
Research Article
Austin J Anal Pharm Chem. 2018; 5(3): 1105.
Nano Graphene Oxide as Solid Phase Extraction Adsorbent Coupled with Dispersive Liquid-Liquid Microextraction to Determine Ultra-Trace Quantities of Propranolol from Urine Samples
Gholami A1, Bahrami F1* and Faraji M2
1Department of Analytical Chemistry, Faculty of Science, University of Kashan, Kashan, Iran
2Faculty of Food Industry and Agriculture, Department of Food Science & Technology, Standard Research Institute (SRI), Iran
*Corresponding author: Fahimeh Bahrami, Department of Analytical Chemistry, Faculty of Science, University of Kashan, Kashan, Iran
Received: August 28, 2018; Accepted: September 18, 2018; Published: September 19, 2018
Abstract
In this research, a new nano graphene oxide based solid phase extraction followed by Dispersive Liquid-Liquid Microextraction was applied as simple, rapid and sensitive determination of trace amounts of Propranolol (PRO) in urine samples with HPLC-UVD. Several factors influencing the extraction of PRO, such as pH, adsorbent amounts, extraction time, organic solvent type and the composition of solvent and desorption conditions were studied and optimized. Under optimum condition, the limit of detection (LOD) and limit of quantification (LOQ) of the proposed method were 2ngmL−1 and 6.6ng/mL, respectively. Good linear behaviour over the investigated concentration ranges (2-2000ngmL-1) and good correlation coefficient of 0.9901 (r2) were obtained. The relative standard deviations (RSDs) based on three determinations at 2, 20, 200 ngml-1 levels of PRO was less than 9.7%. The findings of the present study may provide clinical and diagnostic laboratories.
Keywords: Propranolol (PRO); Nano Grapheme oxide (NGO); Solid Phase Extraction (SPE); High Performance Liquid Chromatography (HPLC); Dispersive Liquid-Liquid microextraction ( DLLME); Urine analysis
Introduction
Propranolol (PRO), one of the most widely prescribed–blockers in the long-term treatment of hypertension and cardiovascular diseases is usually taken orally, although an intravenous form is available for acute administration [1]. Figure 1 showed the chemical structure and properties of PRO. It is used in the treatment or prevention of many disorders including acute myocardial infarction, arrhythmias, angina pectoris, hypertension, hypertensive emergencies, hyperthyroidism, migraine, pheochromocytoma, menopause, and anxiety. It is chemically described as in (RS)-1-(1-methylethylamino)-3-(1 -naphthyloxy) and its molecular formula is C16H21NO2 [2-4].
Recent years, the application of nanomaterials in extraction procedures, as stationary phases or adsorbents, has undergone rapid growth [5,6]. Graphene (G), a new allotropic member of carbon, due to its excellent physical and chemical properties, has been studied world-wide for several purposes since its discovery in 2004 [7-10]. The layered structure of graphite and GO is the same, but the plane of carbon atoms in graphite oxide is heavily decorated by oxygen containing groups. Since the reason, graphene (G) and graphene oxide (GO) has attracted attention from many researchers [7].
Blockers (known also as adrenergic antagonists) due to their intensive use for the treatment of various cardiovascular disorders, poor degradability and inefficient removal by wastewater treatment processes [11-12] able to affect the balance of ecological systems, even at low concentrations. It is easy to understand the necessity for their determination in the environment.
Various extraction techniques, such as membrane extraction [13], solid–phase extraction, [14,15] solid–phase microextraction [16-19], liquid–phase microextraction [11,13,16,20-22] were used to extract these classes of compounds from aqueous matrices. All these techniques possess pros and cons making each one more preferable than the others under different circumstances.
Dispersive liquid–liquid microextraction is an alternative to the classical liquid–liquid extraction and includes extractant volumes, at microliter levels [23]. Briefly, a mixture of an extracting and a dispersing solvent is rapidly injected in an aqueous sample and a cloudy solution is formed for the analysis of PRO in pharmaceutical and biological samples including spectrophotometry [17,18], spectrofluorometry [24], chromatography [20,21], chemiluminescence combined with electrochemical and electroanalytical methods [25-28].
In the present study, GO used for the adsorption/removal of PRO from urine sample. Afterwards, a simple, rapid and sensitive HPLC method with UV detection (HPLC-UVD), adequate sensitivity and short elution time is described for determination of PRO in these samples.
Experimental
Chemicals and reagents
All of the reagents used were of analytical grade. PRO was purchased from daroupakhsh (Tehran, Iran). The chemical structure properties of PRO is shown in Figure 1. HPLC-grade acetonitrile, methanol, sodium dilydrogen phosphate, sodium hydroxide and hydrochloric acid were all purchased from Merck (Darmstadt, Germany). Nano Graphen Oxide (NGO) were provided from Sigma Aldrich Company (Steinheim, Germany). The diameter of NGO were less than 50nm. The reagent water used was purified with a Milli-Q system from Millipore (Bedford, MA, USA). Stock standard solution of PRO (1000mgL−1) was prepared by dissolving in 5mL methanol and then diluted with reagent water. The working solutions were prepared by proper dilution of the standard solution in the reagent water.
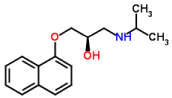
Figure 1: Chemical structure of propranolol.
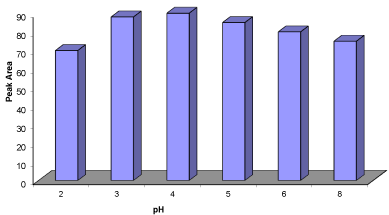
Figure 2: Effect of pH on extraction efficiency. Conditions: Sample volume =
30mL; concentration of the propranolol = 100ngL-1; 20mg NGO; stirring time
= 15min; elution with 1mL (2×0.5ml) acetic methanol; desorption time = 4min.
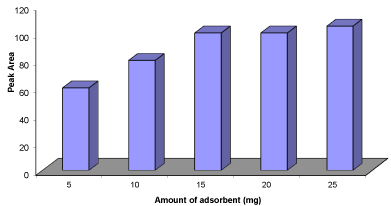
Figure 3: Effect of weight of NGO extraction efficiency. Conditions: Sample
volume = 30mL; concentration of the propranolol = 100ngL-1; sample’s pH =
4.0; stirring time = 15min; elution with 1mL (2×0.5ml) methanol; desorption
time = 4min.
Apparatus
The HPLC-UVD operating mode was isocratic, injection volume was 20μL and column temperature was adjusted to room temperature. The chromatography column was a HPLC column Waters Symmetry C8 15cm × 4.6mm, 3.5μm. The mobile phase used was a combination mixture of phosphate buffer with pH=2.5 and Methanol (50:50 V/V). The flow rate was 1mLmin−1. The mobile phase was filtered through a (0.45μm) pore size filter (Merck Millipore, Billerica, Massachusetts, USA) and degassed by vacuum prior to use. The UV- visible detector was adjusted to 290nm.
A 40kHz and 0.138kW ultrasonic water bath with temperature control (Tecno-GazSpA Italy) was applied for ultrasonication of the samples. All of the pH measurements were performed with a Jenway model 3320 pH meter (Staffordshire ST15 0SA, England) supplied with a combined glass electrode. A Stuart CB162 motor-stirrer (Staffordshire ST15 0SA, England) was applied to stir solutions by a magnet.
Solid phase extraction-dispersive liquid-liquid microextraction procedure
15mg of NGO was dispersed in a 30mL sample in a 50mL beaker and then sonicated for about 2min. The mixture was shaken for about 10min and then the sorbent was separated from the solution by centrifugation (4000rpm, 5min). The preconcentrated target analytes were eluted from the isolated sorbent with 1.0mL (2×0.5 ml) acetic methanol for about 5min. Afterwards, eluted analyts collected into a 10mL screw cap glass test tube with conical bottom afterwards, 100μL chloroform (as the extraction solvent) were added to the test tube. A 5.0mL of deionized water was quickly injected into the test tube through a 5.00mL gastight syringe from Hamilton (Reno, NV, USA). A cloudy solution, resulting from distribution of fine chloroform droplets in the aqueous solution, was formed in the test tube. At this stage, the analyte were extracted into the fine droplets of chloroform in a few seconds. Next, the solution was shaken for about 5min and centrifuged at 4000rpm for 5min. The organic layer was then transferred into a 4mL conical vial and evaporated under a stream of nitrogen. The residue was redissolved in 100μL of a mobile phase, finally, a 20μL portion of this solution was injected into the HPLCUVD system. All experiments were run in triplicates, and the mean values were exploited for optimization.
Sample preparation of real samples
In order to study the feasibility of the proposed SPE method for extraction and determination of PRO in the real samples, the developed technique was applied for the extraction of PRO in urine samples. In order to reduce the matrix effect, the spiked urine sample were diluted to 1:10 using ultra-pure water without further treatment. The pH of urine samples were adjusted to 4 and then centrifuged for 15 minutes until a white lipidic solid sedimented in the bottom of the tube [29]. Then the supernatant was transferred into a clean tube and spiked with PRO. All samples were stored at 4ºC and directly used for MSPE.
Result and Discussion
Optimization of DSPE parameters
To evaluate the effects of the operating parameters on the extraction recovery of the selected analytes and based on preliminary studies, four main variables (adsorption time, sorbent mass, desorption time, pH of sample, organic solvent type and the composition of solvent used for drug desorption) were chosen. The other variables and interactions were insignificant and were therefore neglected in further studies.
Effect of sample’s pH: The pH of the sample influenced the reaction between PRO and NGO. The analyte is electrically neutral, so it can be efficiently adsorbed and desorption is unaffected by the charges on the surface of the adsorbent. The pH of the spiked urine samples were adjusted from 2 to 8 by 0.1 M HCl and NaOH (Figure 2). PRO is secondary amines with acidity constants (pKa=9.5) and therefore it is present in solution primarily in positively charged form at operating pH conditions (pH 2 –8). Thus, at strong acidic conditions (pH=3) where the highest drug removal was observed.
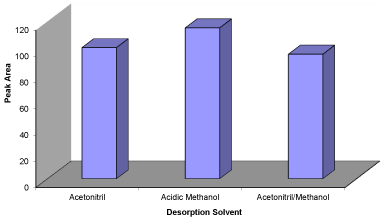
Figure 4: Effect of various extraction solvent on extraction efficiency.
Conditions: Sample volume = 30mL; concentration of the propranolol =
100ngL-1; sample’s pH = 4.0; 15mg NGO; stirring time = 15min; elution with
1mL (2×0.5ml) methanol; desorption time = 4min.
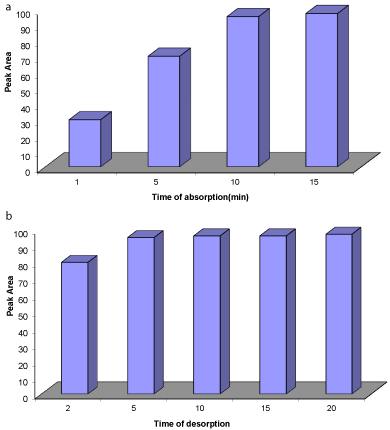
Figure 5: (a) Effect of different time of drug extraction; (b) effect of various
time of desorption, other extraction conditions are as mentioned in Figure 4.
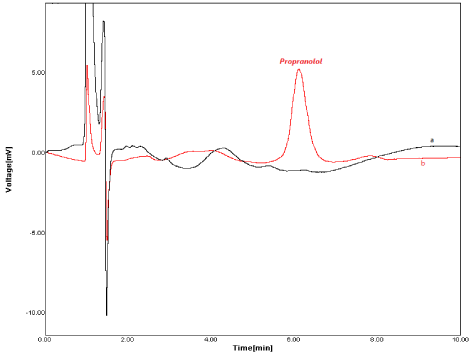
Figure 6: Chromatograms of blank urine (a) positive urine; (b) samples.
Effect of the adsorbent amount: Amount of adsorbent seems to affect drug extraction efficiency; therefore, the amount of NGO was optimized in the range of 5-25 mg (Figure 3). The addition of increasing amounts of adsorbent up to 15mg possibly helped the analyte and adsorbent reaction by providing an adequate surface for drug adsorption, but in high amounts of solid phase, low extraction efficiency was obtained. This is probably a result of NGO aggregation and a decrease in the effective adsorption surface area. Therefore, the remaining experiments were carried out with 15mg of NGO.
Selection of proper eluent: Desorption of the analyte from MSPE adsorbent was carried out using various organic solvents such as acetic methanol, acetic acetonitrile and equal mixture of acetonitrile and methanol. Among these solvents, acetic methanol showed the highest peak area compared with the others. Therefore, acetic methanol was chosen for the rest of the experiments. Methanol is a stronger polar solvent than acetonitrile. Moreover, NGO can be dispersed in polar solvents because of the presence of polar groups on its surface. Maximum extraction efficiency in methanol resulted from the high dispersion of NGO in acetic methanol, which provided the maximum surface for the drug and adsorbent interaction (Figure 4).
Effect of extraction and desorption time: Extraction time is an important parameter in the MSPE procedure, because maximum extraction efficiency depends on the time it takes the extraction to reach equilibrium. A time range of (1-15 minutes) was investigated for spiked urine samples. The highest peak area was achieved in 5min, but extraction efficiency remained constant with subsequent increases in time. This phenomenon may be an expression of extraction equilibrium achievement in 10 minutes (Figure 5a). Desorption time is another important factor in the MSPE method; it seems to affect the amount of drug desorbed from the NGO surface. In the time range of (2-20 minutes), the amount of desorbed drug was studied. Extraction efficiency increased with a time up to 5min, and then peak area decreased (Figure 5b). Therefore, 5min was selected for the rest of the experiments.
Method validation
The quantitative analytical characteristics of linear range, correlation coefficient (r2), limit of detection (LOD), limit of quantification (LOQ), and relative standard deviation (RSD) are shown in Table 1. The calibration curve was constructed by plotting the mean peak area of five concentrations, each in three measurements. The blank urine sample was spiked with PRO at different concentrations (2, 20, 200 ng/mL) and analyzed using the MSPE procedure. Accuracy and precision experiments were performed at three concentrations covering the calibration range (Table 2). Considering the complexity of the biological matrix, these results can be regarded as satisfactory.
Application of MSPE procedure to real sample
In order to study the feasibility of the proposed MSPE method for extraction and determination of PRO in the real sample, the developed technique was applied for the extraction of drug from the urine sample (Table 3). The application of the proposed method was investigated using positive urine samples. Figure 6 presents the blank urine and positive urine chromatograms. For reducing the matrix effect, the spiked urine sample were diluted to 1:10 using ultra-pure water without further treatment.
Analyte
Concentration range(ng/ml)
Linearity (r²)
LOD (ng/ml)
LOQ (ng/ml)
RSD (%)
Propranolol
2-2000
0.9901
2
6.6
3.5
Table 1: Table of figures of merit for MSPE extraction of propranolol.
Linearity is described by the correlation coefficient for the calibration curve. Limit of Detection (LOD): S/N=3; Limit of Quantification (LOQ): S/N=10; RSD: Relative Standard Deviation.
Analyte Concentration (ng/ml)
Intra-day (n=3)
Inter-day (n=3)
Precision
Accuracy
Precision
Accuracy
(RSDª)
(bias)
(RSDª)
(bias)
2
5.1
0.58
6.1
0.39
20
7.6
0.3
5.2
0.7
200
9.7
0.9
8.3
0.95
Table 2: Result of method validation of MSPE extraction.
ªRelative Standard Deviation.
Analyte
Recovery (%)
Subject 1
96.13
Subject 2
97.15
Table 3: Result of method validation of GO-based SPE-DLLME–HPLC-UVD method.
Conclusion
In this study, a new NGO-based SPE-DLLME–HPLC-UVD method was developed for determination of PRO in urine samples. The samples were initially extracted in via the NGO- based SPE, and then the eluents of this stage were exploited as disperser solvent for the following DLLME procedure for further purification and enrichment of the analytes prior to HPLC analysis. As a consequence, High extraction efficiency method was achieved, resulting in a low detection limit. The proposed method offers a simple, sensitive, inexpensive method for extraction and determination the presence of PRO in positive urine samples. Based on the obtained results and due to the commercial availability of NGO, it is anticipated that the proposed method has a great analytical potential in pretreatment of drugs from real samples in the same way. Furthermore, the findings of the present study may provide clinical and diagnostic laboratories with an improved analytical method for determining the presence of PRO.
References
- C Jantarat, N Tangthong, S Songkro, GP Martin, R Suedee. S-Propranolol imprinted polymer nanoparticle-on-microsphere composite porous cellulose membrane for the enantioselectively controlled delivery of racemic propranolol. International Journal of Pharmaceutics. 2008; 349: 212–225.
- GG Oliveira, DC Azzi, FC Vicentini, ER Sartori, OF Filho. Voltammetric determination of verapamil and propranolol using a glassy carbon electrode modified with functionalized multiwalled carbon nanotubes within a poly (allylamine hydrochloride) film. J. Electroanal. Chem. 2013; 708: 73–79.
- J Zhanga, L Dinga, A Wenb, F Wua, L Suna, L Yang. An HPLC–ESI–MS method for the determination of propranolol in human plasma and its application to pharmacokinetic studies. Asian J. Pharm. Sci. 2009; 4: 169–177.
- T Alizadeh, L Allahyari. Highly-selective determination of carcinogenic derivative of propranolol by using a carbon paste electrode incorporated with nano-sized propranolol-imprinted polymer. Electrochim. Acta. 2013; 111: 663–673.
- K Scida, PW Stege, G Haby, GA Messina, CD García. Recent applications of carbon- based nanomaterials in analytical chemistry: critical review. Anal.Chim. Acta. 2011; 691: 6–17.
- M Valcárcel, S Cárdenas, BM Simonet, Y Moliner-Martínez, R Lucena. Carbon nanostructures as sorbent materials in analytical processes. TrAC, Trends Anal. Chem. 2008; 27: 34–43.
- GZ Kyzas, A Koltsakidou, SG Nanaki, DN Bikiaris, DA Lambropoulou. Removal of beta-blockers from aqueous media by adsorption onto graphene oxide. Science of the Total Environment. 2015; 537: 411–420.
- BH Fumes, MR Silva, FN Andrade, CED Nazario, FM Lancas. Recent advances and future trends in new materials for sample preparation. TrAC,Trends Anal. Chem. 2015; 71: 9–25.
- Q Liu, J Shi, G Jiang. Application of graphene in analytical sample preparation. TrAC, Trends Anal. Chem. 2012; 37: 1–11.
- G Liu, W Jiang, Y Wang, S Zhong, D Sun, J Liu. One-pot synthesis of Ag@Fe3O4/reduced graphene oxide composite with excellent electromagnetic absorption properties. Ceram. Int. 2015; 41: 4982–4988.
- AA Salem, IA Wasfi, SS Al-Nassibi. Trace determination of-blockers and 2-agonists in distilled and waste-waters using liquid chromatography–tandem mass spectrometry and solid-phase extraction. J. Chromatogr. B. 2012; 908: 27–38.
- S Zorita, P Hallgren, L Mathiasson. Steroid hormone determination in water using an environmentally friendly membrane based extraction technique. J. Chromatogr. A. 2008; 1192: 1–8.
- B Huang, XJ Pan, X Wan, JL Liu, SM Zhao, P Hu, FR Li. Simultaneous determination of steroid endocrine disrupting chemicals in water by solid phase extraction-derivatization-gas chromatographic–mass spectrometry. Chin. J. Anal. Chem. 2011; 39: 449–454.
- Y Nie, Z Qiang, H Zhang, C Adams. Determination of endocrine-disrupting chemicals in the liquid and solid phases of activated sludge by solid phase extraction and gas chromatography–mass spectrometry, J. Chromatogr. A. 2009; 1216: 7071–7080.
- W Liu, Z Yan, X Huang, J Chen, M Lu, L Zhang, G Chen. Simultaneous determination of blockers and agonists by on-fiber derivatization in self-made solid-phase microextraction coating fiber. Talanta. 2015; 132: 915–921.
- PCF Lima Gomes, BB Barnes, ÁJ Santos-Neto, FM Lancas, NH Snow. Determination of steroids, caffeine and methylparaben in water using solid phase microextraction-comprehensive two-dimensional gas chromatography–time of flight mass spectrometry. J. Chromatogr. A. 2013; 1299: 126–130.
- L Qiu, W Liu, M Huang, L Zhang. Preparation and application of solid-phase microextraction fiber based on molecularly imprinted polymer for determination of anabolic steroids in complicated samples. J. Chromatogr. A. 2010; 1217: 7461–7470.
- K Saito, K Yagi, A Ishizaki, H Kataoka. Determination of anabolic steroids in human urine by automated in-tube solid-phase microextraction coupled with liquid chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2010; 52: 727–733.
- L Ciofi, D Fibbi, U Chiuminatto, E Coppini, L Checchini, M del Bubba. Fully-automated on-line solid phase extraction coupled to high-performance liquid chromatography– tandem mass spectrometric analysis at sub-ng/L levels of selected estrogens in surface water and wastewater. J. Chromatogr. A. 2013; 1283: 53–61.
- F Guo, Q Liu, G Qu, S Song, J Sun, J Shi, G Jiang. Simultaneous determination of five estrogens and four androgens in water samples by online solid-phase extraction coupled with high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2013; 1281: 9–18.
- E Pujos, C Cren-Olivé, O Paisse, MM Flament-Waton, MF Grenier-Loustalot. Comparison of the analysis of-blockers by different techniques. J. Chromatogr. B. 2009; 877: 4007–4014.
- M Rezaee, Y Assadi, MR Milani Hosseini, E Aghaee, F Ahmadi, S Berijani. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A. 2006; 1116: 1–9.
- L Xu, X Qi, X Li, Y Bai, H Liu. Recent advances in applications of nano materials for sample preparation. Talanta. 2016; 146: 714–726.
- K Scida, PW Stege, G Haby, GA Messina, CD García. Recent applications of carbon- based nano materials in analytical chemistry: critical review. Anal.Chim. Acta. 2011; 691: 6–17.
- M Valcárcel, S Cárdenas, BM Simonet, Y Moliner-Martínez, R Lucena. Carbon nanostructures as sorbent materials in analytical processes. TrAC, Trends Anal. Chem. 2008; 27: 34–43.
- BH Fumes, MR Silva, FN Andrade, CED Nazario, FM Lanc. Recent advances and future trends in new materials for sample preparation. TrAC, Trends Anal. Chem. 2015; 71: 9–25.
- Q Liu, J Shi, G Jiang. Application of graphene in analytical sample preparation. TrAC, Trends Anal. Chem. 2012; 37: 1–11.
- M Cruz-Vera, R Lucena, S Cárdenas, M Valcárcel. Sorptive microextraction for liquid chromatographic determination of drugs in urine. TrAC, Trends Anal. Chem. 2009; 28: 1164-1173.
M Caban, P Stepnowski, M Kwiatkowski, N Migowska, J Kumirska. Determination of-blockers and -agonists using gas chromatography and gas chromatography–mass spectrometry–a comparative study of the derivatization step. J. Chromatogr. A. 2011; 1218: 8110–8122.