
Research Article
Austin J Anal Pharm Chem. 2017; 4(3): 1093.
A Novel Thiourea-Based Sensor: Synthesis and Recognition for Acetate Anion
Chen S, Yu Q, Zhang X and Dai Z*
¹Department of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, Nanjing 210009, PR China
*Corresponding author: Zhenya Dai, Department of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, Nanjing 210009, PR China
Received: December 04, 2017; Accepted: December 19, 2017; Published: December 26, 2017
Abstract
Here in a series of novel and colorimetric chemosensors based on (trans)- 1.2-cyclohexanediamine and thiourea group were designed and synthesized, and their application in anion recognition was investigated. The chemsensor 4b showed higher selectivity for the detection of acetate ion than other anion such F-, Br-, I,-, ClO4 -, H2PO4 - in DMSO. The chemsensor 4b displayed immediate visible changes from nearly colorless toyellow and yellow-green upon the addition of acetate anion.
Keywords: Anion Sensor; Thiourea; Acetate Anion; Colorimetry; Trans- 1.2-Cyclohexanediamine
Introduction
The development of colorimetric and fluorescent chemosensors for the detection of various anions has attracted much attention due to the importance of anions in biological, industrial, food and environmental process [1-8]. Among the chemosensors, colorimetric sensors could be close to be applied in practical project, in which the detection of various anions by color change with those receptors needed less instruments and costed less [9], especially for realtime and online analysis of analytes. Most of that artificial receptor for the recognition of anions were mainly based on the combined nonconvalent interactions by using the dominant N-H function groups and neutral and cationic C-H hydrogen bond donors [10]. As a result, those receptors incorporating groups such as amide [11-16], urea [17-18], pyrrole [19-20] and thiourea [21-23] as binding units were designed and reported. Some of these colorimetric receptors for acetate ion in organic media such as DMSO have been reported [24-26]. On the other hand, among various anions, acetate ion has been found to be a possible tracer for maligences and have been extensively investigated in prostate cancer and metastases [27]. Moreover the rate of the acetate ion production and oxidation has been proved to be vital as an indicator of organic eletrocomposition in marine sediments [28], in which context, the development a novel colorimetric and fluorescent chemosensor for the rapid, convenient and low-cost detection of biologically important anions such as acetate was required.
While during the last few years, a large number of sensors for acetate anion have been reported, it was still a challenge to find easily synthesized, highly sensitive and selective to acetate anion and novel receptor.
Experimental
Materials
All reagents for synthesis were obtained commercially and used without further purification. In the titration experiments, all the anions were added in the form of tetrabutylammonium (TBA) salts, which were also commercially available, and stored in vacuum desiccators containing self-indicating silica and dried fully before use. DMSO was dried with CaH2 and then distilled under reduced pressure.
General method
1H NMR spectra were recorded on a Bruker AV-300 spectrometer or a Bruker AV-500 spectrometer at room temperature. Chemical shifts (in ppm) were referenced to tetramethylsilane (d = 0 ppm) in CDCl3 or DMSO-d6 as an internal standard. 13C NMR spectra were obtained by the same NMR spectrometer and were calibrated with DMSO-d6 (d =39.00ppm). Data for 1H NMR were reported as follows: chemical shifts (d ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet or unresolved, br s = broad singlet), coupling constant (Hz) and integration. Data for 13C NMR were reported in terms of chemical shift and multiplicity where appropriate. Mass spectra were performed on an Aglient 6530 Q-TOF for HRMS. The yields were determined on a METTLER TOLEDO ME 104 balance (accuracy: 0.1mg): Flash column chromatography was carried out on silica gel (200-300 mesh). UV-vis spectra were recorded on UV-2450.
Synthesis
Synthesis of 1a: 1a-1 was prepared according to the literature [29].
To a solution of 1a-1 (0.492g 2.4mmol) in anhydrous DMF, were added sequentially at room temperature: N2H4-2HCl (0.106g, 1mmol) HOBt (0.35g, 2.4mmol), DIPEA (1.4ml, 8.0mmol), EDCI (0.46g, 2.4mmol). The mixture was stirred at room temperature for 24 hours and diluted with EtOAc (40ml). The mixture was washed with water, 1% HCl and saturated sodium chloride and dried with Na2SO4. After filtration, evaporation of the solvent gave and the crude product which was further purified by silica gel (eluent: petrol: EA = 2:1) to afford the white solid (yield: 68%)
1a 1H-NMR (DMSO-d6, 500MHz) d 9.74-9.5(2H, s, NH); 7.45- 7.40(4H, d, J =7.43 Hz, ArH); 7.36-7.32(4H, t, J = 7.34 Hz, ArH); 7.29- 7.25(2H, t, J=7.27 Hz, ArH); 4.03-3.97(2H, d, J = 4.0Hz, CH); 3.46- 3.41(2H, d, J = 3.44Hz, CH); 3.22-3.16(2H, m, CH); 2.90-2.85 (2H, m, CH); 2.31-2.23(2H, m, CH); 2.13-2.05(2H, m, CH); 1.90-1.82(2H, m, CH), 1,80-1.71(4H, m, CH2); 13C-NMR(300 MHz, DMSO-d6) d 170.72, 138.75, 128.34, 128.05, 126.864, 67.76, 58.078, 52.480, 29.36, 23.02; ESI-MS: calculated for C24H30N4O2 [M+Na]+: 429.2 ; observed 429.3.
Synthesis of 2a, 3a: 2a and 3a were prepared according to the literature [30-31].
2a, white solid, yield: 98%:
1H-NMR (300MHz, DMSO-d6): d 8.55-8.47 (2H, d, J = 8.59Hz, ArH), 8.26-8.16 (2H, br s, CONH), 8.14-8.00 (4H, d, J = 8.06, ArH), 7.84-7.66 (2H, t, J = 7.73, ArH), 7.40-7.28 (2H, t, J = 7.33Hz, ArH) , 4.17-3.90 (2H, s, CH), 2.27-2.18 (2H, m, CH), 1.89-1.79 (2H, m, CH2), 1.52-1.43 (4H, m CH2); 13C-NMR (300MHz, DMSO-d6): d 163.6, 149.7, 148.2, 137.5, 126.3, 121.7, 52.3, 31.6, 24.4; ESI-MS: calculated for C18H20O2N4 [M+Na] + 347.1, found 347.1.
3a, white solid, yield: 90%:
1H-NMR(CDCl3 500 MHz):d 8.65-8.47 (2H, br s, CONH), 8.28- 8.14 (6H, m, ArH), 7.85-7.79 (2H, d, J = 7.82Hz, ArH) 7.79-7.74(2H, t, J = 7.77 Hz, ArH), 7.63-7.56(2H, t, J = 7.69 Hz, ArH), 4.66-3.98 (2H, s, CH), 2.41-2.32(2H, s, CH), 2.00-1.87(2H, s, CH), 1.72-1.49 (4H, m, CH2); 13C-NMR(CDCl3 300MHz):d 149.8,145.8,137.6,130.4, 129.0, 128.6, 127.9, 118.4, 52.8, 31.6, 24.5, ESI-MS: calculated for C26H24N4O2 [M+Na]+ 447.1, found 447.2
Synthesis of 2b and 3b: To a solution of 2a or 3a (1mmol) in CH2Cl2 (10mL) was added m-CPBA (383mg, 2.2mmol) at 0°C under stirring. After the reaction was completed, the mixture was concentrated and purified by the basic aluminum oxide column chromatography (eluent: EtOAc/MeOH = 3:1) to give a white powder 2b or 3b.
2b white solid, yield: 65%:
1H-NMR(300MHz,CDCl3) d 11.62-11.22(2H, br s, CONH), 8.49- 8.34(2H, d, J = 8.40Hz, ArH), 8.28-8.12(2H, d, J = 8.19Hz, ArH), 7.45-7.26 (4H, m, ArH), 4,42-3.98 (2H, s, CH), 2.30-2.16 (2H, s, CH), 1.87-1.73(2H, s, CH), 1.58-1.40(4H, m, CH2); 13C NMR d 159.4, 140.7, 140.4, 128.9, 127.0, 126.7, 52.5, 31.7,24.2. ESI-MS: calculated for C18H20N4O4 [M+Na]+: 379.1; observed 379.1.
3b white solid, yield: 57%:
1H-NMR(DMSO-d6, 300MHz) d 11.78-11.49 (2H, s, CONH), 8.81-8.63 (2H, m, NH), 8.40-8.33 (2H, m, ArH), 7.86-7.79 (6H, m, ArH); 7.74-7.65(2H, m, ArH), 4.51-4.25 (2H, s, CH), 2.42-2.17 (2H, m, CH2), 1.89-1.80 (2H, m, CH2), 1.66-1.57 (2H, m, CH2); 1.41-1.23 (2H, m, CH2); 13C NMR d160.3, 141.7, 137.4, 130.7, 130.5, 129.5, 127.9, 125.8, 122.7, 120.2, 52.6, 31.9, 24.3; ESI-MS: calculated for C26H24N4O4 [M+Na]+: 479.1; observed 479.0
Synthesis of 4a and 4b: 4a-1 and 4b-1 were preparation according to literature [32].
To a solution of 4a-1 or 4b-1 (1mmol) in anhydrous THF (5ml) was added (1R,2R)-1,2-cyclohenxanediamine (0.5mmol). The resulting mixture was stirred at room temperature for 12 hours. Evaporation of the solvent gave the crude product, which was purified by flash column chromatography.
4a, white solid, yield: 70%:
1H-NMR (500MHz, DMSO-d6) d12.02-11.83(2H, s, NH), 10.48- 10.33(2H, s, NH), 8.25-8.18(2H, d, ArH, J = 8.25 Hz), 7.75-7.66(2H, t, ArH, J = 7.71 Hz), 7.10-7.03(2H, d, ArH, J = 7.07 Hz), 7.03-6.95(2H, t, ArH, J = 7.00Hz), 4.59-4.39(2H, m, NCH), 2,32-2.22(2H, m, CH), 1.76-1.70(2H, m, CH2), 1.48-1.39(4H, m, CH2); 13C-NMR(300 MHz, DMSO-d6) d 179.35, 183.66, 145.55, 138.20, 117.52, 112.28, 63.76, 28.74, 23.49; ESI-MS: calculated C18H22N6S2 [M+Na]+ 409.1, observed 409.1.
4b, yellow-white solid, yield: 69%:
1H-NMR (500MHz,DMSO-d6): d 11.87-11.19(2H, s, NH), 10.93- 10.34(2H, s, NH) 8.52- 8.04(2H, d, ArH, J = 8.25Hz), 7.98-7.88(2H, dd, ArH, J = 7.83 Hz), 7.41-6.90(2H, d, ArH, J = 7.11Hz), 4.77-4,34(2H, s, NCH), 2.38 2.19(2H, s, CH), 1.81-1.69(2H, s, CH2), 1.47-1.32(4H, m, CH2); 13C-NMR(500 MHz, DMSO-d6) d 179.1, 151.9, 143.7, 138.6, 123.5,113.8, 57.7, 31.2, 24.0; ESI-MS: calculated for C18H20Cl2N6S2 [M+H],sup>+
455.0; observed 455.1 (Figure 1).
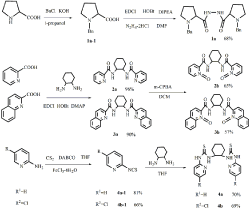
Figure 1: synthesis of 1a, 2a, 2b, 3a, 3b, 4a, 4b.
Result and Discussion
UV–vis spectral observations and Colorimetric studies
To determine the selectivity of those receptors to various anion, UV-vis spectral experiment were conducted using standard solution of the receptors 1a, 2a, 2b, 3a,3b,4a and 4b in dry DMSO by adding up to 10 equiv of tetrabutylammonium salts as acetate anion and recording the absorbance changes.
As was showed in Figure 2, receptors 1a, 2a, 2b, 3a, 3b, 4a in DMSO with AcO-, had only one absorption band, while the receptor 4b with AcO- had two absorption bands, which one absorption band is at 337nm, the other is at 444nm. Additionally, the colorimetric properties of those receptors were explored to establish if there were changes could be observed by naked eye and the findings were depicted in Figure 3. It was observed that receptor 4b with AcOdisplayed a distinct color change, which the solution changed from colorless to yellow or yellow-green.
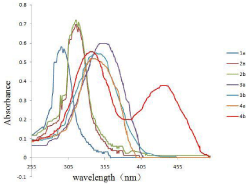
Figure 2: UV-vis absorption spectra of receptors (1a,2a,2b,3a,3b,4a,4b)
(5×10-5 M in DMSO) with 8 equiv of TBA-AcO.
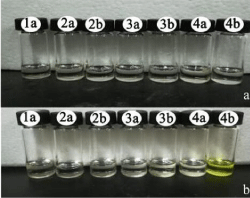
Figure 3: (a) color changes observed for receptors (1a,2a,2b,3a,3b,4a,4b)
(5×10-3 M in DMSO) (b) color changes observed for receptors
(1a,2a,2b,3a,3b,4a,4b) (5×10-3 M in DMSO ) upon addition of 1.0 equiv of
TBA-AcO at room temperature.
Based on receptor 4b with AcO- exhibiting distinct color changes, further experiments about receptor 4b were conducted.
It was observed in Figure 4 that receptor 4b, as the free receptor molecular, exhibited one main absorption band at 337nm. Upon the addition of 10 equiv of the various anions to the receptor 4b solution, the absorption at 337nm decreased gradually and a new absorption band at 444 nm emerged, especially upon the addition of the acetate ion. Other anions (ClO4-, I-, Br-) were faintly responsive at 444nm, while F- and H2PO4 - did not show noticeable decrease in absorbance at 337nm.
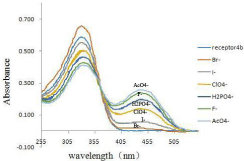
Figure 4: UV-vis spectra of 4b (2.0×10-5 M solution in DMSO) upon the
addition of 8 equiv of each tetrabutylammonium salts (TBA-X) (X= F- , Br-, I-, AcO-, H2PO4 -, ClO4 -).
Moreover, as was shown in Figure 5, when addition of 0.01 equiv of F-, it did not exhibit a distinct color changes while AcO- still had noticeable color change. Those result showed that 4b was able to detect acetate ion in the range of 10-5-10-3 M. Also, 4b with acetate exhibited yellow, while other anions with 4b did show noticeable color change at 365nm.
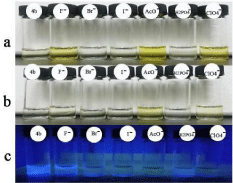
Figure 5: Color observed 4b (Y M solution in DMSO) upon the addition of 1.0 equiv of various anions as TBA-X(X=F-, Br-, I-, AcO-, ClO4 -, H2PO4 -), and the first 4b solution was observed without addition of any anions. (a) Y= 5×10-3 ; (b) Y= 5×10,-5 ; (c) Y= 5×10-3 , detected at 365nm.
On the other hand, on addition of increasing amounts of tetrabutylammmonium salts of acetate ion to the solution of receptor 4b, the absorption band at 337nm decreased while formation a distinct isosbestic point at 361 nm (Figure 6). The observation of an isosbestic point in the UV-vis spectra indicated the formation of a stable complex between the host and guest.
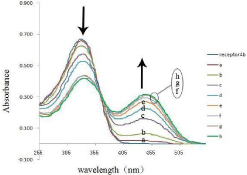
Figure 6: UV-vis absorption spectra titration of receptor 4b (5×10-5 M in
DMSO) with x equiv of TBA-AcO at room temperature: (a ) x= 1.0; (b) x=2.0;
(c) x= 3.0; (d) x=4.0; (e) x= 5.0; (f) x= 6.0; (g) x=7.0; (h) x= 8.0.
To further study the complex of the host and guest, Job spot was also done. The Job plot showed that the ratio of receptor 4b with acetate ion is 1:1 (Figure 7).
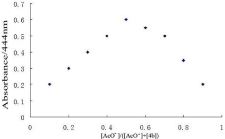
Figure 7: The stoichiometry analysis of the complex [4b-AcO-] in DMSO by
Job plot analysis; [4b]+[AcO-]= 2.0×10-5 M.
1H-NMR titration experiments
The interaction of the anion with the receptor NH protons through hydrogen bond interaction is known to result in line broadening of the concerned 1H-NMR signals, and is usually followed by a downfield shift of the resonances of these protons with increasing concentrations of anions [33]. As was shown in Figure 8, in order to gain an insight into the interaction details of receptor 4b with guest anion, the 1H-NMR titration of 4b with AcO- were conducted. Free receptor 4b exhibited three signal peaks at 11.40ppm, 10.61ppm and 8.22ppm in the far downfield region, attributed to Ha, Hb, Hc respectively. Upon addition of 1.0 equiv of AcO-, clear downshifts (0.05ppm, 0.11ppm, 0.03ppm) for Ha, Hb, Hc respectively were observed. Hence, the added AcO- was bound by 4b, through hydrogen bond interactions, from thiourea and aromatic hydrogen.
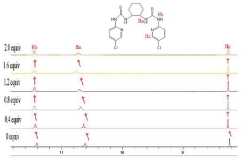
Figure 8: 1H-NMR titration spectra of receptor 4b in DMSO-d6 upon addition
of x equiv of TBA-AcO; x= 0; 0.4; 0.8; 1.2; 1.6; 2.0.
Based on the facts above, we considered the stable complex might attribute to the six hydrogen-binding with the acetate anion (Figure 9).
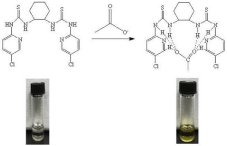
Figure 9: Proposed mechanism of formation the complex of 4b and AcO-.
Conclusion
In summary, a novel colorimetric anion sensor which allowed for “naked eye” detection and UV-VIS detection of acetate anion with high sensitivity and selectivity has been designed and synthesized. The urea group was exploited as an anion binding site in 4b, and it utilizes hydrogen-binding to form a 1:1 host and guest complex as a mode of recognition AcO-, which could be concluded by the Job plot analysis. And 1H-NMR titration of receptor 4b with AcO- confirmed the N-H hydrogen bonding interaction mode.
Acknowledgment
We were thankful to the National Natural Science Foundation of China for their financial support (No. 21102180).
References
- Langton MJ, Serpell CJ, Beer PD. Anion Recognition in Water: Recent Advances from a Supramolecular and Macromolecular Perspective. Angew Chem Int Ed Engl. 2016; 55: 1974-1987.
- Scheiner S. Highly Selective Halide Receptors Based on Chalcogen, Pnicogen, and Tetrel Bonds. Chemistry. 2016; 22: 18850-18858.
- Brown A, Beer PD. Halogen bonding anion recognition. Chem Commun. 2016; 52: 8645-8658.
- Li YJ, Xie DY, Pang XL, Yu XD, Yu T, Ge XT. Highly selective fluorescent sensing for fluoride based on a covalently bonded europium mesoporous hybrid material. Sensors Actuators B-Chem. 2016; 227: 660-667.
- Zhou X, Kim J, Liu ZX, Jo S, Pak YL, Swamy KMK, et al. Selective fluorescent and colorimetric recognition of cyanide via altering hydrogen bonding interaction in aqueous solution and its application in bioimaging. Dyes And Pigments. 2016; 128: 256-262.
- Molina P, Zapata F, Caballero A. Chem Rev. 2017.
- Gale PA. Anion receptor chemistry. Chem Commun. 2011; 47: 82-86.
- Maurya N, Bhardwaj S, Singh AK. A modest colorimetric chemosensor for investigation of CN- in semi-aqueous environment with high selectivity and sensitivity. Sensors Actuators B-Chem. 2016; 229: 483-491.
- Ghorpade TK, Patri M, Mishra SP. Highly sensitive colorimetric and fluorometric anion sensors based on mono and di-calix[4]pyrrole substituted diketopyrrolopyrroles. Sensors Actuators B-Chem. 2016; 225: 428-435.
- Martinez-Manez R, Sancenon F. Fluorogenic and Chromogenic Chemosensors and Reagents for Anions. Chem Rev. 2003; 103: 4419-4476.
- Bondy CR, Loeb SJ. Amide based receptors for anions. Coord Chem. 2003; 240: 77-99.
- Kwon JY, Jang YJ, Kim SK, Lee KH, Kim JS, Yoon JY. Unique Hydrogen Bonds between 9-Anthracenyl Hydrogen and Anions. J.Org.Chem. 2004; 69: 5155-5157.
- Basu A, Dey SK, Das G. RSC Adv. 2013; 3: 6596.
- Chakraborty S, Arunachalam M, Dutta R, Ghosh P. Arene platform based hexa-amide receptors for anion recognition: single crystal X-ray structural and thermodynamic studies. RSC Adv. 2015; 5: 48060-48070.
- Katagiri K, Furuyama T, Masu H, Kato T, Matsumura M, Uchiyama M, et al. supramol .chem. 2011; 23: 125-130.
- Ghosh K, Sarkar AR, Masanta G. Tetrahedron Lett. 2007; 48: 8725-8729.
- Oton F, Tarraga A, Espinosa A, Velasco MD, Molina P. Ferrocene-Based Ureas as Multisignaling Receptors for Anions†. J.Org.Chem. 2006; 71: 4590- 4598.
- Khansari ME, Johnson CR, Basaran I, Nafis A, Wang J, Leszczynski J, et al. Synthesis and anion binding studies of tris(3-aminopropyl)amine-based tripodal urea and thiourea receptors: proton transfer-induced selectivity for hydrogen sulfate over sulfate. RSC Adv. 2015; 5: 17606-17614.
- Samanta R, Mahanta SP, Chaudhuri S, Panda PK, Narahari A. New strapped calix[4]pyrrole based receptor for anions. Inorganica Chimica Acta. 2011; 372: 281-285.
- Linn MM, Poncio DC, Machado VG. An anionic chromogenic sensor based on the competition between the anion and a merocyanine solvatochromic dye for calix[4]pyrrole as a receptor site. Tetrahedron Lett. 2007; 48: 4547-4551.
- Nie L, Li Z, Han J, Zhang X, Yang R, Liu WX, et al. Development of N-Benzamidothioureas as a New Generation of Thiourea-Based Receptors for Anion Recognition and Sensing. J.Org.Chem. 2004; 69: 6449-6454.
- Boyle EM, Comby S, Molloy JK, Gunnlaugsson T. Thiourea Derived Tröger’s Bases as Molecular Cleft Receptors and Colorimetric Sensors for Anions. J.Org.Chem. 2013; 78: 8312-8319.
- Sun XH, Li W, Xia PF, Luo HB, Wei Y, Wong MS, et al. Phenyl-calix[4]arene- Based Fluorescent Sensors: Cooperative Binding for Carboxylates. J Org Chem. 2007; 72: 2419-2426.
- Kado S, Otani H, Nakahara Y, Kimura K. Highly selective recognition of acetate and bicarbonate by thiourea-functionalised inverse opal hydrogel in aqueous solution. Chem Commun. 2013; 49: 886-888.
- Gunnlaugsson T, Davis AP, O’Brien JE, Glynn M. Fluorescent Sensing of Pyrophosphate and Bis-carboxylates with Charge Neutral PET Chemosensors†. Org.Lett. 2002; 4: 2449-2452.
- Kovalchuk A, Bricks JL, Reck G, Rurack K, Schulz B, Szumna A, et al. A charge transfer-type fluorescent molecular sensor that “lights up” in the visible upon hydrogen bond-assisted complexation of anions. Chem Commun. 2004; 1946-1947.
- Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. PNAS. 2000; 97: 3450-3454.
- Kita A, Suehira K, Miura T, Okamura Y, Aki T, Matsumura Y, et al. Characterization of a halotolerant acetoclastic methanogen highly enriched from marine sediment and its application in removal of acetate. J Biosci Bioeng. 2016; 121: 196-202.
- Spiegel J, Cromm PM, Itzen A, Goody RS, Grossmann TN, Waldmann H. Angew Chem Int Ed Engl. 2014; 53: 2498-2503.
- Sprague DJ, Nugent BM, Yoder RA, Vara BA, Johnston JN. Switching Regioselectivity in Crossed Acyloin Condensations between Aromatic Aldehydes and Acetaldehyde by Altering N-Heterocyclic Carbene Catalysts. Org Lett. 2015; 17: 880-883.
- Pignataro L, Benaglia M, Cinquini M, Cozzi F, Celentano G. Readily available pyridine- and quinoline-N-oxides as new organocatalysts for the enantioselective allylation of aromatic aldehydes with allyl(trichloro)silane. Chirality. 2005; 17: 396-403.
- Zhang H, Liu RQ, Liu KC, Li QB, Li QY, Liu SZ. A One-Pot Approach to Pyridyl Isothiocyanates from Amines. Molecules. 2014; 19: 13631-13642.
- Ros-Lis JV, Martínez-Má&nTilde;ez R, Sancenón F, Soto J, Rurack K, Weißhoff H. Signalling Mechanisms in Anion-Responsive Push-Pull Chromophores: The Hydrogen-Bonding, Deprotonation and Anion-Exchange Chemistry of Functionalized Azo Dyes. Eur.J.Org.Chem. 2007; 2007: 2449-2458.