg/mL. Samples were injected in triplicate and calibration curves were plotted against the standard drug concentrations versus peak areas of the individual drugs. The calibration curves for both the drugs were characterized by the equations shown in Table 1; In addition, correlation coefficients > 0.999 confirms method linearity.
% of nominal
CPM
Mean
LCF
Mean
( μg/mL)
AUC (n= 3)
( μg/mL)
AUC (n= 3)
50
2
881.46
10
1412.37
75
3
1320.43
15
2116.27
100
4
1789.49
20
2868.67
125
5
2202.79
25
3531.59
150
6
2673.02
30
4260.36
Slope
446.55
142.23
Intercept
-12.75
-6.67
r2
0.9997
0.9997
Table 1: Result of Linearity study.
Precision
Method precision was verified in its repeatability and intermediate precision. The ICH guideline Q2(R1) [24] offers two alternatives for assessing repeatability. One needs triplicate estimation with independently prepared samples at low, medium and high analyte levels or in other six replicate determinations at 100% level.
Nine independent mixed standards samples containing both analytes at 80%, 100%, and 120% of their corresponding expected concentrations in the pharmaceutical formulation were injected and the RSD (%) of their recoveries were determined. The observed RSD levels (Table 2), which were below 2%, were considered satisfactory.
For verifying intermediate precision, the samples were injected at random during three different days. In all cases results were consistent (RSD<2%), confirmed the precision of the method (Table 2).
Drug
Conc.
Repeatability (n= 6)
Inter-day (n= 3)
(μg/mL)
Area
% R.S.D
Area
% R.S.D
Mean ± S.D. (n=6)
Mean ± S.D. (n=3)
CPM
2
888.13 ± 9.25
1.042
4
1777.84 ± 10.69
0.6
1778.12 ± 13.42
0.755
6
2677.29 ± 29.76
1.111
LCF
10
1425.94 ± 10.05
0.705
20
2843.62 ± 10.18
0.36
2845.13 ± 14.56
0.512
30
4277.60 ± 50.83
1.305
Table 2: Result of Inter-day and Intraday precision.
Accuracy
Method accuracy was demonstrated by standard addition method, by evaluating declared amounts of standard drugs which was fortified to pre-assayed pharmaceutical formulation sample (2.0μg/ mL CPM and 10.0μg/mL LCF), at 80%, 100%, and 120% in triplicates. The %RSD did not exceed 2% (Table 3), meaning that essentially quantitative recoveries were achieved. This confirmed the method enables the accurate determination of the analytes.
Drug
% level
Spiked Conc. (µg/mL)
Recovered Conc. (µg/mL)
Mean Recovery (%)
R.S.D (%)
CPM
(2.0 µg/mL)
80
1.6
1.61
100.63
0.63
100
2
1.99
99.33
1.05
120
2.4
2.41
100.28
1.28
LCF
(10.0 µg/mL)
80
8
8.04
100.46
0.58
100
10
9.91
99.1
0.4
120
12
11.97
99.73
0.93
Table 3: Result of Recovery study (n=3).
LOD and LOQ
The ICH guidelines do not specifically need calculation of the limits of detection (LOD) and quantification (LOQ) for principal analytes in their formulations. However, to assess the confirmed concentration ranges of the analytes were above their LOQ values, the LOD and LOQ were determined employing the ICH method based on the calibration curve [24]. The LOD values were 0.108μg/ mL for CPM and 0.496μg/mL for LCF; the corresponding LOQ values, determined with the same method, were 0.327 and 1.504μg/ mL, respectively. These values fall below the lowest expected analyte concentrations in the samples (Table 4).
LOD (μg/mL)
LOQ (μg/mL)
CPM
0.108
0.327
LCF
0.496
1.504
Table 4: Limit of Detection and Quantification.
Robustness
The robustness of the method was assessed by deliberately causing small changes to the procedure. The flow rate (1mL/min) was altered by ± 2ml/min (0.8-1.2), the pH of the aqueous phase (6.5) was modified in ± 0.2 units (6.3-6.7), and the proportion of the organic mobile phase content (50) was changed in ±2 mL (48–52%). The results were repeated in triplicate and summarized in Table 5. Results indicates its reliability during normal use.
Parameter
Modification
CPM
LCF
Flow rate
0.8
3.20 ± 0.02
3.70 ±0.02
1.2
5.83 ± 0.06
6.27 ± 0.06
pH
6.3
1786.36 ± 9.46
1814.25 ± 7.43
6.7
2870.71 ± 8.40
2909.02 ± 23.70
Organic modifier
48.0
1742.73 ± 14.28
1830.12 ± 15.39
52.0
2800.49 ± 13.88
2928.82 ± 15.94
Table 5: Result of Robustness study.
System suitability
System suitability parameters must be performed to ensure that the system is working correctly during the analysis. The SST parameters were evaluated by injecting six replicates of a solution containing 4μg/mL CPM and 20μg/mL of LCF. The observed RSD values for different parameters were reported, respectively (Table 6), in full compliance with the commonly accepted values (<2%). Method performance data including column efficiencies (N) capacity factors (k), selectivity (a), resolutions between adjacent peeks (Rs), and tailing factors (tf) are also listed. All parameters were within acceptable limits.
Parameter
CPM
LCF
Acceptance Limit
Average (n=6) ± %RSD
Retention Time (tr, min.)
3.319 ± 0.14
6.118 ± 0.18
%RSD < 2 %
Resolution (Rs)
11.655 ± 0.76
Rs > 2
Tailing factors (tf)
1.21 ± 0.05
1.32 ± 0.08
Tf = 2.0
Theoretical plates (N)
5706 ± 47.44
7282 ± 123.55
N = 2000
Table 6: Results for system suitability test (n=6).
Analysis of marketed formulation by developed method
The validated HPLC method was applied to the simultaneous determination of the analytes in syrup formulation (Levotus). Analyses were carried out in triplicate and the results (mean and RSD) are shown in Table 7.
Analyte Dosage form
Label claim (mg)
Assay (% of label claim*)
Mean ± S. D.
CPM
LCF
CPM
LCF
Levotus Syrup
4
20
99.84 ± 0.421
99.57 ± 0.416
Table 7: Results of marketed formulation analysis.
The data showed recoveries between 99.84 % and 99.57% of label claim of the different analytes, with satisfactory precision (RSD =2%, n). These results confirmed the amount of each active principal in the samples was close to the declared content.
No extra peaks which could interfere with the determination of the analytes were observed and the determinations of both drugs were accurately achieved with good recovery and precision. Therefore, the proposed method can be confidently employed for the quality control syrups containing the pharmaceutical combination of CPM and LCF (Table 7).
The test results were comparable to labelled value of each drug in syrup. These results show the developed method is accurate, precise, simple and rapid. It can be used in the routine quality control of dosage form in industries.
Conclusion
A reliable and rapid liquid chromatography method for the simultaneous estimation of CPM and LCF in syrup preparations has been developed and validated. The method is capable of separating the active principles within 10min. In addition, the results suggest the method is sensitive, linear, precise, accurate, specific, and robust for the mixture under investigation. So, the method can be safely applied to the quality control of CPM and LCF in its formulation.
Acknowledgement
The authors wish to express their heartfelt regards to Shri D. D. Patel President, Om Gayatri Education & Charitable Trust and Pioneer Pharmacy Degree College for his valuable guidance and support. Authors are grateful to LGM Pharma, for providing the drug sample and the Management of Pioneer Pharmacy Degree College for providing necessary research facilities.
References
- Drug profile for Chlorpheniramine maleate. 2015.
- DM Paton, DR Webster. Clinical pharmacokinetics of H1- receptor antagonists (the antihistamines). Clin. Pharmacokinet. 1985; 10: 477–497.
- Drug profile for levocloperastine fendizoate.
- MA Catania, S Cuzzocrea. Pharmacological and clinical overview of cloperastine in treatment of cough. Ther. Clin. Risk Manage. 2011; 7: 83–92.
- Nalini K, Narmada P, G Vijaya lakshmi, Gowtham Y, Jogi KV. Simultaneous estimation of paracetamol, guaiphensin, phenylepherine, chlorpheniramine maleate, and Bromhexine HCl in combined tablet dosage form by RP-HPLC. Int. J. of Pharma. Sci. and Res. 2014; 5: 410-416.
- Rajurkar S. Simultaneous determination of chlorpheniramine maleate, paracetamol and Pseudoephedrine HCl, in pharmaceutical preparation by HPLC. Int. J. Life Sci Pharm Research. 2011; 1: 94-100.
- Abdulbari MM, Ihsan MSH. Simultaneous determination and validation of chlorpheniramine maleate, acetaminophen, phenylpropanolamine hydrochloride and caffeine in tablet dosage form by using reverse phase high performance liquid chromatography (RP-HPLC). Int. J. Pharm Pharm. Sci. 2013; 5: 666-670.
- Shah DA, Doshi SS, Baldania SL, Chhalotiya UK, Bhatt KK. Development of LC Method for Estimation of diethyl carbamazine citrate and chlorpheniramine maleate in combined dosage form. Turk J. Pharm. Sci. 2014; 11: 79-86.
- Khalode KD, Waiker SB, Padmane SP. A Validated RP-HPLC Method for the simultaneous estimation of dextromethorphan hydrobromide and chlorpheniramine maleate in syrup formulation. Am. J. Pharm Tech Res. 2012; 2: 392-394.
- Sanchaniya PM, Mehta FA, Uchadadiya NB. Development and Validation of an RP-HPLC Method for Estimation of Chlorpheniramine Maleate, Ibuprofen, and Phenylephrine Hydrochloride in Combined Pharmaceutical Dosage Form, Chromatography Research International. 2013; 6.
- Daravath B, Reddy GS, Kamarupu SK. A Validated RP-HPLC Method for the simultaneous estimation of Diethylcarbamazine citrate and chlorpheniramine maleate in combined pharmaceutical dosage form”. Asian J. of Pharma and Clinical Research. 2014; 7: 98-102.
- Vignaduzzo SE, Kaufman TS. Development and Validation of A HPLC Method for the Simultaneous Determination of Bromhexine, Chlorpheniramine, Paracetamol, and Pseudoephedrine in Their Combined Cold Medicine Formulations. J Liq Chromatography and Related Tech. 2013; 36: 2829-2843.
- Vijay Anand PR, Deepika N, Sam Solomon WD, Venkatanarayan R. RP-HPLC Method development and Validation for Simultaneous determination of Codeine phosphate, Chlorpheniramine maleate and Sodium benzoate in cough syrup formulation. J Pharm Research. 2012; 5: 949-953.
- Qi ML, Wang P, Leng YX, Gu JL, Fu RN. Simple HPLC method for simultaneous determination of acetaminophen, caffeine and chlorpheniramine maleate in tablet formulations. Chromatographia. 2002; 56: 295-298.
- Wanjari DB, Parashar VV, Lulay SN, Tajne MR, and Gaikwad NJ. Simultaneous HPLC Estimation of Acetaminophen, Chlopheniramine Maleate, Dextromethorphan Hydrobromide and Pseudoephedrine Hydrochloride in Tablets. Indian J Pharm Sci. 2004; 66: 345-347.
- Sarve S, Lokhande R, Sutar R, Pandekar S. Simultaneous estimation of Guaiphensin, Ambroxol HCl, pseudoephedrine and chlorpheniramine in combined dosage form by RP-HPLC. J Chem Pharm Res. 2014; 6: 1577-1582.
- Al-Shaalan NH. Determination of phenylephrine hydrochloride and chlorpheniramine maleate in binary mixture using chemometric-assisted spectrophotometric and high-performance liquid chromatographic-UV methods. J Saudi Chem Soc. 2010; 14: 15-21.
- Hassan EM, Mahrous MS, Shdeed RN. Spectrophotometric determination of the active components of a ternary mixture containing Chlorpheniramine maleate, Diclofenac sodium and Paracetamol. J Pharm Biomed Sci. 2011; 7: 1-9.
- Wadher SJ, Kalyankar TM, Panchal PP. Development and validation of simultaneous estimation of chlorpheniramine maleate and phenylepherine HCl in bulk and capsile dosage form by UV spectrophotometer. Int J Chem Tech Research. 2013; 5: 2410-2419.
- Al-Kayasi HN, Salem MS. Simultaneous Quantitative Determination of Codeine Phosphate, Chlorpheniramine Maleate, Phenylephrine Hydrochloride and Acetaminophen in Pharmaceutical Dosage Forms Using Thin Layer Chromatography Densitometry. Analytical Letter. 1986; 19: 915-924.
- Gercia A, Ruperej FJ, Ceppa F, Pellati F, Barbas C. Development of Chromatographic method for the determination of genotoxic impurities in cloperastine fendizoate. J Pharm Biomed Anal. 2012; 61: 230-233.
- Jain NA, Umekar MJ, Lohiya RT. Spectrophotometric method development for determination of levocloperastine fendizoate in bulk and pharmaceutical dosage form. Asian J Research Chem. 2011; 4: 1231-1234.
- LR Synder, JJ Kirkland, JL Glajch (Eds.). Practical HPLC Method Development, Wiley, New York, 1997, p. 295.
- ICH Guide Q2(R1). Validation of Analytical Procedures- Text and Methodology. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), Geneva, Switzerland, 2005.