
Review Article
Austin J Anal Pharm Chem. 2017; 4(3): 1092.
Photobleaching and Its Kinetics of Fluorescein Dyes in PVA Polymer Matrices: A Review
Islam MZ¹*, Noori A² and Paul SC¹
¹Department of Applied Chemistry & Chemical Engineering, Noakhali Science and Technology University, Bangladesh
²Departtment of Chemistry, University of Tasmania, Australia
*Corresponding author: Muhammad Zakarul Islam, Department of Applied Chemistry & Chemical Engineering, Noakhali Science and Technology University, Bangladesh
Received: November 30, 2017; Accepted: December 13, 2017; Published: December 20, 2017
Abstract
This review article aimed providing the theory and mechanism underlying the photobleaching process a most common limitation for fluorescence technique. This report also includes a brief discussion on the kinetics of photobleaching reaction. To discuss this, we choose a xenthene group dye (i.e. fluorescein, FL) embedded in polyvinyl alcohol, PVA (FL-PVA) matrices system. The photobleaching of FL-PVA depends on the fluorophore concentration and also with the temperature. A slower photobleaching process is observed at lower concentration of FL dyes and photobleaching kinetics can be described by mono-exponential. On the other hand, it is a faster process at high concentration (e.g. 0.1% FL in PVA). The photobleaching rate is usually increased with temperature. The photobleaching reaction kinetics is bi-exponential before the glass transition temperature (Tg) of PVA and it becomes mono-exponential above the same. The bi-exponential kinetics (T<Tg) indicates there has two different adsorbent media for guest dye. It is possible due to semi-crystalline nature of PVA. One is isolated by the PVA chain leads to a lower rate and other region allowing triplet-triplet and triplet-ground state dye-dye (D-D) interaction and a faster photobleaching is the consequence. Above Tg, although there has a remarkable increase of non-fluorescent process including photobleaching, internal conversion, intersystem crossing and bimolecular diffusion quenching, the PVA medium becomes viscous homogeneous and responsible for a single exponential kinetics.
Keywords: Fluorescein dye; Photobleaching; PVA polymer; dye-dye interaction; Glass transition temperature
Abbreviations
Tg: Glass Transition Temperature; FL: Fluorescein; PVA: Polyvinyl Alcohol; D-D: Dye-Dye Interaction; FL-PVA: Fluorescein Doped Polyvinyl Alcohol
Introduction
PVA (pure PVA or PVA blended with other polymers) thin films are often encountered on medical devices and as coating a drugs [1,2]. Use of polymers as micrometer thin films (<100) is widespread in biomedical applications such as local drug delivery [3,4], cell sheet engineering [5], microfluidic devices [6-9], bioadhesion mediators [9,10] and bioactuators [11-13]. If one uses the polymer as a thin film then one needs to know not only the thickness of the film but the physicochemical nature of the polymer. Fluorescence spectroscopy is a very powerful tool to monitor microscopic environmental changes in the polymer systems/ polymer thin films because of its high sensitivity and selectivity, high speed measurements and nondestructive nature [14]. The surrounding microenvironment can be responsible for changes in the absorption/emission intensity, shifting of band position, anisotropy and fluorescence lifetime [15-17]. Mainly there are three types of fluorescent probe systems used in polymer science [18]. 1) Some polymers contain intrinsic fluorophores within their structure [19]. 2) At the end/middle of the polymerization process fluorescent group can be introduced into the system by a covalent bond [20,21]. 3) Fluorophores can be doped or inserted into the polymer systems without the formation of covalent bonds (e.g. salicylic acid doped PVA [22]). Category 2 and 3 are known as external probes. FL-PVA thin film is used in this discussion. Bleaching refers to the irreversible conversion of a fluorophore or particle into a non-fluorescent entity. In most cases, this process is photoinduced (and called photobleaching) which ultimately causes the loss of emission or absorption intensity. This is a common phenomenon for fluorophores and ultimately limits practically all fluorescence techniques which may require high sensitivity, high signal rate i.e. all imaging techniques of fluorescence, single molecule fluorescence, fluorescence correlation spectroscopy (FCS).There are a number of chemical pathways by which a fluorophore is going to permanently photobleach. The photobleaching mechanism is yet not a well understood phenomenon. Particularly, it is more complex in solid polymer matrices. This review is aimed to discuss a detailed literature of photobleaching, its mechanism, and kinetics. Here we consider a xanthene group dyes (e.g. FL) doped PVA polymer matrices which was mostly studied in this area.
A general mechanism of photobleaching
Photobleaching reactions usually occur when fluorophores are in the singlet or triplet excited state (Figure 1). There are three types of photobleaching processes [23].
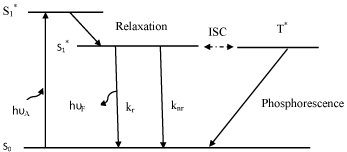
Figure 1: A very simplified form of Jablonski diagram to illustrate the
principles behind fluorescence spectroscopy. After absorption of appropriate
amount of energy, the molecule is electronically excited fromS0to S1. Losing
some of the energy by relaxation, the excited molecules then return to the
ground state by two means (i.e. radioactive and non-radioactive) with the
rate of kr and knr. It can also make an intersystem crossing (ISC) to the lowest
triplet excited state (T*). A triplet state molecule can return to the ground state
by emitting photon and the process is called phosphorescence. Or, it can also
be thermally activated, cross back to the singlet excited state, and then return
to the ground state by emitting delayed fluorescence. Photobleaching is
possible from the excited singlet and triplet state. Reproduced from ref. [17].
Unimolecular
The fluorophore molecules are photochemically fragmented to produce radical or ions (e.g. homo– or heterolytic dissociation reactions). This dissociation pathway is important for the fluorescent aromatic amino acids tyrosine and tryptophan [24].
Multiphoton photolysis
The fluorescence techniques, where high irradiance is used (i.e. Single Molecule Detection or Fluorescence Confocal microscopy) generally there has a greater probability of multi photon absorption. It thus leads to a high probability of photobleaching reaction due to the population of higher excited electronic states. In polar solvents (e.g. water), a subsequent heterolytic dissociation of fluorophore (M) forming a radical ion pair (M +e–) from a higher excited state is specially favoured. The two-photon excitation and the consequence higher excited singlet or triplet state ((1,3) M n ) can be achieved by the following: A successive absorption of photon via an intermediate state (either first excited singlet or triplet).
1. A successive absorption of photon via an intermediate state (either first excited singlet or triplet).
1 (1,3) (1,3) ( ) 0 1 hv hv n M ??? M ??? M ? M+e- Equation 1
1 2 1 ( ) 0 hv n M ??? M ? M+e- Equation 2
where the superscript indicates singlet (1) or triplet (3) state and subscript denotes the respective electronic state (e.g. 0 for ground, 1 for first and n for higher). For more details, the author would suggest ref. [23].
Bimolecular reaction
This type of reaction covers different photoinduced reactions proceeding via the formation of an encounter complex. It can usually takes place either from the first excited singlet or triplet state (Figure 1) and several types of chemical reaction involving energy transfer, electron transfer, proton transfer, and addition reaction can subsequently take place by the following:
(1,3) 1 M + R? X +Y or (1,3) 1 M + R?Z Equation 3
where, R is the solvent molecule, an added reactant (such as proton donor, quencher etc.), an impurities, another dye molecule or atmospheric oxygen.
One possible pathway of photobleaching from the first excited singlet state is: when there has been a large increase in dipole moment during electronic excitation, a greater degree of interaction with the micro-environment could occur and some of this would be irreversible and responsible for photobleaching. On the other hand, photobleaching from the excited triplet state, (ETS) can occur by several reaction pathways [25]. Once an ETS is formed from the singlet state, it may be depopulated by two pathways. Firstly and more importantly, the reaction of ETS with oxygen from the environment [26]. This reaction may involve electron/energy transfers from ETS to molecular oxygen. The electron transfer produces superoxide radical (O2 –) and a cationic non-fluorescent state. On the other hand, energy transfer from ETS to molecular oxygen (T* + 3O2 ? S + 1O2) produces a reactive singlet oxygen. This singlet oxygen is very reactive and immediately reacts with exposed group of fluorophores and produces irreversible non-fluorescent product [27]. And secondly, a fluorophore in the ETS itself is very reactive (triplet state has two unpaired electrons, which can act as a radical) and undergoes several irreversible reactions e.g. triplet quenching, electron transfer between two ETS, reaction between ETS and ground state fluorophore.
According to Talhavini et al. the following reaction may happen once an ETS is formed:
where S is the ground state, S* is the singlet excited state, T* is the triplet excited state, DH semi-reduced fluorophore, D is the semi-oxidized form of fluorophore and M is the electron/H donor group. The reaction a-c is the triplet quenching process and does not responsible for photobleaching. The reaction type d is very dangerous to occur the photobleaching and generally occur when the fluorophore concentration is high. In this case, fluorophore molecules are so close to each other that they can easily undergo interaction with the neighbouring fluorophore molecule. It was called as a dye-dye (DD) interaction. However, at low fluorophore concentration, reaction type e-g is generally occurred. Photobleaching can also be observed in an inert atmosphere where no oxygen is present. The mechanism involves the reaction between the triplet state of a fluorophore and the matrix molecules or impurities present in the local environment. This type of non-oxygen mediated photobleaching was reported by several authors for fluorescein [26,28-30].
Photobleaching of FL-PVA films
The photobleaching of xenthene dye is supposed to happen by either reductive or oxidative mechanisms and its mechanism depends on several factors such as presence of oxygen, the presence and concentration of electron donator groups, the dye concentration, and the temperature. Since photobleaching generally involves some form of chemical reaction and thus activation energy, it means that photobleaching is temperature dependant. Usually the photobleaching rate increases with temperature [31] because of the increase of reaction rates responsible for photobleaching. In addition, additional reaction pathways could be activated at higher temperature leading to greater photobleaching.
M Talhavini et al. [32] reported a temperature dependant photobleaching characteristics of FL-PVA polymer matrix. They observed decrease of intensity with three linear slope (at 130, 260- 270 and 330-350K) when they plotted temperature vs fluorescence intensity. In addition, at 315K a clear increase of intensity was reported. One possible mechanism for this increase of intensity could be presented here: the breaking of the intermolecular H-bonding between PVA polymers at higher temperature (i.e. at 315K) lead to an increase the side chain mobility of polymer [33]. Finally, a relaxation in PVA side chain was observed which involves segmental movement of the macromolecules which were not H-bonded. When the molecular diffusion was induced by the polymer relaxation process, the average distance between the two adjacent FL dye was increased and thus minimized self-quenching [34] and an increased fluorescence intensity is the consequence.
The temperature dependant photobleaching process in a polymer matrix is also dependent on the polymer glass transition temperature, Tg. For example, Dibbern-Brunelli et al. reported the temperature dependent photobleaching of FL doped into 87% - 89% hydrolysed PVA cast on a glass plate. The integrated emission intensity of FL-PVA decreased as a function of temperature. There was a clear inflexion in the plot observed at 350K which is the Tg of PVA. And after that a sharp decrease in intensity was measured which was associated with the increased mobility of polymer chains which caused quenching.
Wang et al. studied the temperature effect on the fluorescence properties of a 4-methyl-7-(2,3-epoxypropoxy) coumarin (MEC) doped PVA polymeric film matrix [25]. They did the experiment over a 273-333K temperature range and reported that fluorescence intensity and quantum yield decreased with the increase in temperature. A good linear correlation between KIC and 1/T was also reported within 273–323K temperature range for a fluorescein fluorophore in aqueous solution which had a KIC = 2.0 × 1011 exp[(- 5.5kcal/mol)/RT] s–1, where kIC stands for the IC rate constant. They explained these temperature dependant fluorescence properties by the photophysical and photochemical dye properties. The fluorescence intensity of MEC bearing PVA is mainly controlled by a radiation less temperature dependant process. Temperature increases collision between molecules and thus quenching of fluorescence intensity. Thermally activated ISC is also responsible for decreasing the quantum yield of fluorescence.
Photobleaching kinetics of FL-PVA
To the author’s knowledge, most of the photobleaching kinetics of fluorophores in polymer thin films systems have been described in literature are fluorophores of xanthene groups (specially fluorescein in PVA polymer matrices) [26,32,35]. This photobleaching kinetics in polymer matrix generally can be described by using two models: B model and exponential model. From B model one could get information about the decay constant of reaction and photobleaching quantum yield (for more details see [26,36] ). On the other hand, the probability of a fluorophore surviving a certain number of excitation cycles before being photobleached is usually exponentially distributed. The exponential model represents the decrease of fluorescence intensity over time and is used to determine the rate constant and activation energy of the active photobleaching reaction. It can be mathematically presented by
F i i I t C A t T - = + S Equation 5
where lF (t) is the time-dependant fluorescence intensity, proportional to the electronic ground state population, Ai is the pre-exponential factor proportional, C is the integration constant and ti is the lifetime defined as the time necessary for the emission to reach 1/e of the initial value. Using the rate constant value, one could determine the apparent activation energy by using Arrhenius equation (ln k = ln A – Ea/RT, where A is a frequency factor related to the relative contribution of corresponding rate constant, k is the rate constant, and Ea is the apparent activation energy). The associated preexponential factor is considered as a weighing factor.
The fitting of experimental photobleaching data to a specific exponential function depends on the fluorophore environments (e.g. fluorophore concentration, temperature etc). In general, a biexponential function can be interpreted by the presence of at least two different sorption sites for the guest molecule where the sorbed dye molecules should assume different spectroscopic, photophysical and photochemical properties. For example, photobleaching of 0.1% fluorescein in PVA is mono-exponentially fitted above the Tg of PVA whereas bi-exponentially fitted at temperatures below the Tg (77°C) [32]. PVA is a semi-crystalline polymer. The possible reason for this bi-exponential relationship is the existence of two different domains below the Tg in PVA. One region contains isolated fluorophores and is responsible for the lower rate of photobleaching. And the other contains closely located fluorophores (separated by Förster radii or less) which allow D-D interaction between two neighbouring molecules and this is responsible for the faster rate of bleaching. They also concluded that this faster process involved triplet- triplet and triplet-ground state fluorophore interactions [32]. However, above the Tg, these two domains are equalized and work as a homogeneous viscous medium. The kinetics of photobleaching then can be described by the single exponential function at above Tg [37,38]. The contribution of the normalised weight factor for these two rates is mostly dependent on temperature [37]. The pre-exponential faster process is predominant at higher temperature and an opposite phenomenon is observed at lower temperatures where the process is slower. For example, at 260K the Aslower=0.60 and Afaster =0.40.
Conclusion
The photobleaching properties of FL-PVA is complex and is a function of FL concentration and temperature of the medium. At high concentration, a heterogeneous distribution of FL-PVA film matrices is taking place, with some molecules isolated than others and residing either in the amorphous or at the interface between crystalline and amorphous domains of the semi crystalline PVA matrices. However, at lower concentration, FL is isolated with the PVA chain. The temperature dependant fluorescence intensity can be described as a polymer relaxation and either photophysical and photochemical processes of dye molecules in polymer matrices. The curve profiles for fluorescence intensity vs temperature should be a result of a convolution among photophysical processes concentration, bimolecular quenching, unimolecular rate processes, and photochemical processes decreasing the dye concentration and mobility of polymer chains. Some of these processes can be minimized at low concentration. The D-D interaction should be the case for higher concentration of fluorophore and specifically for reactive compounds which can form the dimer. When temperature is increased the photobleaching rate is increased and below the Tg it can provide two region and responsible for two rate. Above the Tg, the medium is worked as a homogeneous and the reaction kinetics is mono-exponential.
References
- Xu M, Zhang C, Luo Y, Xu L, Tao X, Wang Y, et al. Application and functional characterization of POVACOAT, a hydrophilic co-polymer poly (vinyl alcohol/ acrylic acid/methyl methacrylate) as a hot-melt extrusion carrier. Drug development and industrial pharmacy. 2014; 40: 126-135.
- Fujii T, Noami M, Tomita K, and Furuya Y. PVA copolymer: the new coating agent. Pharmaceutical Technology Europe. 2008; 20.
- HB. Restenosis: a challenge for pharmacology. Trends Pharmacol Sci, 2000; 21: 274–279.
- Lewis AL, Tolhurst LA and Stratford PW. Analysis of a phosphorylcholinebased polymer coating on a coronary stent pre- and post-implantation. Biomaterials. 2002; 23: 1697-1706.
- da Silva RMP, Mano JF and Reis RL. Smart thermoresponsive coatings and surfaces for tissue engineering: switching cell-material boundaries. Trends in biotechnology. 2007; 25: 577-583.
- Peterson SL, McDonald A, Gourley PL and Sasaki DY. Poly(dimethylsiloxane) thin films as biocompatible coatings for microfluidic devices: Cell culture and flow studies with glial cells. Journal of Biomedical Materials Research Part A. 2005; 72A: 10-18.
- Lahann JB, Mercedes Rodon, Teresa Lee, Jinwook Choi, Insung S, Jensen Klavs F, et al. Reactive polymer coatings: A platform for patterning proteins and mammalian cells onto a broad range of materials. Anal. Chem. 2003; 75: 2117–2122.
- Soper SA, Henry AC, Vaidya B, Galloway M, Wabuyele M and McCarley RL. Surface modification of polymer-based microfluidic devices. Analytica Chimica Acta. 2002; 470: 87-99.
- Prabhakar SKRaB. Bioadhesive Polymeric Platforms for Transmucosal Drug Delivery Systems – a Review. Tropical Journal of Pharmaceutical Research. 2010; 9: 91-104.
- Song J and Vancso GJ. Responsive Organometallic Polymer Grafts: Electrochemical Switching of Surface Properties and Current Mediation Behavior. Langmuir. 2011; 27: 6822-6829.
- Rajeswari Ravichandran SS, Jayarama Reddy Venugopal, Shayanti Mukherjee and Ramakrishna aS. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface. 2010.
- Yoshitake Akiyama aKI, b Yuji Furukawaa and Keisuke Morishima. Longterm and room temperature operable bioactuator powered by insect dorsal vessel tissue. 2009; 9: 140-144.
- Suchaneck G, Guenther M, Sorber J, Gerlach G, Arndt K-F, Deyneka A, et al. Plasma surface modification of hydrogel thin films. Surface and Coatings Technology. 2003; 174–175: 816-820.
- Abboud J-L and Notari R. Critical compilation of scales of solvent parameters. Part I. Pure, non-hydrogen bond donor solvents. Pure and Applied Chemistry. 1999; 71: 645-718.
- Bosch P, Catalina F, Corrales T, and Peinado C. Fluorescent probes for sensing processes in polymers. Chemistry-A European Journal. 2005; 11: 4314-4325.
- Demchenko AP. The red-edge effects: 30 years of exploration. Luminescence. 2002; 17: 19-42.
- Lakowicz JR. Principles of fluorescence spectroscopy. 2009: Springer.
- Itagaki H. Chapter 3 - Fluorescence Spectroscopy, in Experimental Methods in Polymer Science, T. Tanaka, Editor. 2000, Academic Press: Boston. p. 155-260.
- Yang J-S and Swager TM. Porous shape persistent fluorescent polymer films: an approach to TNT sensory materials. Journal of the American Chemical Society. 1998; 120: 5321-5322.
- Liu J, Yan J, Chen X and Fang Y. Studies on the conformational behavior of acenaphthylene-labeled poly (acrylamide-co-acryloyl-6-deoxy-6-amino-β- cyclodextrin). Colloid and Polymer Science. 2007; 285: 881-889.
- Fritzsche M, Barreiro C, Hitzmann B and Scheper T. Optical pH sensing using spectral analysis. Sensors and Actuators B: Chemical. 2007; 128: 133-137.
- Mishra H, Misra V, Mehata MS, Pant TC and Tripathi HB. Fluorescence Studies of Salicylic Acid Doped Poly(vinyl alcohol) Film as a Water/Humidity Sensor. The Journal of Physical Chemistry A. 2004; 108: 2346-2352.
- Rettig W, Strehmel B, Schrader S and Seifert H. Applied fluorescence in chemistry, biology and medicine. 2012: Springer Science & Business Media.
- Baugher J and Grossweiner L. Photolysis mechanism of aqueous tryptophan. The Journal of Physical Chemistry. 1977; 81: 1349-1354.
- Gollnick K, Franken T, Fouda MFR, Paur HR and Held S. Merbromin (mercurochrome) and other xanthene dyes: Quantum yields of triplet sensitizer generation and singlet oxygen formation in alcoholic solutions. Journal of Photochemistry and Photobiology B: Biology. 1992; 12: 57-81.
- Talhavini M and Atvars T. Photostability of xanthene molecules trapped in poly (vinyl alcohol)(PVA) matrices. Journal of Photochemistry and Photobiology A: Chemistry. 1999; 120: 141-149.
- Zheng Q, Juette MF, Jockusch S, Wasserman MR, Zhou Z, Altman RB, et al. Ultra-stable organic fluorophores for single-molecule research. Chemical Society Reviews. 2014; 43: 1044-1056.
- Song L, Varma C, Verhoeven J and Tanke HJ. Influence of the triplet excited state on the photobleaching kinetics of fluorescein in microscopy. Biophysical journal. 1996; 70: 2959.
- Song L, Van Gijlswijk R, Young IT and Tanke HJ. Influence of fluorochrome labeling density on the photobleaching kinetics of fluorescein in microscopy. Cytometry. 1997; 27: 213-223.
- Hoebe R, Van Oven C, Gadella TW, Dhonukshe P, Van Noorden C and Manders E. Controlled light-exposure microscopy reduces photobleaching and phototoxicity in fluorescence live-cell imaging. Nature biotechnology. 2007; 25: 249-253.
- Zondervan R, Kulzer F, Kol’chenk MA and Orrit M. Photobleaching of Rhodamine 6G in Poly(vinyl alcohol) at the Ensemble and Single-Molecule Levels. The Journal of Physical Chemistry A. 2004; 108: 1657-1665.
- Talhavini M and Atvars TDZ. Dye-polymer interactions controlling the kinetics of fluorescein photobleaching reactions in poly(vinyl alcohol). Journal of Photochemistry and Photobiology A: Chemistry. 1998; 114: 65-73.
- Tamai Y, Tanaka H and Nakanishi K. Molecular Dynamics Study of Polymer-Water Interaction in Hydrogels. 2. Hydrogen-Bond Dynamics. Macromolecules. 1996; 29: 6761-6769.
- Wang B, Guan X, Hu Y and Su Z. Preparation and fluorescent properties of poly(vinyl alcohol) bearing coumarin. Polymers for Advanced Technologies. 2007; 18: 529-534.
- Talhavini M, Corradini W and Atvars T. The role of the triplet state on the photobleaching processes of xanthene dyes in a poly (vinyl alcohol) matrix. Journal of Photochemistry and Photobiology A: Chemistry. 2001; 139: 187- 197.
- Dubois A, Canva M, Brun A, Chaput F and Boilot J-P. Photostability of dye molecules trapped in solid matrices. Applied Optics. 1996; 35: 3193-3199.
- Hooker JC and Torkelson JM. Coupling of probe reorientation dynamics and rotor motions to polymer relaxation as sensed by second harmonic generation and fluorescence. Macromolecules. 1995; 28: 7683-7692.
- Levitus M, Talhavini M, Negri RM, Atvars TDZ and Aramendía PF. Novel kinetic model in amorphous polymers. Spiropyran-merocyanine system revisited. The Journal of Physical Chemistry B. 1997; 101: 7680-7686.