
Research Article
Austin J Anal Pharm Chem. 2018; 5(1): 1097.
Utility of Schiff’s Base as a Novel Catalyst for Knoevenagel Condensation: Syntheses of Benzimidazolylthioacrylonitriles Using One-Pot, Step- Wise and Tandem Fashion
Srinivas Rao S*
¹Department of Chemistry, Vidya Jyothi Institute of Technology (Autonomous), Himayatnagar (Vill.), India
*Corresponding author: Srinivas Rao S, Department of Chemistry, Vidya Jyothi Institute of Technology (Autonomous), Himayatnagar (Vill.), Hyderabad, India
Received: February 13, 2018; Accepted: March 12, 2018; Published: March 19, 2018
Abstract
In this article, we have developed an efficient one pot, step wise and tandem syntheses of benzimidazolylthioacrylonitriles 6a-p by Knoevenagel condensation of 2/4 with aromatic aldehydes 5 and aniline in methanol. In one-pot synthesis, an insitu generated schiff base (i.e. reaction of aniline and aldehydes) acts as one of the reagent for the formation of unsaturated nitriles. Schiff base acting as a proton abstracting agent which abstracts the acidic proton from 2/4 to generate a carbanion, followed by formation of an adduct which leads to formation of an unsaturated nitriles by eliminating aniline as byproduct. In this reaction, Schiff base is acting as a reaction intermediate.
Keywords: Schiff’s base; Aromatic aldehydes; Chloroacetonitrile; Benzimidazole; 2-mercaptobenzimidazole
Introduction
Chemistry of multiple bonds has achieved a dramatic development in the past decades [1-4] because these compounds have been used as substrates in the synthesis of industrial and biological active compounds via condensation reactions [5-6]. Moreover, the compounds containing carbon-nitrogen, carbon-oxygen and/or carbon-carbon double bond are also possess biological activity [7].
a,β-unsaturated nitriles play an unique role in drug discovery programs. Various unsaturated nitrile derivatives are also reported to exhibit a wide spectrum of biological properties such as Cardiotonic agents. Analogs possessing a,β-unsaturated nitriles at the 17-β position had high activity in natural products. In Digoxin, the unsaturated 17-lactone plays an important role in receptor binding. Saturation of the lactone ring dramatically reduced the biological activity. In this the lactone at 17th position is not required. For example a,β-unsaturated nitriles (-C=C-CN group) the lactone could be replaced with little or no loss in biological activity [8,9] (Figure 1).
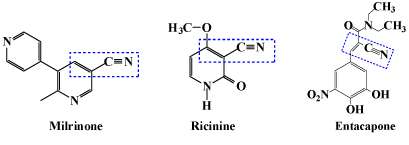
Figure 2:
Sidhu et al reported [10] that chemoselective reaction of cyanoacetic acid with benzal-4-acetylanilines gave a, β-unsaturated nitriles. Hello et al synthesized N-a-saccharylbenzyl benzanilide and some of its derivatives via Schiff’s bases [11].
Schiff base is an imine which contains an azomethine group synthesized by the simple condensation of aldehydes or ketones with primary amines. Schiff bases are having highly synthetic value in the organic synthesis. Schiff bases are readily participate in addition reactions cyclization reactions, act as precursors for the synthesis of various important intermediates and products [12-15].
Based on the literature citations given above and in continuation of our earlier studies [16]herein we reported the synthesis of a, β-unsaturated nitriles i.e. 2-((1H-benzimidazol-2-yl)thio)-3- arylacrylonitrile 6a-p via Schiff’s base, which acts as a reaction intermediate.
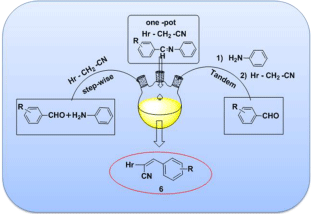
Figure 1: Graphical Abstract:
Results and Discussion
2-mercaptobenzimidazole 1 on reaction with chloroacetonitrile in DMF containing K2CO,3 & TBAB at RT for 3h followed by processing to obtain respectively 1H-(benzimidazol-2-ylsulfanyl)acetonitrile 2. o-phenylenediamine 3 with cyanoethylacetate under reflux for 3h gave 1H-(benzimidazol-2-yl)acetonitrile 4 (Figure 2).
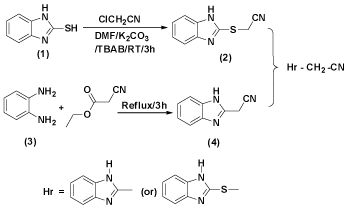
Figure 3:
Our one-pot synthesis of 6a-p was conducted as outlined in Figure 2. The required unsaturated nitriles 6a-p were prepared in onepot synthesis by taking 2 or 4 with an aromatic aldehydes 5 followed by aniline in methanol at RT for 15min. In this method, an insitu generated schiff base (i.e. reaction of aniline and aldehydes) acts as one of the reagent which leads to the formation of unsaturated nitriles 6a-p. The structure interpretation (i.e. IR, NMR and LC-MS) for the compounds 6i-p were found to be in agreement with the structures assigned to them in literature [17,18] and the structures of 6a-h was assigned on the basis of its spectral properties IR, NMR & Mass spectra (For Details, Please See Experimental Section) (Table 1, 2).
S.No.
SM
Ar-CHO
Reagent
Product
Yield* (%)
One-pot
Step-wise
tandem
a
2
-C6H5
Aniline
6a
88
79
85
b
2
-C6H4 –NO2 -p
Aniline
6b
86
81
83
c
2
-C6H4 –NO2 -m
Aniline
6c
85
77
80
d
2
-C6H4 –CH3 –p
Aniline
6d
82
73
80
e
2
-C6H4 –CH3 –o
Aniline
6e
85
75
81
f
2
-C6H4 –OH –p
Aniline
6f
80
77
75
g
2
-C6H4 –OH –o
Aniline
6g
82
72
76
h
2
-C6H4 –(OCH3)-p
–(OCH3)-m
Aniline
6h
84
78
82
* refers to recrystallized products only.
Table 1: Synthesis of 6a-h from 2 via Schiff’s base.
S.No.
SM
Ar-CHO
Reagent
Product
Yield* (%)
One-pot
Step-wise
tandem
i
4
-C6H5
Aniline
6i
86
78
82
j
4
-C6H4 –NO2 -p
Aniline
6j
84
80
77
k
4
-C6H4 –NO2 -m
Aniline
6k
83
78
79
l
4
-C6H4 –CH3 –p
Aniline
6l
85
71
80
m
4
-C6H4 –CH3 –o
Aniline
6m
80
73
75
n
4
-C6H4 –OH –p
Aniline
6n
80
77
75
o
4
-C6H4 –OH –o
Aniline
6o
84
72
74
p
4
-C6H4 –(OCH3)-p
–(OCH3)-m
Aniline
6p
86
78
72
* refers to recrystallized products only.
Table 2: Synthesis of 6i-p from 4 via Schiff’s base.
6a-p could also be prepared in step-wise synthesis involving the sequencies (5 → 7 → 6a-p) (Figure 3). In this method, an aromatic aldehyde 5 was treated with aniline at RT for 10min gave the corresponding Schiff’s base 7. The product was characterized by comparison of its physical data with that of the same product reported [18] earlier. 7 were converted to unsaturated nitriles 6a-p by treatment of 7 with 2/4 in methanol at RT for 10min. The mixture was then processed to obtain 2-((1H-benzimidazol-2-yl)thio)-3- arylacrylonitrile 6a-p identical with the same product obtained earlier in the one-pot route (Figure 2).
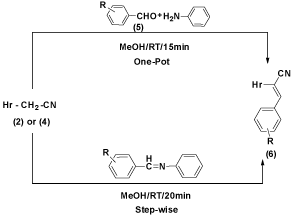
Figure 4:
In this step-wise reaction, an aromatic aldehyde was treated with aniline gave the previously reported Schiff bases. The C-N double bond of Schiff bases like the Carbon-oxygen double bond, readily participates in condensation reactions of the aldol type. Schiff bases in general, and N-substituted aryl aldimines in particular, react readily with active methylene compounds under a variety of compounds to give adducts which tend to eliminate the elements of an amine, affording the corresponding alkene.
In this reaction, the Schiff base acting as a proton abstracting agent which abstracts the acidic proton from 2/4 to generate a carbanion, followed by formation of an adduct which leads to formation of an unsaturated nitriles by eliminating aniline as by-product. In this reaction, Schiff base is acting as a reaction intermediate.
In tandem reaction, 2-((1H-benzimidazol-2-yl)thio)-3- arylacrylonitrile 6a-p were synthesized by taking an aromatic aldehydes 5 with aniline was stirred for 10 min yielded Schiff’s base until the disappearance of 5 was found on TLC. To the same mixture, 2/4 was added and then the reaction mixture in methanol at RT for 10min until the disappearance of 2/4 took place. Structure of the final product was established by its comparison with authentic sample prepared earlier prepared earlier in step-wise and one-pot route shown in Scheme 2 (Figure 4).
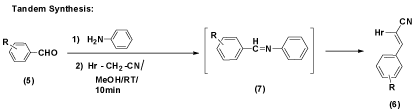
Figure 5:
Experimental part
General: All chemicals were purchased from commercial suppliers and used further without purification. IR Spectra were recorded with Jasca FT-IR 5300. 1H NMR and 13C NMR were recorded in CDCl3 / DMSO using Varian 400-MHz instrument. Mass spectra were recorded on an Agilent LC-MS instrument giving only M+ values in Q+1 mode. Thin-layer chromatography (TLC) analyses were carried out on glass plates coated with silica gel GF-254 and visualization was achieved using iodine vapours or UV lamp. Starting materials 5 were obtained from commercial sources and used as such. 2/4 has been prepared by literature method [17,18].
Preparation of 6a-p from 5 (General Procedure) (Tandem Synthesis)
A mixture of an aromatic aldehyde (10mM), aniline (10mM) in methanol (20mL) was stirred at RT for 10min when colorless solid separated out from reaction mixture. Then, to this solution 2/4 (10mM) was added and the mixture was stirred for 10min. Another colorless solid separated out from reaction mixture which was collected by filtration. The isolated solid was washed with water (10mL) and dried. The crude product was recrystallized from a suitable solvent to obtain 6a-p.
2-((1H-benzimidazol-2-yl)thio)-3-phenylacrylonitrile (6a): m.p. 192-1940C; IR (KBr): 3400 – 2800 cm-1 (br, m, -NH-); 1H- NMR (400 MHz, DMSO-d6/ TMS): d 8.20 (s, 1H), 8.10 (d, J= 4.6 Hz, 2H), 7.66 -7.33 (m, J= 6.6 Hz, 3H), 7.65–7.34 (m, J= 6.4 Hz, 4H), 7.38- 7.27 (m, 2H), 10.6 (s, 1H, -NH-), 13C NMR: d 140.5 (C=N), 138.5 (2 Ar-C) , 135.0 (C=CH), 132.4 (C=N), 128.5 (2 Ar-C), 128.3 (Ar-C), 126.9 (2 Ar-C), 124.5 (2 Ar-C), 115.5 (C=CH) ppm; MS (CI): m/z 278 [M.++1]; HRMS (C16H11N3S): Calcd for [M.++H], 278.4641 found 278.4638.
Conclusion
In summary, the methods developed for the synthesis of 6a-p in one-pot, step-wise and tandem synthesis using Schiff base. Of all the methods discussed, one-pot synthesis (Figure 3) appears to be the better, less time and efficient method of products obtained, compared to the other two methods. The present protocol is also equally efficient under a scale-up condition with cost-effective, large-scale production of these medicinally privileged heterocyclic compounds.
Acknowledgments
The author is highly indebted to University Grants Commission (UGC), New Delhi for sanctioned Minor Research Project and also thankful to Principal, Vidya Jyothi Institute of Technology (Autonomous), Hyderabad.
References
- Chen GM, Brown HC. An Efficient Synthesis of N-Unsubstituted Imines as Organoborane Adducts Stable at Room Temperature: New Promising Intermediates for Synthesis. J. Am. Chem. Soc. 2000; 122: 4217-4218.
- Hothi HS, Makkar A, Sharma JR, Manrao MR. Synthesis and antifungal potential of Co(II) complexes of 1-(2'-hydroxyphenyl) ethylideneanilines. Eur. J. Med. Chem. 2006; 41: 253-255.
- Antonov L, Fabian WMF, Nedeltcheia D, Kamounah FS. F.t.i.r. spectral study of intramolecular hydrogen bonding in thromboxane A2 receptor antagonist S-145 and related compounds. Part I. Perkin II. 2000; 6: 1173-1179.
- Christoper MV, Liliya GN, David WN, Andreas AS, Mark OB, Stephen AW. Synthesis and antifungal properties of benzylamines containing boronate esters. Can. J. Chem. 2001; 79: 1115-1123.
- Sidhu A, Rai M. Indian J. Chem. 2009; 48: 735-738.
- Raj M, Kumar S, Krishan K, Singh A. Chem. Ind. 1979; 211.
- Kumar MT, Shivasubramanianjan S, Ponnuswamy A, Mohan P. Synthesis and evaluation of pharmacological CNS activity of a-hydroxy O-alkyl etheroximes. Eur. J. Med. Chem. 1996; 31: 905-909.
- Sandhar RK, Sharma JR, Manrao MR. J. Synthesis and fungitoxicity of aldimines and 4-thiazolidinones derived from 4-bromoaniline. Indian Chem. Soc. 2008; 85: 220-223.
- Manrao MR, Kaur G, Kaul VK. Indian J. Nem. 2007; 37: 205-208.
- Sidhu A, Sharma JR, Rai M. Chemoselective reaction of cyanoacetic acid with benzal-4-acetylanilines and fungitoxicity of products. J. Chem. Sci. 2009; 121: 449-456.
- Hello KM, Nahi RJ, Mitab HH. The Scientific J. for Vet. Medicine. 2006; 5: 21-27.
- Layer RW. The Chemistry of Imines. Chem. Rev. 1963; 63: 489-510.
- Sprung M. A Summary of the Reactions of Aldehydes with Amines. Chem. Rev. 1940; 26: 297-338.
- Harada K. Interscience. 1955; 255-267.
- Morath RJ, Stacy GW. Interscience. 1970; 327-338.
- Rao SS, Dubey PK, Kumari YB. Phosphorus, Sulfur, and Silicon and the Related Elements. Rao SS, Reddy ChVR. Dubey PK. J. Het. Chem.
- Siya R. Synthesis of 2-thiobenzimidazole derivatives as potential antifilarial agents. J Het. Chem.1985; 22: 1269-1274.
- Dubey PK, Reddy PVV, Srinivas K. Syn. Comm. 2008; 38: 613-618.