
Research Article
Austin J Anal Pharm Chem. 2017; 4(2): 1085.
Evaluating Metabolic Responses in Mice to Nanosized Titanium Dioxide Particles Using Gas Chromatography- Mass Spectrometry Based Metabolomics
Singh AK1,2,$, Ratnasekhar Ch1,$, Chaudhari BP3, Singh D4, Chattopadhyay BD2, Mudiam MKR5*
¹Analytical Chemistry Laboratory, Regulatory Toxicology Group, CSIR-Indian Institute of Toxicology Research, Lucknow, India
2Department of Physics, Jadavpur University, Kolkatta, India
3Biochemical Sciences Division, CSIR-National Chemical Laboratory, Pune, India
4Animal House Facility, Regulatory Toxicology Group, CSIR-Indian Institute of Toxicology Research, Lucknow, India
5Chromatography Unit, Analytical Chemistry & Mass Spectrometry Division, CSIR-Indian Institute of Chemical Technology (CSIR-IICT), Hyderabad, India $These authors contributed equally to this work
*Corresponding author: Mohana Krishna Reddy Mudiam, Chromatography Unit, Analytical Chemistry & Mass Spectrometry Division, CSIR-Indian Institute of Chemical Technology (CSIR-IICT), Tarnaka, Uppal Road, Hyderabad – 500 007, Telangana, India
Received: April 20, 2017; Accepted: May 18, 2017; Published: May 25, 2017
Abstract
Titanium dioxide nanoparticle (TiO2 NP) is one of the most commonly used engineered nanoparticles. It has attracted lot of interest to analytical toxicologists in recent past due to its toxicity on human health and environment. The present study was aimed to explore the GC-MS based metabolomics as a tool to investigate the toxicity of TiO2 NP in comparison to TiO2 BP with doses of 300, 600, 1200 mg/kg respectively in both gender(s) of Swiss Albino mice for 7 and 14 days. Serum biochemistry and histopathology parameters were performed. Chemometric analysis by supervised PLS-DA was performed to identify the discrimination/classification between exposed and non-exposed samples due to metabolic perturbations. The morphological, biochemical, haematological and metabolomic analysis revealed that, TiO2 NP has induced toxicological effects to both female and male mice. The results showed that metabolomics along with biochemical analysis can be employed as a comprehensive tool to identify the toxicity of NPs in the model organisms at molecular level.
Keywords: Toxicity; Nanoparticles; Gas chromatography-Mass Spectrometry; Metabolomics
Abbreviations
GC-MS: Gas Chromatography-Mass Spectrometry; NP: Nano Particles; BP: Bulk Particles; TiO2 NP: Titanium Dioxide Nano Particles; TiO2 BP: Titanium Dioxide Bulk Particles; PLS-DA: Partial Least Square Discriminant Analysis; NMR: Nuclear Magnetic Resonance; AMDIS: Automated Mass Spectral Deconvolution and Identification System; NIST: National Institute of Standards and Technology; MS: Mass Spectrometry
Introduction
Titanium dioxide nanoparticles (TiO2 NPs), one of the most widely engineered nanoparticles has many industrial applications in the areas of cosmetics, drug delivery, pigment in paints, ceramics and pharmaceuticals [1,2]. Globally, TiO2 NPs are abundantly produced and widely used because of their smaller size and larger surface area with high stability and anticorrosion properties. Nowadays, a large number of nanoparticles are entering into our environment due to their usage due to advances in nanotechnology, thus, causing concern as they have potential impacts on human and environmental health [3]. The conventional toxicological experiments has revealed that, TiO2 NPs can produce free radicals with strong oxidizing ability which thus induce oxidative stress and finally resulted in apoptotic cell death, fibrosis, DNA damage and pulmonary inflammation [4-6].
In recent years, metabolomics has been shown as a valuable tool to identify and quantify the global changes in small molecular weight metabolites (amino acids, organic acids, sugar, fatty acids etc.,) of an intra-cellular system to therapeutic intervention or toxicant and diseases [7]. Thus, metabolomics is considered as a potential tool in functional genomics, disease diagnosis, toxicology and pharmacology research [8,9]. This approach has been successfully used in toxicological sciences to understand the mechanism of action and to identify the biochemical responses to toxicant exposure.
Nuclear magnetic resonance (NMR) and mass spectrometry (MS) are considered to be two complementary analytical platforms to study the metabolic responses in any organism [10,11]. The separation efficiency and identification potential of mass spectrometry based metabolomics have improved by coupling it with separation techniques like gas and liquid chromatography [12-15]. The gas chromatography-mass spectrometry (GC-MS) based metabolomics has various advantages over other hyphenated analytical techniques which include, high chromatographic resolution, analyte-specific detection and quantification as well as capability to identify unknowns made it a suitable tool for metabolomics in the fields of toxicity and biomarker discovery. Therefore, the study has been carried out to evaluate the molecular events following oral dose of nanosized and bulk sized TiO2 particles in Swiss Albino mice at three different doses 300, 600 and 1200 mg/kg body weight for 7 and 14 days by using GC-MS combined with pattern recognition approaches. Serum biochemistry and haematology tests were also performed.
Materials and Methods
Chemicals and reagents
All chemicals used were analytical grade. Methoxyamine hydrochloride, N-methyl-N-trimethylsilyl trifluoracetamide (MSTFA) and all standards were procured from Sigma- Aldrich (St. Lous, MO, USA). Methanol was obtained from Sigma Aldrich (St. Lous, MO, USA). The ultra-pure water was prepared by RiOsTM water purification system (Millipore, Billerica, MA, USA). IMECO ULTRA SONICS (Bombay, India) was used as sonicator. Heto GD-2 maxi dry plus (Germany) was used as lyophilizer.
Particle characterization
A stock solution of 1mg/ml of TiO2 NP in Milli-Q water was prepared and subjected to 15 min ultrasonic vibration (Sonics & Material Inc.) for dispersion. Furthermore, the surface morphology of the TiO2 NP was confirmed by using a scanning electron microscope (SEM with EDAX – Apollo XL, FEI, Eindhoven, Netherlands).
Animal selection
The present study was carried out on male and female Swiss Albino mice weighing 25-30gm. The animals were housed in polypropylene cages with stainless steel grids under optimal conditions (humidity 50 ±10%), temperature 22 ±3°C and light intensity 12-h light/dark cycle). Animals were provided with fed water and standard pellet diet ad libitum. The study protocol was approved by institutional ethics committee at CSIR-IITR.
Experiment design
Adult female and male mice were divided into 13 groups ((six groups for 7 days, six groups for 14 days and one control groups). Each group consists of 5 male and 5 female animals. These groups were given 300, 600, and 1200 mg/kg TiO2 NP and TiO2 BP administered by a single oral gavage according to the Organization for Economic Co-operation and Development, 420 (OECD, 1992) to Swiss Albino female and male mice for 7 and 14 days. Body weight and clinical signs of toxicity were recorded throughout the period of experiment [16,17].
Sign of toxicity and mortality
Signs of toxicity such as diarrhea and body weight loss were observed once daily throughout the experiment. After 7 and 14 days of dosing mice were sacrificed and blood was collected in 10% ethylene diamine tetra acetic acid (EDTA) anticoagulant containing tubes for hematology analysis and non-oxalate tubes for the separation of serum for metabolomic and biochemistry analysis.
Clinical biochemistry
The clinical biochemistry parameter of serum samples were carried out with fully automated biochemical analyzer (Clinical chemistry analyzer Randox-daytona UK) using standard kits. The following parameters were tested: glucose (GLU), creatinine (CREA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), triglycerides (TG), alkaline phosphate (ALP), uric acid (URCA) and cholesterol (CHOL).
Hematological parameters
Blood collected in 10% EDTA was analyzed for white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), hematocrit (HCT), mean cell volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT), neutrophils (NEUT) lymphocyte (LYM), monocyte (MONO), eosinophils (EO) and basophils (BASO) through automated cell counter Hematology analyzer (Sysmex XT-2000iV Analyzer-IDEX America) using standard kits.
Metabolite extraction and derivatization
Extraction of metabolites from serum and derivatization was implemented in a similar approach as previously described with little modifications [18]. Serum metabolites were extracted by adding 800μl of MeOH (80%, v/v) for deproteinization. After vortexing, samples were centrifuged at 10,000rpm for 15min. Then the extraction procedure with 80% MeOH was repeated thrice and then all supernatants were pooled. The pooled extract was freeze dried using Scanvac system (Labogene, Denmark). To the resultant residue, an amount of 40μl of methoxyamine hydrochloride was added and mixed vigorously for 1min and then incubated at 65°C for 30min. To this, 90μl of N-methyl- N-(trimethylsilyl)-trifluoroacetamide was added and incubated at 65°C for 1hr under agitation using a thermo mixer (BR BIOCHEM Life sciences, India). Samples were made up to the volume of 400μl using hexane for further analysis using GC-MS.
GC-MS instrumentation and data acquisition
The GC-MS analysis was performed for metabolomics as previously described in studies [19]. Metabolite profiling was performed on Trace GC ultra (Thermo Scientific, FL, USA) coupled to TSQ Quantum XLS mass spectrometer (Thermo Scientific, FL, USA). TG-5MS fused silica capillary column (30m x 250μm i.d; Thermo Scientific), chemically bonded with 5% phenyl 95% methyl polysiloxane cross linked stationary phase (0.25μm film thickness) was utilized to separate the peaks. GC injector was used in split less mode. The injector temperature was set at 2600C. Helium, the carrier gas, was maintained at a constant flow rate of 1.1ml/min during the analysis. The column temperature was initially kept at 650C for 2min, then ramped to 2300C at a rate of 60C/min and then finally increased to 2900C at a rate of 100C/min, where it was held for 20min. The interface temperature and ion source were set at 2900C and 2200C respectively. Electron impact ionization (EI+) mode was used for mass detection with electron energy of 70eV. Mass spectra were acquired with a scan range of m/z 45-800. Solvent delay was set at 7min. The sample volume of 1μl was injected in GC-MS for analysis [19].
Data pre-processing
Serum used for metabolomic analysis as a single batch in random order. AMDIS software (version 2.0) was used to identify the metabolites in serum. The mass spectra of all the detected compounds were compared with spectra in NIST library (version 2.0) or standards for confirmation. All GC-MS raw data files were exported into Net CDF format using XCalibur software (Thermo Fisher Scientific, FL, USA).
Deconvolution of the Net CDF format files was performed using the XCMS software [20]. The data was arranged on a three dimensional matrix consisting of arbitrary peak index (RT-m/z pair), sample names and peak area. The total area normalization was performed in order to reduce the systematic biases within the experiment. Normalized data was used for multivariate analysis to remove the offsets and adjust the importance of high and low abundance metabolites to an equal level.
Multivariate pattern recognition analysis
Metabo Analyst tool was used for data processing and statistical analysis [21]. To identify the differential metabolites that account for the separation between groups, supervised PLS-DA was applied. PLS-DA model was validated using the leave one out cross validation method [22]. Quality of model is assessed on R2 and Q2 scores [23]. Further, model validation was performed by 500 times permutation tests [24]. Metabolites with variable importance in projection (VIP) values of greater than 1 were taken as potential marker metabolites.
Results and Discussion
In-life parameters
Oral administration of TiO2NP at 300 and 600 mg/kg did not produce any signs of toxicity and mortality during 7 and 14 days exposure in both male and female mice. However, there was significant reduction in body weight of animals at high dose (1200mg/kg) at 14 days along with significant toxicity symptoms (Table 1).
Dose (mg/kg)
Body weight (g)
Total deaths
Signs of toxicity
0 days
7days
14 days
Female
Control (n=5)
25.3±0.8
27.3±0.8
31.8±0.8
0
Nil
300 (n=5)
26.2±0.7
28.3±0.7
32.2±0.7
0
Nil
600 (n=5)
25.4±0.6
27.5±0.6
33.5±0.8
0
Nil
1200 (n=5)
27.6±0.7
28.9±0.7
26.2±0.9
0
Diarrhea, weight loss
Male
Control (n=5)
25.8±0.8
26.9±0.6
32.8±0.7
0
Nil
300 (n=5)
25.7±0.9
26.7±0.7
33.2±0.9
0
Nil
600 (n=5)
26.6±0.7
27.4±0.3
33.5±0.6
0
Nil
1200 (n=5)
26.8±0.5
28.6±0.6
25.2±0.5
0
Diarrhea, weight loss
Table 1: Morbidity and mortality in mice after orally administered TiO2 NPs.
Characterization of TiO2 NPs
Characterization of NPs is one of the critical factors responsible for nanoparticles property and their mechanism of cellular interaction. We also measure the average size of NPs by scanning electron microscope (SEM). It was ranged from 16.03 to 22.05 nm (Figure 1).
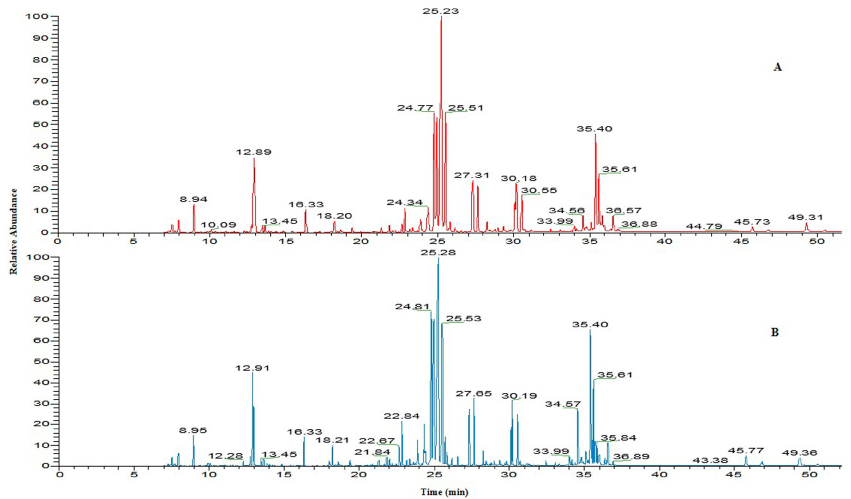
Figure 1: SEM image of TiO2 NP (< 25 nm).
Animal observation and body weight
Adverse signs, symptoms and mortality were not observed after 7 and 14 days of oral treatment with 300, 600 and 1200 mg/kg BW of nanosized and bulk sized TiO2 particles in mice. However, mice treated with high dose (1200mg/kg) of TiO2NPs showed some irritation, dullness and the significant reduction in feed intake, body weight gain.
Clinical biochemistry
The results of serum biochemical parameters of female and male mice after orally administered TiO2 NP for 14 days were shown in Table 2. Serum biochemistry parameters such as Glucose, AST, ALT, triglycerides and alkaline phosphate were significantly changed in both female and male mice exposed to TiO2 NP at high concentration (1200mg/kg) dose as compared to controls for 14 days. No changes were observed in biochemistry parameters after exposure to TiO2NP for 7 days. In the study, we found increased levels of serum enzymes AST, ALT and alkaline phosphatase in treated mice at high concentration (1200mg/kg) of TiO2 NP at 14 days. It is considered to be potential biomarker of liver damage. Therefore, our data suggest that liver damage might have occurred in mice due to exposure of TiO2 NPs.
TiO2 NP(mg/kg)
Parameters
Control
600
1200
2400
600
1200
2400
Female
Male
GLU (mg/dL)
120.47
±7.51
125.00
±2.65
127.67
±6.12
127.67
±5.78
129.67
±4.48
115.33
±2.02
105.33
±1.85*
CREA (mg/dL)
0.37
±0.06
0.40
±0.06
0.39
±0.10
0.38
±0.03
0.43
±0.06
0.48
±0.15
0.37
±0.03
AST (μ/L)
176.00
±23.26
180.00
±7.94
198.33
±9.21
210.00
±17.50
251.33
±12.57
277.67
±20.51
298.67
±9.83*
ALT (μ/L)
177.00
±4.93
182.33
±16.22
178.00
±3.21
164.10
±0.95
175.67
±4.41
190.33
±3.67
219.33
±4.70*
TP (g/dL)
6.37
±0.45
4.27
±0.09
4.53
±0.18
5.03
±0.22
4.50
±0.35
5.20
±0.04
5.37
±13.81
TG (mg/dL)
50.67
±2.60
56.67
±7.54
54.00
±6.51
57.00
±2.52
53.63
±0.35
51.33
±3.38
44.33
±5.69*
ALP (μ/L)
373.67
±38.20
345.67
±6.74
354.67
±12.33
354.67
±0.33
382.00
±10.26
409.67
±46.96
416.33
±16.49*
URCA (mg/dL)
1.37
±0.15
0.57
±0.18
0.50
±0.15
0.83
±0.28
1.43
±0.18
14.33
±1.20
5.77
±4.11
CHOL (mg/dL)
60.67
±6.23
61.67
±0.33
58.67
±5.36
55.33
±1.76
61.00
±7.81
58.67
±3.38
40.00
±1.00
GLU (mg/dL)
120.47
±7.51
125.00
±2.65
127.67
±6.12
127.67
±5.78
129.67
±4.48
115.33
±2.02
105.33
±1.85*
*Significant at the level of p < 0.05.
Table 2: Biochemical data of mice orally administered TiO2NP for 14 days.
Haematological parameters
There were no significant changes in haematological parameters in both gender orally administered to TiO2 NP and TiO2 BP (300,600,1200 mg/kg) for 7 days. However, a significant changes (p<0.05) were noted in WBC, HCT, NEUT and BASO in animals after exposed to TiO2 NP (1200mg/kg) as compared to control for 14 days (Table 3). WBC, HCT, NEUT and BASO significant increased in TiO2 NP treated mice at high dose (1200mg/kg) for 14 days, suggesting that nanosized TiO2 particles may induce inflammation and it may also generate oxidative stress via activation of oxidative stress responsive transcription factors [25]. Therefore increased level of some haematology parameters like WBC resulting from administration of TiO2 NPs may induce oxidative stress. Earlier studies also reported that TiO2 NP can produce free radicals which can exert a strong oxidizing ability and produce oxidative stress in rodents [26-29].
TiO2 NP (mg/kg)
Parameters
Control
600
1200
2400
600
1200
2400
Female
Male
WBC (103/μL)
14.07
±4.81
17.04
±0.76
16.08
±0.86
15.02
±0.91
16.36
±0.75
19.99
±3.71
26.91
±2.86
RBC (106/μL)
5.93
±0.45
6.19
±0.31
5.17
±0.45
5.67
±0.47
5.64
±0.52
6.15
±0.69
6.46
±0.64
Hb (g/dl)
11.93
±0.55
10.80
±1.51
11.37
±2.17
13.33
±0.88
10.13
±0.95
13.20
±0.80
10.83
±2.00
HCT (%)
37.97
±1.55
37.90
±1.42
32.70
±3.08
39.60
±1.80
31.67
±3.01
33.23
±1.52
29.20
±5.90*
MCV (fl)
64.50
±4.22
62.07
±2.99
67.47
±4.21
81.50
±6.21
62.93
±0.44
63.00
±5.03
57.50
±1.12
NEUT (%)
9.97
±1.93
10.00
±2.98
13.97
±3.25
14.87
±4.79
15.20
±2.55
14.67
±2.35
24.43
±1.63*
EO (%)
0.90
±0.15
1.17
±1.82
1.23
±052
1.97
±0.32
1.87
±0.91
1.90
±2.41
0.77
±0.12
BASO (%)
0.83
±0.55
0.67
±0.33
1.00
±0.31
0.80
±0.40
0.63
±0.15
0.30
±0.10
1.03
±0.60*
LYM (%)
84.03
±3.68
64.77
±1.34
79.03
±3.81
68.77
±8.10
81.50
±4.25
61.73
±12.09
108.00
±10.75*
MONO (%)
4.27
±1.30
5.07
±0.75
5.13
±0.29
4.07
±0.39
3.80
±0.76
3.40
±0.64
4.23
±1.57
*Significant at the level of p < 0.05.
Table 3: Hematology data of mice orally administered TiO2 NP for 14 days.
Metabolic responses of mice after exposure to TiO2 NP
All mice were survived in the experimental conditions and no mortality was found in treated group of mice after exposure for a period of 7 and 14 days. The results of metabolomic studies were carried out at three different doses 300, 600, 1200 mg/kg BW of TiO2 NP respectively. Figure 2 shows that a representative total ion chromatogram of serum of control and exposed mice at high concentration (1200mg/kg) of TiO2 NP.

Figure 2: The total ion chromatogram of serum samples of (A) control; (B) 1200 mg/kg TiO2 NP exposed mice.
Multivariate analysis was performed to reduce the data to low dimensional space, where discrimination of metabolic profiles between sample classes can be modelled. Supervised PLS-DA was performed to find a small number of linear combinations of the original variables, which was predictive for the class membership and that, described most of the variability of the metabolic profiles of control and exposed samples. PLS-DA loadings plots were used to identify the metabolites that were responsible for the observed separation between scores of the control and treated mice. The PLS-DA results were displayed as scores plots indicating scatter of samples, which indicate similar metabolomic compositions when clustered together and compositionally different metabolomic compositions when dispersed. In female mice, the supervised PLSDA model obtained from GC-MS analysis of the samples revealed that the general structure of the complete data set, in which two components cumulatively accounted for 35.8% of the total variance with Component 1 (C1) explained 23.6% and Component 2 (C2) explained 12.2% (Figure 3A).
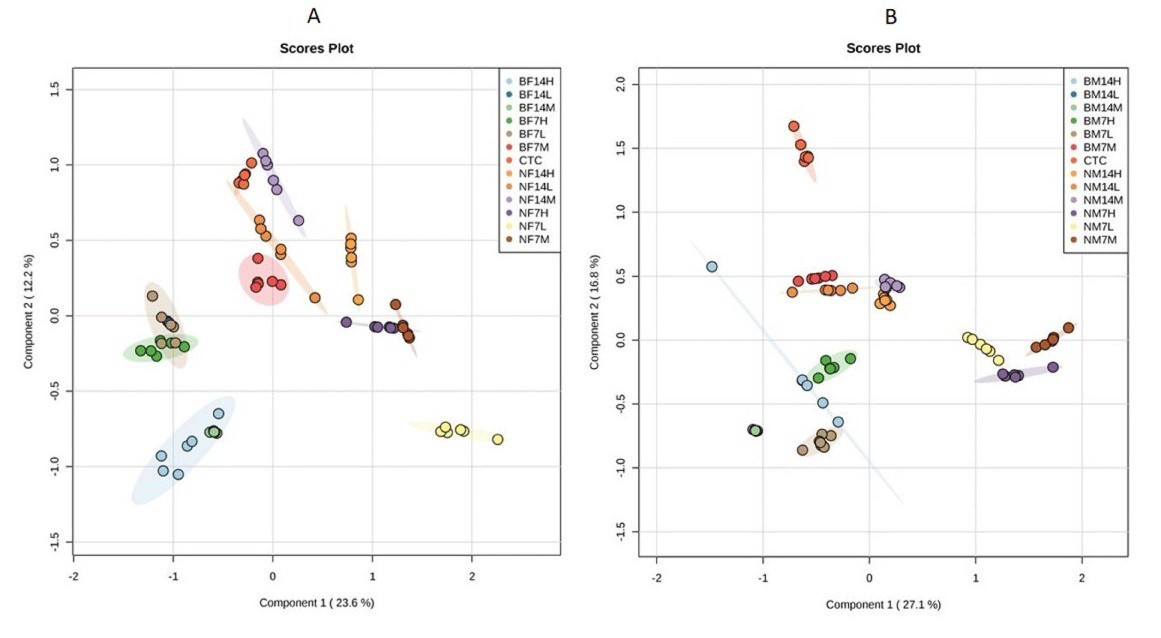
Figure 3: Partial least square discriminate analysis (PLS-DA) scores plot of first component Vs second component for (A) female mice; (B) male mice extracts
showing the separation of control group, from TiO2NP and TiO2 BP exposed mice for 7 and 14 days at concentration of 300 mg/kg (L; Low dose), 600 mg/kg (M;
Medium dose), 1200 mg/kg (H; High dose). BF14; Bulk particle in female mice for 14 days, BF7; Bulk particle in female for 7 days, CTC; control, BM14; Bulk particle
in male mice for 14 days, BM7; Bulk particle in male mice for 7 days, NF14; Nanoparticle in female mice for 14 days, NF7; Nanoparticle in female mice for 7 days,
NM14; Nanoparticle in male mice for 14 days, NF7; Nanoparticle in male mice for 7 days.
In male mice, two components cumulatively accounted for 43.9% of the total variance with C1 explained 27.1% and C2 explained 16.8% of the total variance respectively (Figure 3B). In both male and female mice, clear clusters were identified in PLS-DA scores plot. PLS-DA scores plot showed that, there was significant separation (p<0.05) among control and TiO2 NP and TiO2 BP treated group of mice along first component. A clear, linear dose trend with separation becoming more obvious the greater the dose (Figure 3). According to obtained results from PLS-DA, it is clearly suggested that changes of metabolic responses in treated mice were concentration dependent.
Visual examination of PLS-DA score plots is not a reliable method for determining predictive power. Therefore internal cross validation was performed to find out the predictive accuracy and fit of the polynomial model. The cumulative values of PLS-DA model with R2Xcum = 0.349, R²Ycum = 0.431, Q²Xcum = 0.297, Q²Ycum = 0.352 shows good fit of the model in female mice (Figure 4A). The cumulative values of PLS-DA model with R²Xcum = 0.474, R²Ycum = 0.674, Q²Xcum = 0.388, Q²Ycum = 0.599 shows good fit of the model in male mice (Figure 4B). To assess the statistical significance of these apparently highly predictive multivariate models, permutation testing was conducted. The supervised models were further validated with 1000 times permutation tests (p<0.001) (Figure 4C).
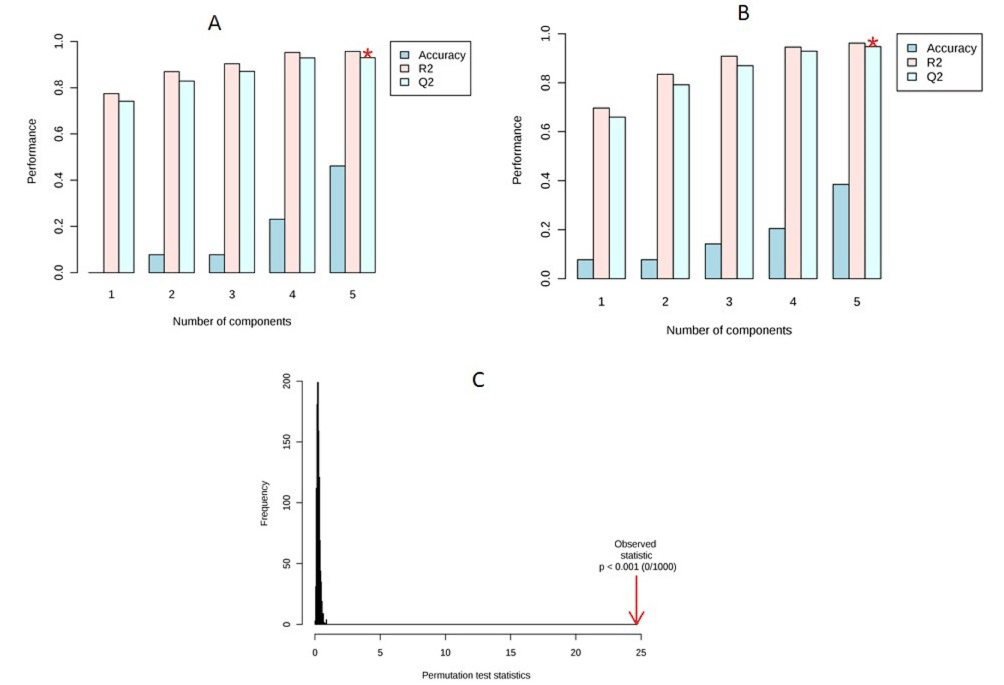
Figure 4: (A) Cross validation of female mice; (B) Cross validation of male mice; (C) Permutation analysis of PLS-DA models derived fromTiO2 exposed and
controls of female and male mice.
Among all the differential variables selected according to the VIP values from the PLS-DA model (VIP score greater than 1), 15 metabolites in female and 17 metabolites in male mice were found in treated group respectively showed in Table 4 and Table 5. In present study, glucose levels were down regulated in serum of treated groups of TiO2 NP in comparison to control. This down regulation in the level of glucose suggested being as results of increase in the energy requirements of mice exposed to TiO2 NP. These results showed that disturbance of energy metabolism after exposure to TiO2 NP [30].
S.No
CheBI ID
Name of metabolite
RT
(min)
m/z
VIP score
p- value
Content
variance
1
CID:22600307
Silanamine
15.16
188
1.0218
5.10E-30
Decreased
2
CHEBI:6650
Malic acid
17.92
245
1.3999
8.73E-34
Increased
3
CHEBI: 18183
Pyroglutamic acid
18.47
156
1.5802
9.20E-30
Decreased
4
CHEBI:30915
Ketoglutaric acid
19.62
288
1.0597
1.03E-17
Decreased
5
CHEBI:18257
Ornithine
22.81
174
1.6673
8.25E-35
Increased
6
CHEBI:30887
Isocitric acid
24.21
465
1.7452
1.27E-27
Increased
7
CHEBI:28875
Tetradecanoic acid
24.30
285
1.7662
4.85E-26
Increased
8
CHEBI: 18186
Tyrosine
25.05
179
1.554
6.85E-24
Increased
9
CHEBI:28757
Glucose
25.36
217
1.054
2.01E-14
Decreased
10
CHEBI:59265
Palmitelaidic acid
27.14
311
1.5171
3.51E-23
Decreased
11
CHEBI:17351
Linoleic acid
28.21
95
1.5967
7.14E-38
Increased
12
CHEBI:16196
Oleic acid
28.23
96
1.4572
1.74E-38
Increased
13
CHEBI:28842
Stearic acid
28.69
298
1.7235
2.34E-57
Decreased
14
CHEBI:32265
Heptadecanoic acid
28.99
327
1.0286
9.01E-35
Decreased
15
CHEBI:15843
Arachidonic acid
30.51
91
1.4786
3.87E-34
Increased
Table 4: Differential metabolites in female mice between TiO2 NP exposed and control groups.
S.No
CheBI ID
Name of metabolite
RT
(min)
m/z
VIP
score
p- value
Content
variance
1
CHEBI:28358
Lactic acid
8.46
190
1.2567
5.22E-31
Increased
2
CHEBI:20067
3-Hydroxybutyric acid
10.65
130
1.4375
3.84E-39
Decreased
3
CHEBI:15428
Glycine
13.98
174
1.2489
4.23E-24
Increased
4
CHEBI:18012
Fumaric acid
14.76
245
1.4406
3.38E-37
Increased
5
CHEBI:17822
Serine
15.24
204
1.4898
7.98E-23
Decreased
6
CHEBI:22660
Aspartic acid
16.50
168
1.285
6.53E-34
Decreased
7
CHEBI:6650
Malic acid
17.92
245
1.0865
6.20E-31
Increased
8
CHEBI: 18183
Pyroglutamic acid
18.47
156
1.7314
8.08E-46
Decreased
9
CHEBI:30915
Ketoglutaric acid
19.62
288
1.5741
1.22E-30
Decreased
10
CHEMSPIDER: 10709816
Phosphopyruvate
19.91
211
2.0992
4.90E-38
Increased
11
CHEBI:26078
Phosphoric acid
22.62
445
1.0075
6.59E-18
Increased
12
CHEBI:18257
Ornithine
22.81
174
2.1934
2.52E-40
Increased
13
CHEBI:30887
Isocitric acid
24.21
465
1.5379
7.77E-36
Increased
14
CHEBI:28875
Tetradecanoic acid
24.30
285
1.7954
1.52E-47
Increased
15
CHEBI:59265
Palmitelaidic acid
25.05
179
1.4911
1.75E-30
Decreased
16
CHEBI:33198
Gluconic acid
27.14
311
1.2888
8.59E-28
Decreased
17
HMDB41480
Cis-13-octadecenoic acid
30.04
339
1.6197
4.41E-27
Decreased
Table 5: Differential metabolites in male mices between TiO2 NP exposed and control groups.
We found, lactic, citric, malic and fumaric acids were significantly (p<0.05) increased in serum after exposed to TiO2 NPs in comparison to control group. Lactic acid acts as a precursor in Krebs cycle (TCA cycle). Citric, malic and fumaric acids were said to be key products in TCA cycle. Citric and lactic acids were closely related to glycolysis and energy metabolic pathway in aerobic condition (Figure 6) [31]. The abnormality of citric and lactic acids may be due to induced oxidative stress in the mice due to TiO2 NPs, which indirectly causes metabolic perturbations in energy metabolism through glycolysis process.
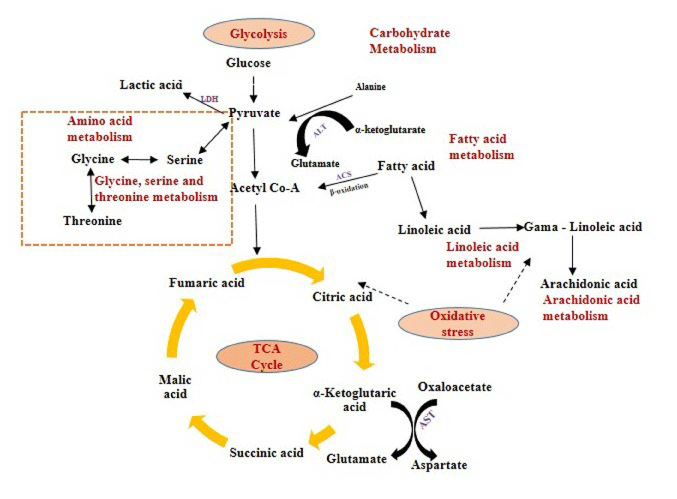
Figure 6: Schematic representation of the affected pathways in Swiss Albino mice due to TiO2 NP exposure.
In the study, the level of 3-hydroxybutyric acid was significantly (p<0.05) decreased in exposed mice after treatment of TiO2 NP. Our findings were supported by previous study where, 3-hydroxybutyric acid said to be an end product of fatty acid beta-oxidation pathway produced mainly in the liver and used as an alternative energy source during tissue damage.
The levels of tyrosine and glycine were significantly increased and the levels of serine and aspartic were significantly decreased in treated mice in comparison to control. Glycine is derived from serine with the help of serine hydroxymethyl transferase enzyme and it acts as a cytoprotective agent by scavenging the reactive oxygen species (ROS) and inhibits inflammatory response due to oxidative stress induced by the NPs. Increased serine levels in serum have been related to cellular proliferation and it may decline the cell injury caused by the reactive oxygen species (ROS).
The affected biological pathways in mice after exposure to TiO2 NP were obtained by using MetPa, a free web-based tool [32]. MetPa reveals importantly, 14 metabolic pathways in mice which showed in Table 6 (Figure 5). Schematic representation of the affected pathways in Swiss Albino mice due to TiO2 NP exposure showed in Figure 6. Fatty acids are important source of energy. In this study, tetradecanoic, arachidonic, oleic and linoleic acids were significantly increased while, palmitelaidic, stearic, heptadecanoic and cis-13- octadecenoic acids were significantly decreased in serum after exposed to TiO2 NP. MetPA results showed two pathways related to fatty acid metabolism such as linoleic acid metabolism and arachidonic acid metabolism was significantly affected in treated mice. GC-MS based metabolomic approach can be used as a potential analytical tool to understand the systemic toxicity of NPs in model organisms [33-35].
S.No
Pathway
Metabolite hits
Impact
1
Citrate cycle (TCA cycle)
3
0.1134
2
Glutathione metabolism
3
0.02004
3
Methane metabolism
2
0.4
4
Aminoacyl-tRNA biosynthesis
4
0.12903
5
Arginine and proline metabolism
3
0.12736
6
Alanine, aspartate and glutamate metabolism
2
0.1962
7
Phenylalanine, tyrosine and tryptophan
biosynthesis
1
0.5
8
Glycine, serine and threonine metabolism
2
0.50732
9
Linoleic acid metabolism
1
1
10
Tyrosine metabolism
2
0.14045
11
Starch and sucrose metabolism
1
0.00233
12
Cysteine and methionine metabolism
1
0.02533
13
Arachidonic acid metabolism
1
0.32601
14
Primary bile acid biosynthesis
1
0.02976
Table 1: Laboratory values at hospital admission.
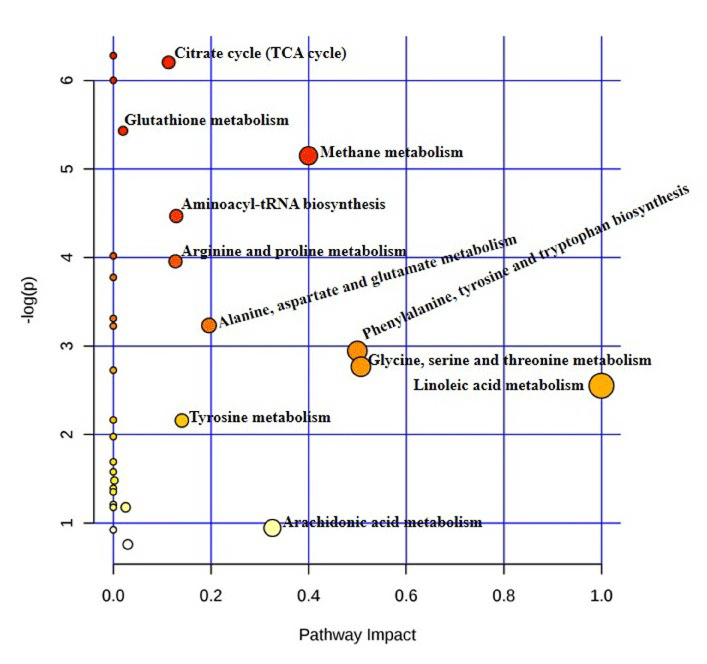
Figure 5: Summary of the pathway analysis of mice exposed to TiO2NP with MetPa.
Conclusion
In conclusion, this is the first comparative metabolomics study on both male and female mice using GC-MS based metabolomics approach to identify the perturbations in metabolic profile after exposed to TiO2NP. Result of multivariate pattern recognition analysis showed clear molecular group responses to TiO2NP at metabolic level. The study revealed that the metabolic perturbations occurred in TiO2 NP exposed mice are both concentration and time dependent. The integrated data set indicated clear indication of oxidative stress and alteration of energy metabolism as a result of TiO2NP exposure. Moreover, our results support the fact that metabolomics approach is a more sensitive than serum biochemistry and haematology analysis. These results showed that metabolomics have a great potential to emerge as a powerful tool to comprehensively understand the toxicity of NPs in model organisms.
Acknowledgment
The authors are thankful to Directors of CSIR-IITR, Lucknow and CSIR-IICT, Hyderabad for scientific discussions and providing the necessary infrastructural facilities to carry out this work. The author(s) received financial support from SERB, New Delhi and CSIR, New Delhi through fast track scheme and NanoSHE (BSC0112) network projects to carry out this research work.
References
- Hayauchi Y. Precise colour determination method for tablets – an application of instrumental colour measurement in the pharmaceutical development. Pharmeur Sci Notes. 2005; 1: 21-26.
- Salthammer T, Fuhrmann F. Photocatalytic surface reactions on indoor wall paint. Environ. Sci. Technol. 2007; 41: 6573-6578.
- Federici G, Shaw BJ, Handy RD. Toxicology of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquat Toxicol. 2007; 84: 415-430.
- Zhang DD, Hartsky MA, Warheit DB. Time course of quartz and TiO (2) particle-induced pulmonary inflammation and neutrophil apoptotic responses in rats. Exp Lung Re. 2002; 8: 641-670.
- Rehn B, Seiler F, Rehn S, Bruch J, Maier M. Investigations on the inflammatory and genotoxic lung effects of two types of titanium dioxide: untreated and surface treated. Toxicol Appl Pharmacol. 2003; 189: 84-95.
- Baan RA. Carcinogenic hazards from inhaled carbon black, titanium dioxide, and talc not containing asbestos or asbestiform fibers: recent evaluations by an IARC Monographs Working Group. Inhal Toxicol. 2007; 19: 213-218.
- Ryan D, Robards K. Metabolomics: the greatest omics of them all? Anal chem. 2006; 78: 7954-7958.
- Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of Metabolomics in Oncology: a review. Clin Cancer Res. 2009; 15: 431-440.
- Johnson CH, Patterson AD, Idle JR, Gonzalez FJ. Xenobiotic Metabolomics: major impact on the Metabolome. Annu Rev Pharmacol and Toxicol. 2012; 52: 37-56.
- Dettemer K, Aronov PA, Hammock BD. Mass spectrometry-based metabolomics. Mass Spectrum Rev. 2007; 1: 51-56.
- Lindon JC, Nicholson JK, Holmes E, Everett JR. Metabonomics: metabolic process studied by NMR spectroscopy of biofluids. Concepts Magn Reson. 2006; 12: 289-320.
- Mudiam MK, Ch R, Saxena PN. Gas chromatography-mass spectrometry based metabolomic approach for optimization and toxicity evaluation of earthworm sub-lethal responses to carbofuran. PLoS One. 2008; 8: e81077.
- Bujak R, Garcia-Alvarez A, Ruperez FJ, Nuno-Ayala M, Garcia, Ruiz- Cabello J. Metabolomics reveals metabolite changes in acute pulmonary metabolism. J Protome Res. 2014; 13: 805-816.
- Hu ZP, Browne ER, Liu T, Angel TE, Ho PC, Chan EC. Metabolomic profiling of TASTPM transgenic Alzheimer’s disease mouse model. J Proteome Res. 2012; 11: 5903-5913.
- Koek MM, Jellema RH, Greef JVD, Tas AC, Hankemeier T. Quantitative metabolomics based on gas chromatography mass spectrometry: status and perspectives. Metabolomics. 2011; 7: 307-328.
- OECD. OECD Guidelines for Testing of Chemicals. No 420: Acute Oral Toxicity-fixed Dose Method. Organisation for Economic Co-operation and Development. Paris. 2001.
- Dasal V, Venugopal R, Reddy GA. Oral toxic exposure of titanium dioxide nanoparticles on serum biochemical changes in adult male Wistar rats. Nanomedicine. 2014; 2: 46-53.
- Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc. 2011; 6: 1060-1083.
- Ch R, Sonane M, Satish A, Mudiam MK. Metabolomics reveals the perturbations in the metabolome of Caenorhabditis elegans exposed to titanium dioxide nanoparticles. Nanotoxicology. 2015; 9: 994-1004.
- Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G. XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem. 2012; 84: 5035-5039.
- Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009; 37: W652-W660.
- Rubingh CM, Bijlsma S, Derks EP. Assessing the performance of statistical validation tools for megavariate metabolomics data. Metabolomics. 2006; 2: 53-61.
- Broadhurst DI, Kell DB. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics. 2006; 2: 171-196.
- Xuan J, Pan G, Qiu Y. Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. Proteome Res. 2011; 10: 5433-5444.
- Dalton JS, Janes PA, Jones NG, Nicholson JA, Hallam KR, Allen GC. Photocatalytic oxidation of NOx gases using TiO2: a surface spectroscopic approach. Environmen Pollution. 2002; 120: 415-422.
- Cho I, Park J, Kim Y. Oxidative degradation and toxicity reduction of trichloroethylene (TCE) in water using TiO2/solar light: comparative study of TiO2 slurry and immobilized systems. Environ Sci Health A. 2005; 40: 1033- 1044.
- Warheit DB, Brock WJ, Lee KP, Webb TR, Reed KL. Comparative pulmonary toxicity inhalation and instillation studies with different TiO2 particle formulations: impact of surface treatments on particle toxicity. Toxicol Sci. 2005; 88: 514-524.
- Bermudez E, Mangum JB, Wong BA, Asgharian B, Hext PM, Warheit DB, et al. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol Sci. 2004; 77: 347-357.
- Gu N, Hu H, Guo Q, Jin S, Wang C, Oh Y, et al. Effects of oral administration of titanium dioxide fine-sized particles on plasma glucose in mice. Food Chem Toxicol. 2015; 86: 124-131.
- Tang M, Zhang T, Xue Y, Wang S, Huang M, Yang Y, et al. Dose dependent in vivo metabolic characteristics of Titanium dioxide nanoparticles. Nanosci Nanotechnol. 2010; 10: 8575-8583.
- Liu S, Xu L, Zhang T, Ren G, Yang Z. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology. 2010; 267: 172-177.
- Xia J, Wishart DS. MetPa: a web based metabolomics tool for pathway analysis and visualization. Bioinformatics. 2010; 26: 2342-2344.
- Griffin JL, Bollard ME. Metabonomics: it’s potential as a tool in toxicology for safety assessment and data integration. Curr Drug Metab. 2004; 5: 389–398.
- Coen M, Holmes E, Lindon JC, Nicholson JK. NMR-based metabolic profiling and metabonomic approaches to problems in molecular toxicology. Chem Res Toxicol. 2008; 21: 9-27.
- Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 2005; 18: 143-162.