
Research Article
Austin J Anal Pharm Chem. 2018; 5(2): 1101.
Method Validation and Stability Evaluation for the Simultaneous Estimation of Clopidogrel and Atorvastatin in Tablet Dosage Forms by RP-HPLC
Sivagami B1*, Pavani Reddy PC1, Chandrasekar R2 and Niranjan Babu MN2
¹Department of Pharmaceutical Analysis, Seven Hills College of Pharmacy, 517561, AP, India
²Department of Pharmacognosy, Seven Hills College of Pharmacy, 517561, AP, India
*Corresponding author: Sivagami B, Department of Pharmaceutical Analysis, Seven Hills College of Pharmacy, Venkataramapuram, Tirupati, Chitoor Dist, 517561, AP, India
Received: July 25, 2018; Accepted: September 07, 2018; Published: September 14, 2018
Abstract
Objectives: The study aims to develop and validate a simple, accurate, precise, economical method for the simultaneous estimation of the Clopidogrel and Atorvastatin in tablet dosage form by RP-HPLC method.
Methods: The chromatogram was run through Standard Discovery C8 (250x4.6mm, 5m.) The Mobile phase containing Buffer 0.1% Potassium Dihydrogen Phosphate: Acetonitrile taken in the ratio 45:55 was pumped through column at a flow rate of 1 ml/min. pH was adjusted to 3.8 with dil. Orthophosphoric acid solution. Buffer used in this method was potassium dihydrogen phosphate solution. Temperature was maintained at 30°C. Optimized wavelength selected Clopidogrel and Atorvastatin was 247nm.
Results: Retention time of Clopidogrel and Atorvastatin were found to 2.555min and 3.224min. With the optimized chromatographic conditions, the drug was linear in the concentration range of 0 – 150 μg/ml. The correlation coefficient was found to be 0.999. The average percentage assay in formulation was found to be 99.60% and 99.14% for Clopidogrel and Atorvastatin respectively. %RSD of the Clopidogrel and Atorvastatin were and found to be 0.4 and 0.3 respectively. %Recovery was obtained as 99.13% and 99.40% for Clopidogrel and Atorvastatin respectively. LOD, LOQ values obtained from regression equations of Clopidogrel and Atorvastatin were 0.26, 0.79 and 0.04, 0.11 respectively. Regression equation of Clopidogrel is y = 16467x + 5787, and y =34860x+5859 of Atorvastatin.
Conclusion: Hence the suggested RP-HPLC method can be used for routine analysis of Clopidogrel and Atorvastatin in API and Pharmaceutical dosage form.
Keywords: Atorvastatin; Clopidogrel; RP-HPLC; Stability; Simultaneous Estimation; Validation
Introduction
Clopidogrel, an antiplatelet agent structurally and pharmacologically similar to ticlopidine, is used to inhibit blood clots in a variety of conditions such as peripheral vascular disease, coronary artery disease, and cerebrovascular disease. Clopidogrel is sold under the name Plavix by Sanofi and Bristol-Myers Squibb. The drug is an irreversible inhibitor of the P2Y12 adenosine diphosphate receptor found on the membranes of platelet cells. Clopidogrel use is associated with several serious adverse drug reactions such as severe neutropenia, various forms of hemorrhage, and cardiovascular edema. Chemically clopidogrel is methyl (2S)-2-(2-chlorophenyl)-2- {4H,5H,6H,7H-thieno[3,2-c]pyridin-5-yl}acetate (Figure 1).
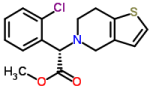
Figure 1: Structure of Clopidogrel.
Atorvastatin (Lipitor) is a member of the drug class known as statins. It is used for lowering cholesterol. Atorvastatin is a competitive inhibitor of hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, the rate-determining enzyme in cholesterol biosynthesis via the mevalonate pathway. HMG-CoA reductase catalyzes the conversion of HMG-CoA to mevalonate. Atorvastatin acts primarily in the liver. Decreased hepatic cholesterol levels increases hepatic uptake of cholesterol and reduces plasma cholesterol levels Chemically atorvastatin is known as (3R,5R)-7-[2-(4-fluorophenyl)- 3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5 dihydroxyheptanoic acid (Figure 2).
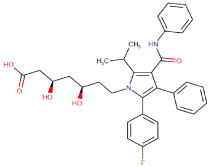
Figure 2: Structure of Atorvastatin.
Many methods like UV, RP-HPLC have been developed but with high retention time and run time in this study an accurate, precise, economic, method was developed for the assay related substance for the simultaneous estimation of Atorvastatin and Clopidogrel in pharmaceutical dosage forms. RP-HPLC methods [1-5]. By UV method [6] in bulk and combined dosage formulations [7-9]. In capsule dosage forms [10] in human plasma, Human Serum and Rat plasma [11-13]. This method seems to be economic with less retention time. Since the retention time was less the run time also decreased. The method developed can be applicable for quality control studies in industries.
Materials and Methods
Materials
Clopidogrel and Atorvastatin pure drugs (API), Combination Clopidogrel and Atorvastatin tablets (Axigrel AT), was obtained from Axis Life Science Pvt Ltd. Distilled water, Acetonitrile, Phosphate buffer, Methanol, Potassium dehydrogenate ortho phosphate buffer, Ortho-phosphoric acid. All the above chemicals and reagents used were analytical grade and procured from Rankem Laboratories Pvt Ltd India.
Instrumentation
Electronics Balance-Denver, pH meter -BVK enterprises, India, Ultrasonicator-BVK enterprises, WATERS HPLC 2695 SYSTEM equipped with quaternary pumps, Photo Diode Array detector and Auto sampler integrated with Empower 2 Software. UV-VIS spectrophotometer PG Instruments T60 with special bandwidth of 2mm and 10mm and matched quartz cells integrated with UV win 6 Software was used for measuring absorbances of Clopidogrel and Atorvastatin solutions.
Methods
Diluent: Based up on the solubility of the drugs, diluent was selected, Acetonitrile and Water taken in the ratio of 50:50.
Preparation of Standard stock solutions: Accurately weighed 18.75mg of Clopidogrel, 2.5mg of Atorvastatin and transferred to individual 25ml volumetric flasks separately. 3/4th of diluents was added to both of these flasks and sonicated for 10 minutes. Flasks were made up with diluents and labeled as Standard stock solution 1 and 2. (750μg/ml of Clopidogrel and 100μg/ml of Atorvastatin).
Parameters
Trial 1
Trial 2
Trial 3
Trial 4
Optimized Method
Mobile phase
Water and Acetonitrile (50:50)
Water: Acetonitrile (50:50)
OPA: Acetonitrile (50:50)
50% OPA buffer: 50% Acetonitrile
55% 0.1% OPA buffer: 45% Acetonitrile
Flow rate
1 ml/min
1 ml/min
1.2 ml/min
1 ml/min
1 ml/min
Column
BDS C18 (4.6 x 150mm, 5µm)
Waters C18 (4.6 x 150mm, 5µm)
Waters C18 (4.6 x 150mm, 5µm)
Discovery C8 (4.6 x 250mm, 5µm)
Altima C18 (4.6 x 150mm, 5µm)
Detector wave length
247nm
247nm
247nm
247nm
220nm
Column temperature
30°C
30°C
30°C
30°C
30°C
Injection volume
10mL
10mL
10mL
10mL
10mL
Run time
8.0 min
14 min
10 min
10 min
6min
Diluent
Water and Acetonitrile in the ratio 50:50
Water and Acetonitrile in the ratio 50:50
Water and Acetonitrile in the ratio 50:50
Water and Acetonitrile in the ratio 50:50
Water and Acetonitrile in the ratio 50:50
Table 1: Method development: Method development was done by changing various, mobile phase ratios, buffers etc.
Trials
Results
Trial 1
Both were eluted but resolution was less & peak shape also not good so, further trial is carried out.
Trial 2
Both peaks were eluted but peak shapes were good but retention time was more than literature review So, Further Trial is carried out
Trial 3
Clopidogrel & Atorvastatin both peak are eluted but Retention was more so, further trail was carried.
Trial 4
Clopidogrel & Atorvastatin both peak are eluted but retention time was more so, further trial is Carried out
Optimized Method
Both peaks have good resolution, tailing factor, theoretical plate count and resolution.
Table 2: Chromatographic conditions.
Preparation of Standard working solutions (100% solution): 1ml from each stock solution was pipetted out and taken into a 10ml volumetric flask and made up with diluent. (75μg/ml Clopidogrel of and 10μg/ml of Atorvastatin).
Preparation of Sample stock solutions: 5 tablets were weighed and the average weight of each tablet was calculated, then the weight equivalent to 1 tablet was transferred into a 100ml volumetric flask, 50ml of diluents was added and sonicated for 25min, further the volume was made up with diluent and filtered by HPLC filters (750μg/ ml of Clopidogrel and 100μg/ml of Atorvastatin).
Preparation of Sample working solutions (100% solution): 1ml of filtered sample stock solution was transferred to 10ml volumetric flask and made up with diluent. (75μg/ml of Clopidogrel and 10μg/ ml of Atorvastatin).
Preparation of buffer 0.01N Kh2po4 Buffer: Accurately weighed 1.36gm of Potassium dihyrogen Ortho phosphate in a 1000ml of Volumetric flask add about 900ml of milli-Q water added and degtas to sonicate and finally make up the volume with water then added 1ml of Triethylamine then PH adjusted to 3.8 with dil. Orthophosphoric acid solution.
Results and Discussion
Clopidogrel and Atorvastatin were eluted at 2.555min and 3.207min respectively with good resolution. Plate count and tailing factor was very satisfactory, so this method was optimized and to be validated (Figure 3).
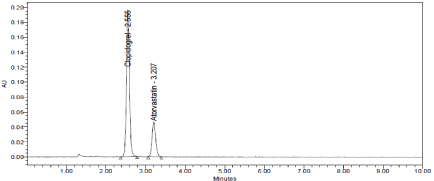
Figure 2: Optimized Chromatogram.
System suitability
All the system suitability parameters were within the range and satisfactory as per ICH guidelines (Table 3) [14].
S no
Clopidogrel
Atorvastatin
Resolution
Inj
RT(min)
USP Plate Count
Tailing
RT(min)
USP Plate Count
Tailing
1
2.535
6881
1.07
3.221
6702
1.11
4.3
2
2.543
6609
1.03
3.223
6810
1.08
4.3
3
2.544
6620
1.03
3.224
6907
1.13
4.7
4
2.548
6826
1.05
3.224
6975
1.1
4.7
5
2.584
6830
1.04
3.233
6947
1.1
4.9
6
2.589
6484
1.05
3.244
6850
1.11
4.8
Table 3: System suitability parameters for Clopidogrel and Atorvastatin.
According to ICH guidelines plate count should be more than 2000, tailing factor should be less than 2 and resolution must be more than 2. All the system suitable parameters were passed and were within the limits.
Linearity
Six linear concentrations of Clopidogrel (18.75-112.5μg/ml) and Atorvastatin (2.5-15μg/ml) were injected in a duplicate manner. Average areas were mentioned above and linearity equations obtained for Clopidogrel was y =16467.x + 5787 and of Atorvastatin was y = 34860x + 5859 Correlation coefficient obtained was 0.999 for the two drugs (Table 4 Figure 4 & 5).
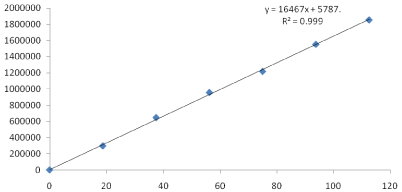
Figure 4: Calibration curve of Clopidogrel.
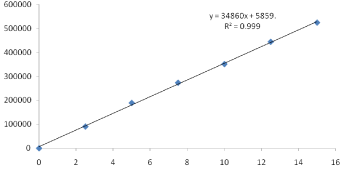
Figure 5: Calibration curve of Atorvastatin.
Clopidogrel
Atorvastatin
Conc (μg/ml)
Peak area
Conc (μg/ml)
Peak area
0
0
0
0
18.75
296899
2.5
90425
37.5
646222
5
189018
56.25
956626
7.5
273091
75
1218095
10
351010
93.75
1552319
12.5
443823
112.5
1854090
15
523808
Table 4: Linearity data for Clopidogrel and Atorvastatin.
System Precision
From a single volumetric flask of working standard solution six injections were given and the obtained areas were mentioned above. Average area, standard deviation and %RSD were calculated for two drugs. %RSD obtained as 0.6% and 0.8% respectively for Clopidogrel and Atorvastatin. As the limit of Precision was less than “2” the system precision passed in this method and the results were within the limits (Table 5).
S. No
Area of Clopidogrel
Area of Atorvastatin
1
1208220
352812
2
1203948
352344
3
1210969
356964
4
1224754
359020
5
1214415
353644
6
1204448
351917
Mean
1211126
354450
S.D
7760.5
2877.1
%RSD
0.6
0.8
Table 5: System precision data of Clopidogrel and Atorvastatin.
Repeatability
Multiple sampling from a sample stock solution was done and six working sample solutions of same concentrations were prepared, each injection from each working sample solution was given and obtained areas were mentioned in the above table. Average area, standard deviation and %RSD were calculated for two drugs and obtained as 0.4% and 0.3% respectively for Clopidogrel and Atorvastatin. As the limit of Precision was less than “2” the system precision passed in this method and the results were within the limits (Table 6).
S. No
Area of Clopidogrel
Area of Atorvastatin
1
1208503
351049
2
1204001
352413
3
1207132
350097
4
1206232
352874
5
1201708
352019
6
1217089
352012
Mean
1207444
351744
S.D
5300.4
1006.7
%RSD
0.4
0.3
Table 6: Repeatability data of Clopidogrel and Atorvastatin.
Multiple sampling from a sample stock solution was done and six working sample solutions of same concentrations were prepared, each injection from each working sample solution was given on the next day of the sample preparation and obtained areas were mentioned in the above table. Average area, standard deviation and %RSD were calculated for two drugs and obtained as 0.3% and 0.3% respectively for Clopidogrel and Atorvastatin. As the limit of Precision was less than “2” the system precision passed in this method and the results were within the limits.
Intermediate precision (Day_ Day Precision)
Table 7.
S. No
Area of Clopidogrel
Area of Atorvastatin
1
1208783
351572
2
1210503
352049
3
1204001
352413
4
1207132
352874
5
1201708
350019
6
1207089
352012
Mean
1206536
351823
S.D
3199.5
985.6
%RSD
0.3
0.3
Table 7: Intermediate precision data of Clopidogrel and Atorvastatin.
Accuracy
Three levels of Accuracy samples were prepared by standard addition method. Triplicate injections were given for each level of accuracy and mean %Recovery was obtained as 99.13% and 99.40% for Clopidogrel and Atorvastatin respectively (Table 8, Figure 6-8).
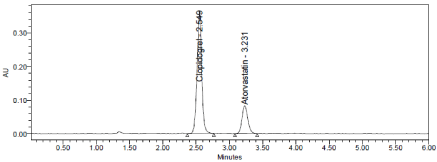
Figure 6: Accuracy 50% Chromatogram of Clopidogrel and Atorvastatin.
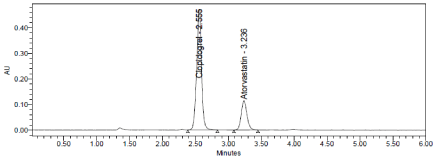
Figure 7: Accuracy 100% Chromatogram of Clopidogrel and Atorvastatin.
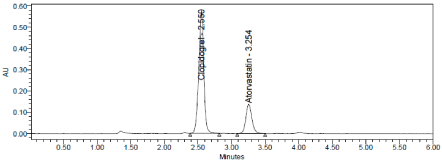
Figure 8: Accuracy 150% Chromatogram of Clopidogrel and Atorvastatin.
% Level
Amount Spiked (μg/mL)
Amount recovered (μg/mL)
% Recovery
Mean %Recovery
50%
37.5
37.207992
99.22
99.13%
37.5
36.985183
98.63
37.5
37.425518
99.8
100%
75
74.537074
99.38
75
73.998057
98.66
75
73.880428
98.51
150%
112.5
110.82893
98.51
112.5
112.23417
99.76
112.5
112.11265
99.66
Table 8a: Accuracy data of Clopidogrel.
% Level
Amount Spiked (μg/mL)
Amount recovered (μg/mL)
% Recovery
Mean %Recovery
50%
5
4.9640562
99.28
99.40%
5
4.9474182
98.95
5
4.9121916
98.24
100%
10
9.9978199
99.98
10
9.9918531
99.92
10
9.911331
99.11
150%
15
14.85066
99
15
14.998537
99.99
15
15.020683
100.14
Table 8b: Accuracy data of Atorvastatin.
Sensitivity
Table 9 Figure 9 & 10.
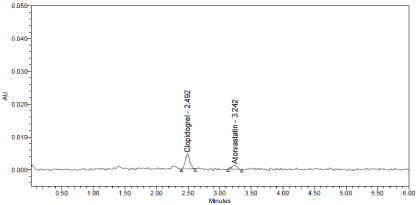
Figure 9: LOD Chromatogram of Standard.
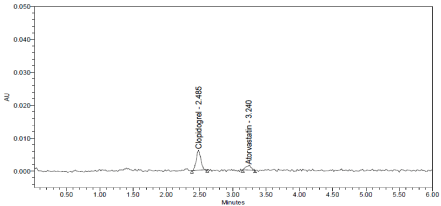
Figure 10: LOQ Chromatogram of Standard.
Molecule
LOD
LOQ
Clopidogrel
0.26
0.44
Atorvastatin
0.79
0.11
Table 9: Sensitivity table of Clopidogrel and Atorvastatin.
Robustness
Robustness conditions like Flow minus (0.9ml/min), Flow plus (1.1ml/min), mobile phase minus (60B:40A), mobile phase plus (55B:45A), temperature minus (25°C) and temperature plus (35°C) was maintained and samples were injected in duplicate manner. System suitability parameters were not much affected and all the parameters were passed. %RSD was within the limit (Table 10, Figure 11-13).
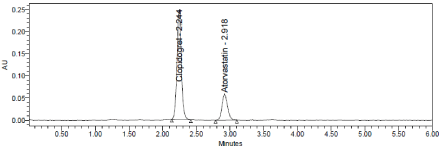
Figure 11: Flow plus Chromatogram of Clopidogrel and Atorvastatin.
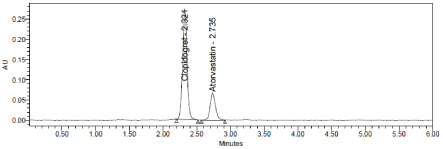
Figure 12: Mobile phase plus Chromatogram of Clopidogrel and Atorvastatin.
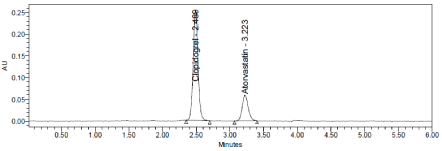
Figure 13: Temperature plus Chromatogram of Clopidogrel and Atorvastatin.
S.no
Condition
%RSD of Clopidogrel
%RSD of Atorvastatin
1
Flow rate (-) 0.9ml/min
0.7
1.4
2
Flow rate (+) 1.1ml/min
0.8
0.3
3
Mobile phase (-) 60B:40A
0.2
0.7
4
Mobile phase (+) 45B:45A
0.7
0.7
5
Temperature (-) 25°C
0.9
1.2
6
Temperature (+) 35°C
0.4
0.4
Table 10: Robustness data for Clopidogrel and Atorvastatin.
Assay
Axis Life Science Pvt Ltd (Axigrel AT), bearing the label claim Clopidogrel 75mg, Atorvastatin 10mg. Assay was performed with the above formulation.. Average % Assay for Clopidogrel and Atorvastatin obtained was 99.60% and 99.14% respectively (Table 11 Figure 14 & 15).
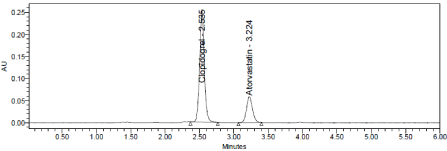
Figure 14: Chromatogram of working standard solution.
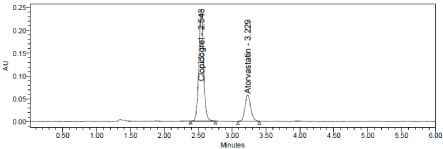
Figure 15: Chromatogram of working sample solution.
S.no
Standard Area
Sample area
% Assay
S. no
Standard Area
Sample area
% Assay
1
1208220
1208503
99.68
1
352812
351049
98.94
2
1203948
1204001
99.31
2
352344
352413
99.33
3
1210969
1207132
99.57
3
356964
350097
98.67
4
1224754
1206232
99.5
4
359020
352874
99.46
5
1214415
1201708
99.12
5
353644
352019
99.21
6
1204448
1217089
100.39
6
351917
352012
99.21
Avg
1211126
1207444
99.6
Avg
354450
351744
99.14
Stdev
7760.5
5300.4
0.44
Stdev
2877.1
1006.7
0.2837
%RSD
0.6
0.4
0.44
%RSD
0.8
0.3
0.3
Table 11: Assay Data of Clopidogrel & Atorvastatin.
Degradation
Degradation Studies: Degradation studies were performed with the formulation and the degraded samples were injected. Assay of the injected samples was calculated and all the samples passed the limits of degradation (Table 12, Figure 16 & 17).
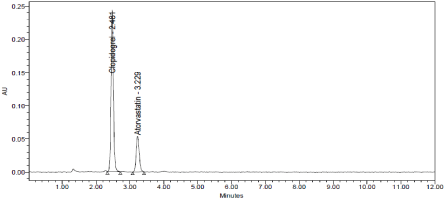
Figure 16: Acid chromatogram of Clopidogrel and Atorvastatin.
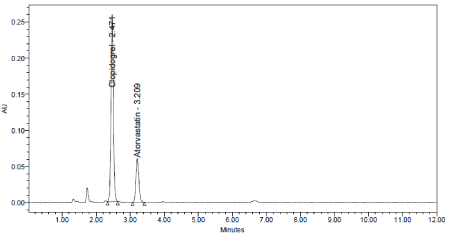
Figure 17: Base chromatogram of Clopidogrel and Atorvastatin.
S.NO
Degradation Condition
% Drug Degraded
Purity Angle
Purity Threshold
1
Acid
4.61
0.544
0.589
2
Alkali
2.93
0.461
0.529
3
Oxidation
1.81
0.523
0.592
4
Thermal
0.51
0.427
0.532
5
UV
0.62
0.321
0.421
6
Water
0.62
0.357
0.522
Table 12a: Degradation Data of Clopidogrel.
S.NO
Degradation Condition
% Drug Degraded
Purity Angle
Purity Threshold
1
Acid
4.76
0.546
0.772
2
Alkali
2.95
0.6
0.733
3
Oxidation
1.91
0.578
0.635
4
Thermal
0.62
0.536
0.756
5
UV
0.98
0.632
0.698
6
Water
0.62
0.535
0.725
Table 12a: Degradation Data of Atorvastatin.
Conclusion
A new method was established for simultaneous estimation of Clopidogrel and Atorvastatin by RP-HPLC method. The proposed HPLC method was found to be simple, specific, precise, accurate, rapid and economical for simultaneous estimation of Clopidogrel and Atorvastatin in pharmaceutical dosage form. The developed method was validated in terms of accuracy, precision, linearity, robustness and ruggedness, and results will be validated statistically according to ICH guidelines. The Sample recoveries in all formulations were in good agreement with their respective label claims. Hence the suggested RPHPLC method can be used for routine analysis of Clopidogrel and Atorvastatin in API and Pharmaceutical dosage form.
Acknowledgements
The authors are thankful to the management of Seven Hills College of Pharmacy for providing the necessary facilities to carry out the research work and for their encouragement and support.
References
- SV Londhe, RS Deshmukh, SV Mulgund and KS Jain, et al. Development and validation of a reversed-phase HPLC method for simultaneous determination of aspirin, atorvastatin calcium and clopidogrel bisulphate in capsules. Indian Journal pharm sci. 2011; 73: 23–29.
- Shrivastava PK, Basniwal PK, Jain D, Shrivastava SK. Concurrent Estimation of Clopidogrel Bisulfate and Aspirin in Tablets by Validated RP-HPLC Method. Indian J Pharm Sci. 2008; 707: 667-669.
- Jain N, Raghuwanshi R, Jain D. Development and Validation of RP-HPLC Method for Simultaneous Estimation of Atorvastatin Calcium and Fenofibrate in Tablet Dosage Forms. Indian J Pharm Sci. 2008; 70: 263-265.
- Shah DA, Bhatt KK, Mehta RS, Baldania SL, Gandhi TR. Stability Indicating RP-HPLC Estimation of Atorvastatin Calcium and Amlodipine Besylate in Pharmaceutical Formulations. Indian J Pharm Sci. 2008; 70: 754-760.
- Peraman R, Mallikarjuna S, Ammineni P, Kondreddy Vk. RP-HPLC method development and validation for simultaneous estimation of atorvastatin calcium and pioglitazone hydrochloride in pharmaceutical dosage form. J Chromatogr Sci. 2014; 52: 1038-1042.
- Game MD, Gabhane KB, Sakarkar DM. Quantitative analysis of clopidogrel bisulphate and aspirin by first derivative spectrophotometric method in tablets. Indian J Pharm Sci. 2010; 72: 825-828.
- Sultana N, Arayne MS, Ali KA, Nawaz M. Simultaneous determination of clopidogrel and aspirin by RP-HPLC from bulk material and dosage formulations using multivariate calibration technique. J Chromatogr Sci. 2011; 49: 165-169.
- Ramesh D, Habibuddin M. Stability Indicating RP-HPLC Method for the Simultaneous Determination of Atorvastatin Calcium, Metformin Hydrochloride, and Glimepiride in Bulk and Combined Tablet Dosage Form. Int Sch Res Notices. 2014; 2014: 754695.
- Nalini K Sahoo, Madhusmita Sahu, Podilapu S Rao, Jajula N Indira, Sandhya N Rani, Goutam K Ghosh, et al. Validation of assay for bulk clopidogrel and for some tablet forms by Reverse-Phase High-Performance Liquid Chromatography. Journal of Taibah University for Science. 2014; 331–336.
- Panchal HJ, Suhagia BN. Simultaneous determination of atorvastatin calcium and ramipril in capsule dosage forms by high-performance liquid chromatography and high-performance thin layer chromatography. J AOAC Int. 2010; 93: 1450-1457.
- Croitoru O, Spiridon AM, Belu I, Turcu-Stiolica A, Neamtu J. Development and Validation of an HPLC Method for Simultaneous Quantification of Clopidogrel Bisulfate, Its Carboxylic Acid Metabolite, and Atorvastatin in Human Plasma: Application to a Pharmacokinetic Study. J Anal Methods Chem. 2015; 2015: 892470.
- Shah Y, Iqbal Z, Ahmad L, Khan A, Khan MI, Nazir S, Nasir F. Simultaneous determination of rosuvastatin and atorvastatin in human serum using RPHPLC/ UV detection: method development, validation and optimization of various experimental parameters. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879: 557-563.
- Pawan K Porwal Akhalaque, Ahmad RA, Santosh S Chhajed. Liquid Chromatographic Method for Simultaneous Quantitation of Clopidogrel, Aspirin and Atorvastatin in Rat Plasma and Its Application to the Pharmacokinetic Study Journal of Chromatographic Science. 2015; 53: 1155–1162.
- ICH Harmonised Tripartite Guideline, validation of analytical procedures: Text methodology, Q2 (R1). International Conference on Harmonization, Geneva. 2005; pp: 1-13.